Introduction
Laurencia coelenterata D. L. Ballantine and Aponte (Ballantine et Aponte, 1995) is a diminutive species, originally collected from the Dry Tortugas, Florida, at 9 m depth. According to the authors, the name given to it was due to the superficial resemblance of juvenile plants to an anemone. Despite this characteristic, the species is not easily found in the natural environment because of its small thalli.
The taxonomy of the red algal genus Laurencia J. V. Lamouroux sensu lato is extremely complicated because of the large degree of morphological plasticity and worldwide distribution from temperate to tropical oceans. Consequently, the taxonomic position of the species in this group has changed rapidly as new morphological and molecular data are processed. In the roughly two hundred years since the creation of the genus Laurencia (Lamouroux, 1813), many changes have been proposed in what we currently refer to as the Laurencia complex, made up of seven formally proposed genera: Laurencia sensu stricto, Osmundea Stackhouse (Stackhouse, 1809), Chondrophycus (Tokida et Saito) Garbary et J. T. Harper (Garbary & Harper, 1998), Palisada (Yamada) K. W. Nam ( Nam, 2007), Laurenciella Gil-Rodríguez, Sentíes, Díaz-Larrea, Cassano et M. T. Fujii (Cassano et al., 2012), and most recently Coronaphycus Metti, (Metti et al., 2015).
In the Cuban archipelago, the Laurencia complex has 17 species, distributed into 3 genera: Laurencia sensu stricto (11), Palisada (5), and Yuzurua (1 with two varieties) (Suárez et al., 2015).
During the Laurencia complex expedition to the eastern coast of the Cuban archipelago, Laurencia coelenterata was found growing on the consolidated substrata in a biogenic sand beach, with limestone and beds of the seagrass Thallasia testudinum Banks ex König at the bottom. The detailed morphological studies carried out on this material revealed vegetative and reproductive characteristics belonging to the genus Osmundea, and not to the genus Laurencia (Ballantine & Aponte, 1995). In this study, previously unknown morphological features of L. coelenterata are revealed that justify its transfer to Osmundea. Emendation to the species’s original description is provided. The new combination is corroborated by chloroplast-encoded rbcL gene-sequence analysis.
Material and methods
Study area. Samples were collected at the Cazonal beach, Santiago de Cuba (19° 53’39’’ N and 75° 31’8 .7’’ W), Cuba. Cazonal is a biogenic sand beach, 60 cm deep, with isolated limestone and beds of the seagrass Thallasia testudinum at the bottom. The beach is located 52 km east of the city of Santiago de Cuba.
Morphological study. Transverse and longitudinal hand sections were made with a stainless-steel razor blade and stained with 0.5% aqueous aniline blue solution, acidified with 1N HCl, to highlight the diagnostic morphological features.
The microscopic measurements were obtained using a calibrated ocular micrometer. Photographs were taken with a Panasonic FT3 Lumix digital camera and the photomicrographs were obtained using a Zeiss Axiocan ERC-5S digital camera (Göttingen, Germany) coupled to an Axioskop 2 Zeiss microscope (Göttingen, Germany). The vouchers were deposited at the herbarium of the Instituto de Botânica, at São Paulo, Brazil (SP) and at the phycological collections of the Oriente University, at Santiago de Cuba, Cuba (FCA 191-ah).
Molecular study. Samples for molecular analysis were cleaned, dried, and preserved in silica gel. Total DNA was extracted using a DNeasy Plant Mini Kit (QIAGEN, Valencia, CA, USA), according to the manufacturer’s instructions. The rbcL gene was amplified in three overlapping fragments with the primer pairs suggested by (Freshwater & Rueness, 1994), using the Taq polymerase chain reaction (PCR) Core Kit (QIAGEN). All PCR products were analyzed by electrophoresis in 1% agarose to confirm product size. The PCR products were purified with the QIAquick Purification Kit (QIAGEN) according to the manufacturer’s recommendations. Cycle sequencing was carried out on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA), using the BigDye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems). Primers were used for PCR amplification and cycle sequencing. Sequences were analyzed with Sequence Navigator software, version 1.0.1 (Applied Biosystems).
