Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Hidrobiológica
versión impresa ISSN 0188-8897
Hidrobiológica vol.20 no.1 Ciudad de México abr. 2010
Artículos
Shrimp postlarvae immigration during the high current velocity period of the flood tide in the Southern Gulf of Mexico
Inmigración de larvas de camarón durante el periodo de mayor velocidad de flujo de marea en el sur del Golfo de México
César Flores–Coto1,*, José A. Becerril–Martínez1, Faustino Zavala–García1, Adolfo Gracia1 and John S. Burke2
1 Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México. Ciudad Universitaria. Circuito Exterior. Apdo. Post. 70–305. México D.F. 04510, México. *E.mail: coto@cmarl.unam.mx
2 NOAA National Oceanic Service. Center for Coastal Fisheries and Habitat Research. 101 Pivers Island Road. Beaufort, North Carolina, USA.
Recibido: 20 de agosto de 2008.
Aceptado: 15 de diciembre de 2009.
ABSTRACT
The immigration pattern of shrimp postlarvae was analysed in the Santana inlet (Tabasco State, Mexico) during the high current velocity period of the flood tide at the time of the full Moon, throughout the period of greatest postlarvae abundance (April–November). Nets with a 50 cm diameter mouth, 1.5 m long, and a 505 µm mesh were used for simultaneous sampling at three depth levels: 1, surface (0.5 m), 2, mid–water (3.5 m) and 3, bottom (6.5 m). A total of 15,530 postlarvae of Farfantepenaeus duorarum and Litopenaeus setiferus were collected in 101 samples: 33 at level 1, 34 at level 2, and 34 at level 3. More than 75% of L. setiferus larvae were larger than 8 mm and more than 81% of F. duorarum were larger than 9 mm. Temperature and salinity varied only slightly, indicating that they do not play a strong role in postlarvae immigration. Postlarvae entered at all water depths. A larvae immigration pattern may be established for both species, with the greatest postlarvae density at the start of the high current velocity period of the flood tide, and a marked decrease towards the end of the period. It is assumed that this immigration pattern allows a deeper penetration into nursery areas and enhances the success of the process of recruitment into estuaries.
Key words: Immigration, Santana inlet, Tabasco.
RESUMEN
El patrón de inmigración de postlarvas de camarón en la Boca de Santana (Tabasco, México) fue analizado durante el lapso de alta velocidad de corriente del flujo de marea durante luna llena, en el periodo de mayor abundancia de larvas (abril–noviembre). Se emplearon redes de 50 cm de diámetro de boca, 1.5 m de largo y 505 µm de luz de malla, con muestreos simultáneos en tres niveles: 1, superficie (0.5 m), 2, media agua (3.5 m) y 3, fondo (6.5 m). Se colectaron 15,530 postlarvas de Farfantepenaeus duorarum y Litopenaeus setiferus en 101 muestras: 33 en el nivel 1, 34 en el nivel 2, y 34 en el nivel 3. Más del 75% de larvas de L. setiferus fueron mayores de 8 mm y más del 81% de F. duorarum fueron mayores de 9 mm. La temperatura y la salinidad tuvieron pequeñas fluctuaciones y no parecen tener un papel determinante en el proceso de inmigración. Las larvas entraron indistintamente por cualquier nivel de la columna de agua. Se determinó el patrón de inmigración de ambas especies, con la mayor densidad de postlarvas al inicio del periodo de alta velocidad de corriente del flujo y una densidad marcadamente menor hacia el final de dicho periodo. Puede asumirse que este patrón de inmigración permite una mayor penetración dentro del área de crianza y propicia el éxito del reclutamiento a los estuarios.
Palabras clave: Inmigración, Boca de Santana, Tabasco.
INTRODUCTION
Many marine species of commercial importance are estuarine–dependent (McHugh, 1975). In the southern Gulf of Mexico, there are several fish and shrimp species of which the larvae migrate from the spawning areas on the continental shelf to the estuaries and lagoons in the region. Among these species, the most relevant, from the fisheries point of view, are Litopenaeus setiferus (Linnaeus, 1767), Farfantepenaeus duorarum (Burkenroad, 1839) and Farfantepenaeus aztecus (Ives, 1891).