Phylogenetic analyses. Phylogenetic relationships were inferred with PAUP* version 4.0b10 (Swofford 2002) and Mr. Bayes v.3.0 beta 4 (Huelsenbeck & Ronquist, 2001). Maximum parsimony (MP) trees were constructed by applying the heuristic search option, tree-bisection-reconnection (TBR) branch swapping, with unordered and unweighted characters, and gaps treated as missing data. Modeltest software, version 3.7 (Posada & Crandall, 1998), was used to find the model of sequence evolution least rejected in each data set by a hierarchical likelihood ratio test. Once the evolution model had determined a Maximum Likelihood (ML), searches were performed by applying the estimated parameters (substitution model, gamma distribution, proportion of invariant sites, and frequencies of the bases). Maximum likelihood analysis was then employed to construct the most likely tree from the data set. Maximum Likelihood and MP branch supports were calculated by nonparametric bootstrapping analysis (Felsenstein, 1985), as implemented in PAUP*.
The general time-reversible model of nucleotide substitution with invariant sites and gamma-distributed rates for the variable sites (GTR + I + G) were used for Bayesian analysis (BI). This model was selected based on an ML ratio test implemented by the software Modeltest, version 3.06 (Posada & Crandall, 1998), with a significance level of 0.01. For Bayesian analysis, we ran four chains of Markov Chain Monte Carlo, sampling one tree every 1,000 generations for 4,000,000 generations, starting with a random tree. A 50% consensus tree-majority rule (as implemented by PAUP*) was computed after the burn-in point. The range of rbcL divergence values within and between species was calculated using uncorrected “p” distances obtained from PAUP*.
Results
Osmundea coelenterata (D.L. Ballantine et Aponte) M.T. Fujii, Sentíes et Areces comb. nov. (Figs 1-9).
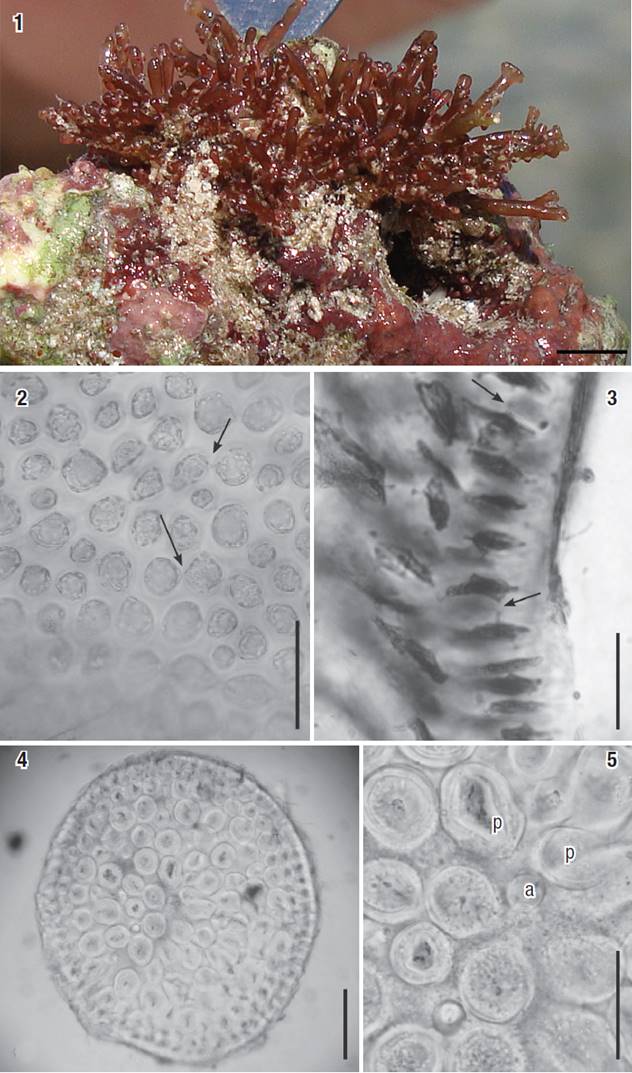
Figures 1-5: Osmundea coelenterata comb. nov. 1 Aspect of a tuft from Cuba on the natural substrate. Scale bar: 2 mm. 2-3) Cortical cells in surface view and inlongitudinal section view, respectively. Arrows indicate secondary pit connections. 4) Transverse section of the thallus (note thick-walled medullary cells). 5) Detail of medulla in transverse section, showing an axial cell (a) with two pericentral cells (p). Scale bars = 100 μm (Figs 2, 5), 50 μm (Fig. 3) and 200 μm (Fig. 4).