The mechanisms responsible for the movement of larvae from the spawning areas towards the estuaries and lagoons vary according to the location of the larvae and their developmental stage. However, the immigration of larvae through the estuarine inlets is regulated by the tidal currents, and these become the main mechanism for the recruitment of a variety of fish and shrimp (Rothlisberg et al., 1995; Blanton et al., 2001; Forward & Tankersley, 2001).
Several aspects of the immigration process have been well documented. First, the number of larvae is generally greater during the flood tide than during the ebb tide (DeLancey et al., 1994; Rothlisberg et al., 1995; Burke et al., 1998; Forward et al., 1999; Jager & Mulder, 1999). In the case of eels, these differences can be as big as eight to 30 times greater abundance during the flood tide compared with that during the ebb tide (McCleave & Kleckner, 1982). However the number of larvae that migrate from coastal areas into estuaries aided by flood tide transport depends on the number that arrives at the inlet of the estuary (Blanton et al., 1999; Forward & Tankersley, 2001).
A second aspect is that many larvae of estuarine–dependent species concentrate outside the inlets in an optimal position to insure the best transport during the next flood cycle (Young & Carpenter, 1977; Calderón–Pérez & Poli, 1987; Poli & Calderón–Pérez, 1987; Rothlisberg et al., 1995; Blanton et al., 1999; Condie et al., 1999).
A third issue is that larvae enter estuaries mainly during the time of greatest flood velocity (Young & Carpenter, 1977; Rothlisberg et al., 1995; Wenner et al., 1998). Most of the research on shrimp migration involves recording a complete tidal cycle in order to allow this aspect to become evident; however, no study has focused specifically on immigration during the high flood tide velocity period. Thus, the main purpose of this study was to establish whether there is an immigration pattern during the high current velocity period of the flood tide during the full Moon, as well as to determine the possible relationship between postlarvae densities at different depths levels, and salinity, temperature and current velocity.
MATERIALS AND METHODS
Sampling site. The Carmen–Pajonal–Machona lagoon system was chosen as it is the most important estuarine system in the state of Tabasco, in the southern Gulf of Mexico (18°14'–18°25' N, 93°34'–93°54' W). This lagoon complex receives freshwater from the San Felipe and Santana rivers. It has two inlets, the "Panteones inlet" that is an artificial inlet in the Machona lagoon to the east, and the Santana inlet to the west of the system (Gutiérrez–Estrada & Galaviz–Solís, 1983). The latter, chosen as the sampling site, has a width of 170 m (Vázquez–Gutiérrez, 1994) and a 7 m deep channel at its westernmost third section (Fig. 1).
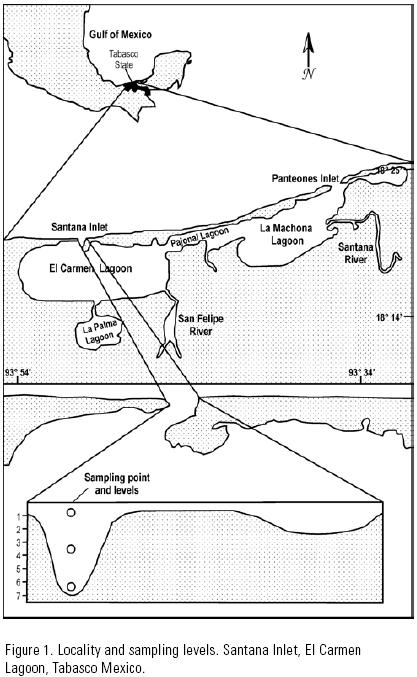
Sample collections. The biological material was collected considering the following: a) April–November (1997) covers the main spawning period of the shrimp species in the study area (Yap et al., 1987; Gracia, 1997), b) sea water enters the lagoon of El Carmen predominantly through the main channel of the Santana inlet (Vázquez–Gutiérrez, 1994), and c) shrimp postlarvae enter the lagoon mainly during the high current velocity period of the flood tide (Rothlisberg et al., 1995; Wenner et al., 1998; Forward et al., 1999). Thus, sampling was carried out from April to November, at the main channel of the Santana inlet, and only during the high current velocity period of the flood tide.