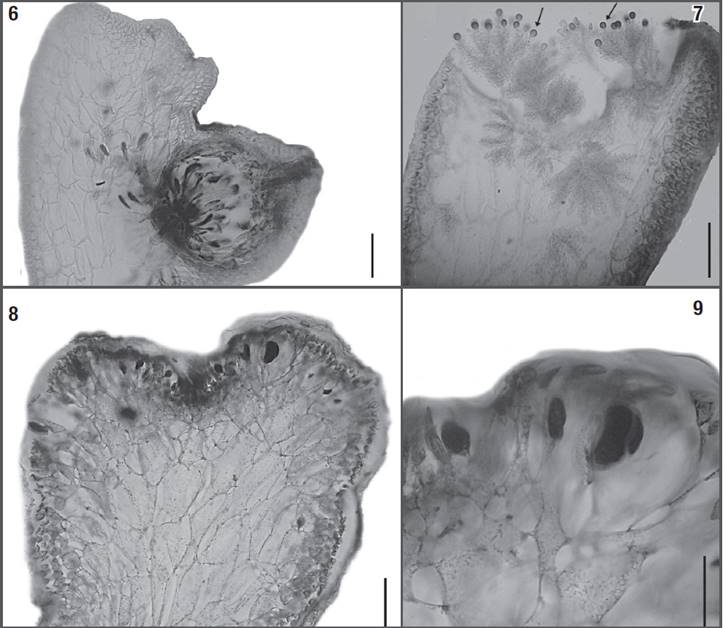
Figures 6-9: Osmundea coelenterata comb. nov. 6) Longitudinal section through a mature cystocarp (note the cystocarp partially immersed in the thallus). 7) Longitudinal section through a cup-shaped spermatangial pit, showing “filament-type” spermatangial branches originated from cortical cells, and terminating in a single vesicular sterile cell (arrows). 8-9) Longitudinal sections through the tetrasporangial branches, with tetrasporangia originated from cortical cells, and showing right angle arrangement in relation to the branch. Scale bars = 100 μm (Figs 6, 8), 50 μm (Figs 7, 9).
BASIONYM: Laurencia coelenterata D.L. Ballantine et Aponte, 1995, Bot. Mar. 38: 417-418, figs. 1-2).
HOLOTYPE: US (D.L. Ballantine # 3908)
PARATYPE: Herbario Marino Puertorriqueño # 4451!
TYPE LOCALITY: Pulaski Shoals, Dry Tortugas, Florida, USA (24o41.661” N, 82o42.296”W).
Amended diagnosis: vegetative axial segment with two pericentral cells, whose diameters are slightly smaller than those of the surrounding cells; male gametophytes with 'filament-type' spermatangial branches originated from apical and cortical cells; tetrasporangia are randomly produced from cortical cells at the apex of the branches.
Habit: plants forming tufts, strongly attached to the substrate by a discoid holdfast, rigid, cartilaginous in texture, not adhering to herbarium paper when dried. The thalli are reddish-brown, the axes terete to slightly compressed, claviform, 3-4 mm high and 500-800 µm diameter. The axes are sparsely branched and the branches are produced alternately. Short branchlets are present in the apical portions of some branches and axes, giving the species a very characteristic appearance (Fig. 1).
Vegetative structures: in surface view, cortical cells are rounded, regularly arranged throughout the thalli, 10-25 µm diameter, with secondary pit connections frequently present between the cortical cells (Fig. 2). Corps en cerise absent. In transverse section, thalli with one-two layers of quadratic to slightly radially elongated pigmented cortical cells, not arranged as palisade, 20-35 µm high x 20-30 µm wide in the middle portions of the main axes (Fig. 3). Medullar region with three or four layers of colorless cells, rounded, (50)90-130 µm diameter, where the pericentral cells are smaller than those in the other layers. Medullary cell walls are extremely thick and can almost completely fill the lumen of the cells. However, lenticular thickenings are absent (Figs 4-5). Each vegetative axial segment cuts off two pericentral cells, rounded, 90-130 µm diameter (Fig. 5). In median longitudinal section through a branchlet, cortical cell walls near apices not projecting beyond the surface.
Reproductive structures: female gametophytes are characterized by laterally bearing cystocarps partially immersed in the thalli. The cystocarps are pyriform, 350-500 µm diameter, bearing a prominent carpostome. Carpospores are claviform, 40-45 μm high x 10-15 μm wide, and are produced abundantly (Fig. 6). Male gametophytes have filament-type spermatangial branches originating in cortical cells in the cup-shaped spermatangial pit. Spermatangial branches bear spermatangial mother cells that give rise to several elliptical spermatangia, 4-8 μm diameter, each with a single distally located nucleus, and usually terminating in one elliptical vesicular sterile cell, 7-8.5 µm high x 6.0-6.5 µm wide. (Fig. 7). Tetrasporangia are cut off randomly from the cortical cells at the apex of the branches and have a diameter of 50-80 μm, displaying right-angle arrangement in relation to the longitudinal axes. The fertile pericentral cells cut off two presporangial cover cells distally and the tetrasporangia. The post-sporangial cell was not discernible (Figs. 8-9).
Habitat and distribution: The holotype specimens were collected by SCUBA at 9 m depth at the Dry Tortugas, Florida, USA. In Santiago de Cuba, however, Osmundea coelenterata was found growing on the consolidated substrata in a biogenic sand beach, with isolated limestone and beds of the seagrass Thallasia testudinum at the bottom.
Material examined: CUBA: IDO-161, Cayo Paredon Grande, Archipiélago Jardines del Rey (intertidal), 21.x.1992, Leg. A. Areces (SP 401499). Santiago de Cuba, Playa Cazonal, 12.xi.2008, Leg. A. Jover Capote, female, male, and tetrasporangial specimens (FCA 191-ah).
Additional material examined: Laurencia coelenterata, USA, Dry Tortugas, Pulaski Shoals, 9 m, 17.ix.1991, Leg. David L. Ballantine, (Herbario Marino Puertorriqueño # 4451 - Paratype) (Figs 10-13).