Sampling was initially to take place every month, however it was only possible to sample for six of the eight months that were planned. The sampling dates were: April 23, May 23, June 21, August 19, October 15 and November 15, 1997, and they corresponded to the full–Moon phase. Samples were collected always at night, except for November. July was not sampled as abundant jellyfish filled and blocked the nets, and neither was September due to the strong norther winds that did not allow sampling to take place.
Each month, the height of the tide and the current velocity were measured a day ahead of sampling in order to anticipate the period of high current velocity. During sampling, the current velocity was measured with a electronic flow meter (General Oceanic Mod. 2031H) one meter below the surface, in order to determine when to begin and end sampling. Sampling began when the current velocity registered 0.5 m s–1, and ended when it fell below this value (Fig. 2).
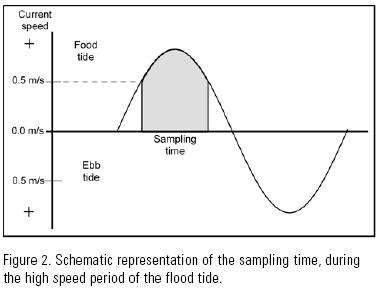
On the other hand, current velocity data were also obtained at each cast (= sampling event), with a General Oceanic model 2030 flowmeter fixed to the mouth of the net.
Sampling was simultaneous at three levels: surface (0.5 m), mid–water (3.5 m) and bottom (6.5 m). The sampling system consisted of three trapeze–type nets, with a 50 cm mouth diameter, a 1.5 m length and a 505 µm mesh size, without bridles, with a ballast down to the bottom, and parallel cables to attach the system to a bridge to enable fishing only during the tidal current (Flores–Coto & Zavala–García, 1994).
Each cast lasted 10–15 minutes. Sampling frequency varied each month (minimum four, maximum eight), depending on the time taken to recover and prepare the equipment, and on the duration of the high current velocity period. This varied from 3 h 7 min to 4 h 17 min, measured from the start of the first to the last cast.
Water samples were obtained at each sampling from the three depth levels with 3–liter van–Dorn bottles, and temperature and salinity were measured with a field thermometer and a refractometer, respectively.
Samples were initially preserved in 4% formalin neutralised with sodium borate, and changed into 70% alcohol 48 h later. Penaeid shrimp postlarvae were separated in the laboratory, identified to species level and measured for total length. Density data were standardised as postlarvae per 100 m–3.
Data analysis. Data analysis was carried out according to McCleave et al. (1987). Density of immigrating postlarvae was assumed to be uniform during the high current velocity period of the flood tide at all sampling depths. For each month, the expected values were calculated based on the filtered volume, and considering the total number of postlarvae (Ni) in each cast as the sum of the number of postlarvae in the three levels, or the sum of the number of postlarvae of all cast when the levels were analysed.
It was also assumed that the swimming of the organisms had an insignificant effect, i.e. that the shrimp postlarvae essentially float with the current, and the expected number of postlarvae in a particular cast or level is proportional to the total filtered water. Hence, the total number of expected postlarvae (Ci, ) is:
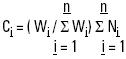
where:
Ci = expected postlarvae in the cast or level i
Wi= amount of filtered water in the cast or level i
ΣWi= sum of the amount of water filtered in the cast or level i
Ni = number of organisms collected in the cast or level i
The fraction i, is constituted, for the casts, by the sum of the three levels, and in the case of levels by the number of casts, which varied from month to month.
The values of the standardised residual were obtained with:
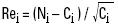
where:
Rei = standardised residuals,
and from the standardised residuals values, the χ2 is calculated as:
χ2 = Σ Rei2
RESULTS
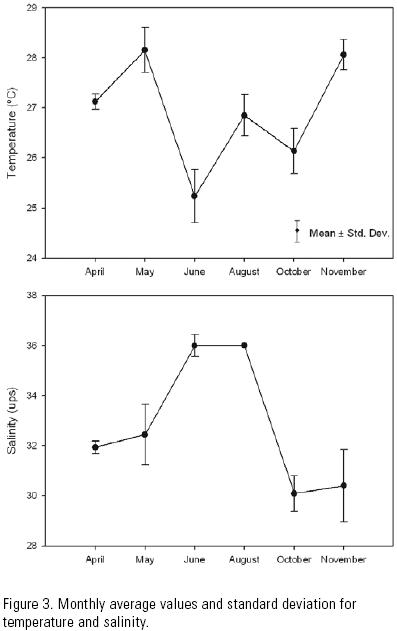
Temperature, salinity and current velocity. The monthly average temperature during the sampling period varied from 25.2°C in June to 29°C in May. During sampling months the temperature variation ranged from a minimum of 0.5°C in April to a maximum of 2.1°C in June (Fig. 3). Temperature was slightly higher during the first than during the last cast, all months. The monthly average salinity varied from 30 ups in October to 36 ups in June and August, with the values varying from a minimum of 0.1 in August to a maximum of 5.5 in May (Fig. 3). Salinity was slightly lower most months during the first than during the last cast. Variations in temperature and salinity at the different depth levels throughout each sampling period were not statistically significant.
Current velocities recorded at each depth level throughout the study period varied from 0.26 to 1.14 m s–1 (Fig. 4). The highest average velocities were recorded in October and November with values of 0.82 and 0.65 m s–1 respectively, and the lowest was recorded in August with 0.41 m s–1.
The tidal current in the water column presented a general pattern in April, June and November, with the greatest velocities at the bottom and the lowest at the surface. Differences among depth levels (surface, mid water and bottom) were significant (ANOVA; p<0.05) only in April and May.
Correlation between postlarvae density of both species and temperature were positive but only statistically significant in June, August and November, moths of marked differences in abundance. Correlations with salinity varied from positive to negative values. These were statistically significant for both species only in June, and for F. duorarum only in November (Table 1). Correlations with current velocity fluctuated from positive to negative values. The values were generally low and not significant, except in the case of August for both species, of June for L. setiferus, and of November for F. duorarum (Table 1).
Seasonal variation in composition and abundance. Samples collected were 101: 33 at level 1 (0.5 m), 34 at level 2 (3.5 m) and 34 at level 3 (6.5 m). Cast number varied with six in April, May and November, four in August and October, and eight in June.
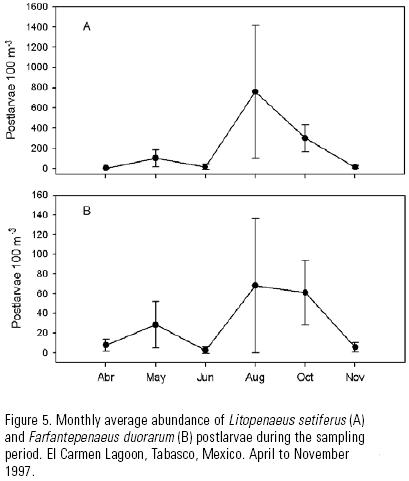
A total of 13,332 postlarvae of L. setiferus and 2,198 post–larvae of F. duorarum were collected. Despite the difference in abundance, both species presented a similar pattern of variation during the sampling period (Fig. 5). The lower values were recorded in April, June and November, and the higher values occurred in October and August, with the maximum variability in August (Table 2).
Vertical distribution. The χ2 test revealed significant differences in postlarvae abundance of the two species among the three sampling levels (0.5, 3.5 and 6.5 m) throughout the flood periods (Table 3). The standardised residual values indicate that the largest immigration of postlarvae of both species occurred along the bottom during April and May, in mid–water during August and October, and along the surface during June and November.
During June and November, when the abundance was the lowest, the largest immigration of postlarvae of both species occurred at the surface level, although the highest velocities were recorded along the bottom.
In August and October, when the greatest abundances are recorded, the largest immigration of postlarvae took place at the mid–water level, whereas the highest velocities occurred at the surface.