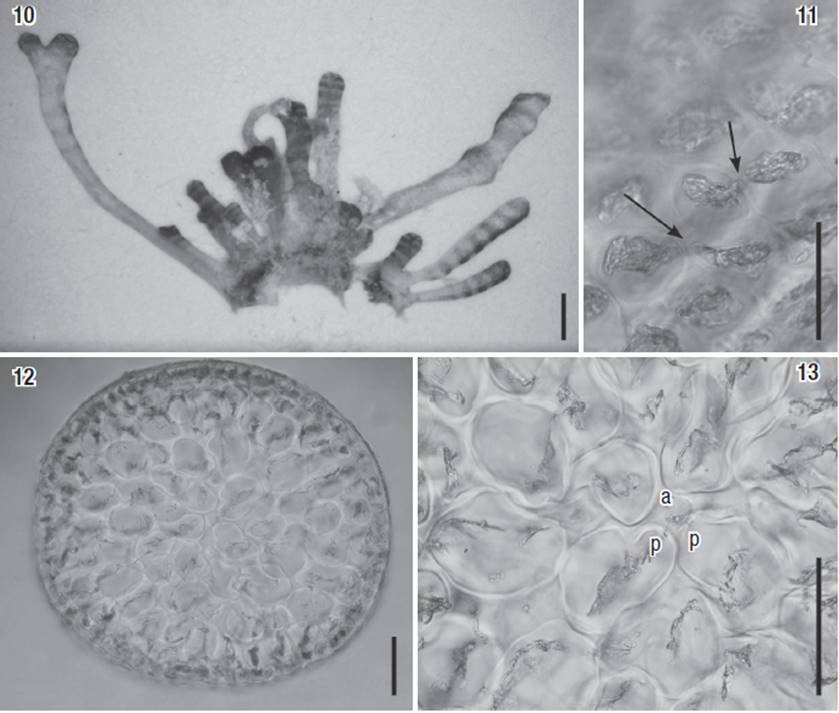
Figures 10-13: Paratype of Laurencia coelenterata (# 4551). 10) Habit of the plant. 11) Cortical cells in surface view, showing a secondary pit-connection between them (arrows). 12) Transverse section of the thallus. 13) Detail of the upper portion of a branch with an axial cell (a) and two pericentral cells (p). Scale bars: 1 mm (Fig. 10), 50 μm (Figs 11 and 13) and 100 μm (Fig. 12).
Molecular analyses. A total of 37 sequences were analyzed including five outgroup taxa, Bostrychia radicans (Montagne) Montagne, Chondria collinsiana M. A. Howe, C. dasyphylla (Woodward) C. Agardh, C. californica (Collins) Kylin in the Rhodomelaceae, and Ceramium brevizonatum H. E. Petersen in the Ceramiaceae. Maximum Parsimony, ML and BI topologies were similar. The first 50 nucleotides and the last 40 bp of all rbcL sequences were removed, producing a data set of 1377 base pairs, and the rest of the sequences were aligned without ambiguity.
Intergeneric divergence varied from 9.2 to 13.7% for Laurencia and Osmundea from 8.5 to 12% for Chondrophycus and Laurencia, from 9 to 11.5% for Chondrophycus and Osmundea, from 8 to 10% for Laurenciella and Laurencia, from 8.2 to 10% for Laurenciella and Palisada, from 10 to 11% for Laurenciella and Chondrophycus, from 10 to 13% for Laurenciella and Osmundea, from 9.8 to 10.2% Laurenciella and Yuzurua, from 7 to 10.5% for Laurencia and Palisada, from 8.5 to 10% for Laurencia and Yuzurua, from 8 to 9.2% for Palisada and Chondrophycus, from 8.5 to 12.5% for Palisada and Osmundea, from 8 to 10.5% for Palisada and Yuzurua, from 9.5 to 10.8% for Chondrophycus and Yuzurua, and from 10.5 to 11.9% for Osmundea and Yuzurua.
Interspecific divergence obtained for the species of Laurencia varied from 1 to 6%, from 1.3 to 6% for Palisada, from 1.8 to 7% for Osmundea, from 1.3 to 1.5% for Laurenciella, 0.5% for Yuzurua, and from 1.6 to 6% for those of Chondrophycus.
Finally, the divergence of the Cuban specimen from other Osmundea species was 3.0-8.5%.
The topology of the majority rule Bayesian tree is shown in Figure 14. The data set consisted of 907 constant characters, 170 parsimony informative sites, and 300 parsimony non-informative sites.