It was only during April and May that the largest immigration of postlarvae and the greatest current velocities coincided along the bottom depth level, and the smaller values of both parameters occurred at the surface.
Abundance pattern during the sampling period. The χ2 test also indicated that the abundance of postlarvae of both species was significantly different among cast in all months (Table 3). The postlarvae number was higher during the first cast and decreased towards the end of the high velocity period, except for F. duorarum in May (Fig. 6). In fact, during the months of highest abundance, August and October, the standardised residuals reveal that immigration occurred essentially at the beginning of the period. This pattern was practically the same, with some small exceptions, despite the differences in density of the two species.
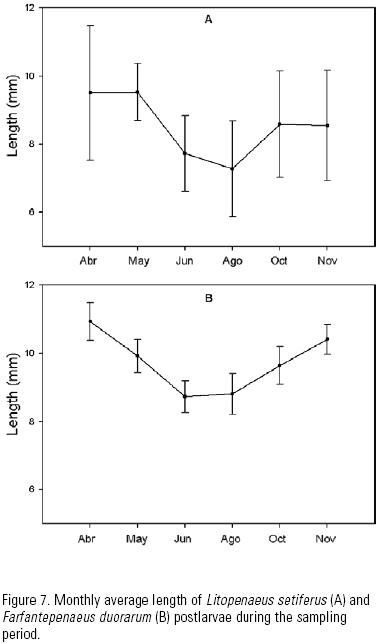
Size variation. The monthly average size of both species increased in April and May, decreased in June and August, and increased again towards October and November (Fig. 7). The average size of F. duorarum was bigger than that of L. setiferus throughout the sampling season. Immigration sizes ranged for L. setiferus and F. duorarum from 4.1–12.6 mm and from 7–12.5 mm, with averages of 8.76 and 9.71 mm total length, respectively. More than 75% of L. setiferus postlarvae were larger than 8 mm, and more than 81% of F. duorarum postlarvae were larger than 9 mm. There was little variation in specimen size between cast.
DISCUSSION
Parameters affecting postlarvae immigration. Correlations between larval abundance of both species, and salinity and temperature, were highly variable and mostly not statistically significant. The only significant values were recorded during the months that presented a marked difference in larvae abundance (Table 1).
These results make it possible to consider unlikely that salinity and temperature did play an important role in the immigration of shrimp postlarvae through the Santana inlet. This inlet is considered well mixed given the small variations recorded in salinity and temperature. The relative homogeneity in the water column may be due to a mixing process generated by the tidal currents, which is common in this type of coastal lagoon (Zijlstra, 1988).
Studies conducted in a variety of systems have indicated that neither salinity nor temperature seem to play a relevant role in the migration of postlarvae through the inlets (Pietrafesa & Janowitz, 1988; Joyeux, 1999).
The main spawning period of L. setiferus, F. duorarum and F. aztecus in the southern Gulf of Mexico starts in March or April, and may last up to November or even December (Yap et al., 1987; Gracia et al., 1997). The period of greatest abundance recorded in this study was August to October, and it coincides with the largest discharges of continental waters in the area (July–October), according to data from the Comisión Nacional del Agua (CNA, 1998). Also, it is possible to expect high postlarvae densities in July and September, months for which this study did not gather data.
The relationship between the high river discharge period and the main shrimp postlarvae immigration pulse supports the notion that salinity plays a more important role outside the inlet as a cue or stimulus for larvae to initiate migration towards the estuaries, as Boehlert & Mundy (1988) and Pietrafesa & Janowitz (1988) mentioned previously. However, this relationship is most likely due to a reproductive strategy that includes an optimal use of seasonally changing resources that ensures larval survival and recruitment to nursery areas, considering that river discharges play an important role through the promotion of offshore primary productivity that may benefit larval survival (Gracia, 1997).
Correlations between current velocity and larval density of both species were very irregular and changed from positive to negative, even during the months with statistically significant values (Table 1). This led to the conclusion that there is no correlation pattern or trend between these parameters.