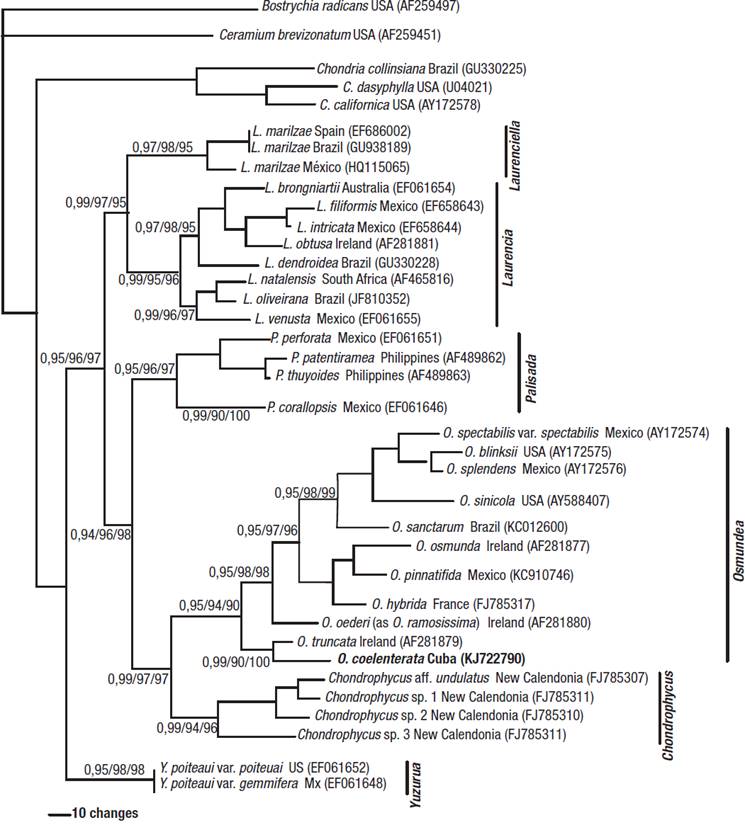
Figure 14: Phylogenetic relationships in the Laurencia complex based on Bayesian analysis of rbcL DNA sequences. Bayesian posterior probability/MP bootstrap/and ML bootstrap values are indicated at the nodes. Taxa marked in bold indicate newly determined sequence.
The analyses show a monophyletic Laurencia complex with high bootstrap support in relation to the members of the outgroups. The Laurencia complex separated into six clades with high bootstrap support, corresponding to the following genera: Laurencia, Chondrophycus, Osmundea, Palisada, Laurencia, and Laurenciella.
The monophyletic clade that corresponded to the genus Osmundea included eleven species. Within the Osmundea assemblage, the Cuban specimen formed a well-supported clade with O. truncata (Kützing) K.W.Nam et Maggs from Ireland.
Discussion
The Cuban specimens formed a well-supported clade with other species of Osmundea corroborating the morphological data. The divergence of the Cuban specimen from other Osmundea species was high, which confirms that it constitutes an authentic taxonomic entity. The intergeneric and interspecific divergence values obtained in the present work are similar to those reported by other authors for the Laurencia complex (McIvor et al., 2002; Cassano et al., 2012a,b; Machín-Sanchez et al., 2014; Metti et al., 2015).
The genera Laurencia and Osmundea are clearly distinguished genetically as well as morphologically. Some features, such as the presence of the secondary pit connections between cortical cells and the presence/absence of lenticular thickening are shared by both genera, but the majority of diagnostic morphological features are distinct, such as four pericentral cells per vegetative segment in Laurencia against two in Osmundea; the origin of spermatangia and tetrasporangia is random from cortical cells in Osmundea, whereas in Laurencia they are from a particular fertile pericentral cell.
The tetrasporangial arrangement in superficial view of the branches is parallel in Laurencia, whereas it can be parallel or right-angled in Osmundea.
(Ballantine and Aponte, 1995) had already noticed that L. coelenterata does not conform to Saito’s (1967) concept of the subgenera Laurencia and Chondrophycus, since the species possesses tetrasporangia with a right-angle arrangement (a character of the subgenus Chondrophycus), and secondary pit connections between cortical cells (a character of the subgenus Laurencia). Currently, we know that the right-angle arrangement of tetrasporangia is shared mainly by the species of Chondrophycus, Palisada, and Yuzurua, and pit connections between adjacent cortical cells could be present in all members of the Laurencia complex (Nam 2006, Cassano et al. 2012, Metti et al. 2015).
Osmundea truncata from Ireland is the closest species to this species, although their gross morphology is completely distinct. The thallus in O. truncata is up to 5 cm high, slightly or strongly compressed and abundantly branched (Machín-Sanchéz et al., 2012), whereas in O. coelenterata it is smaller (3-4 mm), terete, and branching is rare. In relation to the anatomical comparison, Osmundea truncata and O. coelenterata have in common the presence of longitudinally arranged secondary pit connections between adjacent cortical cells and cup-shaped spermatangial pit. Lenticular thickenings are present in the former but absent in the latter. Furthermore, the genetic divergence between these species is 1.5%, clearly representing two distinct species of Osmundea.
The geographic distribution of the Osmundea species was analyzed based on dataset presented by (Guiry and Guiry, 2015). In this database, there are 24 species (and infraspecific) names, of which 20 have been flagged as currently accepted taxonomically. Eight species (40%) of these occur exclusively in the North American Pacific: O. blinksii (Hollenberg et I. A. Abbott) K. W. Nam, O. crispa (Hollenberg) K. W. Nam, O. estebaniana (Setchell & N. L. Gardner) J.N. Norris, O. multibulba (E.Y. Dawson, Neushul et Wildman) K. W. Nam, O. purepecha Sentíes, Mendoza-González et Mateo-Cid, O. spectabilis (Postels et Ruprecht) K. W. Nam, and O. splendens (Hollenberg) K. W. Nam.
Osmundea lata (M. Howe et W.R. Taylor) Yoneshigue-Valentin, M. T. Fujii et Gurgel and O. sanctarum M. T. Fujii et Rocha-Jorge occur only in Brazil and they have restricted and timely distribution in subtidal conditions, under the influence of cold waters of the South Atlantic Central Waters (Howe & Taylor, 1931; Yoneshigue-Valentin et al., 1995, 2003; Rocha-Jorge et al., 2013).
The Northern hemisphere of the Atlantic Ocean, including the Mediterranean, Adriatic Sea, and Macaronesia houses 50% of the Osmudea species known to date: O. hybrida (A. P. de Candolle) K. W. Nam, O. maggsiana Serio, Cormaci et G. Furnari, O. oederi (Gunnerus) G. Furnari, O. osmunda (S. G. Gmelin) K. W. Nam and Maggs, O. pedicularioides (Børgesen) G. Furnari, Serio et Cormaci, O. pelagiensis G. Furnari, O. pelagosae (Schiffner) K. W. Nam, O. pinnatifida (Hudson) Stackhouse, O. truncata (Kützing) K. W. Nam et Maggs, and O. verlaquei G. Furnari (Gury & Gury 2015). Except Osmundea maggsiana and O. pelagiensis that are reported only from the Mediterranean and/or Adriatic Sea (Cormaci et al., 1994; Serio et al., 2008), all others are widely distributed in the Northern Atlantic Ocean and beyond but have never occurred in the North American Pacific.
Osmundea pinnatifida is the most widely distributed species in the northern hemisphere of the Atlantic Ocean and also in the Indian and Pacific Oceans (Guiry & Gury, 2015), characterizing the Hommersand’s late Tethyan distribution pattern, according to (McIvor et al., 2002), corroborated by (Furnari et al., 2004). The reference to this species in Brazil was never confirmed nor was O. hybrida (Fujii et al., 2011).
The geographic distribution pattern found for the species of Osmundea suggests their affinity to temperate and warm temperate conditions. Therefore, this is the first report of Osmundea in the Caribbean, occurring in a typical tropical environment. This fact indicates that further studies are needed in the Caribbean region and in the Western Atlantic.











 nova página do texto(beta)
nova página do texto(beta)