Sizes. The average immigration size was largest during the first months, decreased in August and increased again in October and November, in agreement with the coastal circulation that is predominant in the study area. Zavala–Hidalgo et al. (2003) have indicated that the currents on the continental shelf travel westward and northward along the coastline from May to August. In September, a coastal current develops in the state of Veracruz that flows south along the coastline. This current collides with an onshore flow and forms a front near the Carmen–Machona lagoons, where our study site was located.
Under this circulation pattern, the larvae that migrate westwards from the spawning areas on the Campeche shelf, and perhaps also the Yucatán shelf (Soto & Gracia 1987), may reach the lagoon inlets at a smaller size. In contrast, in September, and once the circulation has changed eastward, the larvae require more time to reach the coastal areas in front of the lagoon inlets.
Alarcón–Daowz (1986) found a similar pattern of seasonal variation in the immigration size of shrimp larvae in the southern Gulf of Mexico, with the larger sizes in Spring and Fall and the smaller sizes in Summer.
Exactly the opposite has been recorded along the Atlantic coast of the United States for larvae of several fish species including Leiostomus xanthurus, with smaller sizes at the beginning of the season, that increase and then decrease again towards the end of the period (Warlen & Burke, 1990). The spawning areas of these fish shift offshore as Winter sets in, and the advection of larvae towards the coast takes longer.
The consistent greater size of F. duorarum compared with L. setiferus may be attributed to differences is spawning localities, with these being farther away from the study inlet in the case of the first species. Gracia (1989) indicated that L. setiferus mainly spawns on the inner and mid shelf of the states of Campeche and Tabasco, whereas F. duorarum spawns on the outer shelf and further north–east.
Vertical distribution. The data obtained suggests that F. duorarum and L. setiferus postlarvae may enter indistinctively throughout the whole water column. Statistical analyses indicate that the greatest immigration occurs along the surface in some months (0.5 m), in other months in mid–water (3.5 m), and in still other months along the bottom (6.5 m). Other authors, including Blanton et al. (2001), have recorded a mean density of L. setiferus an order of magnitude greater at the surface than at the bottom during the night flood tides, as well as the opposite during the day.
Abundance pattern during the high current velocity period of the flood tide. Results for both species indicate that postlarvae density varies significantly throughout the high current velocity flood tide period. The largest immigration occurs at the beginning of the period and declines significantly over time.
These results are expected because larvae immigrations occur together with flood tide streams, and it is logical that the greater transport should occur at the time of the strongest tidal currents during the Spring tides (Forward & Tankersley, 2001). Previous studies such as that of Wenner et al. (1998) that covered a full cycle of neap–spring tides, found that an abundance peak coincided with an increase in water level of more than 0.2 m that induced an additional influx of water that reinforced the flood current.
Also, the greatest larval densities of several fish species occur when high speed currents are present. Jager (1999) for instance, observed that the highest concentration of flounder larvae were recorded in the fast moving surface layer during the flood tide, and Forward et al. (1999) observed the greatest larvae densities of several fish species during the periods of greater flood speed currents.
Two phases may be considered for the migration process of larvae of estuarine–dependent species of fish and crustaceans from spawning to nursery areas. In the first phase, the larvae are typically planktonic and are subjected to a totally passive displacement towards the coast (Boehlert & Mundy, 1988; Condie et al., 1999; Jager, 1999; Rothlisberg et al., 1995), and the second phase includes both a change in habitat when the larvae become epibenthic (Garcia & LaReste, 1981) and a process of concentration of larvae near the estuary inlets (Boehlert & Mundy, 1988; DeLancey et al., 1994; Rothlisberg et al., 1995; Blanton et al., 1999).
The greater abundance at the beginning of the high current velocity period may be conditioned by the process of accumulation of postlarvae near the inlets at a place that is optimal for immigration with the aid of tidal currents. An immigration at the start of the high velocity period ensures a large dispersion into the nursery area that favours the movement of the postlarvae to safe sites before the ebb tide may return them to the ocean.
For many species, immigration into estuaries is not a purely passive process, their behaviour is related to the tidal stage and the result is a selective tidal stream transport (STST) of larvae into estuaries towards their nursery areas (Burke et al., 1998; Forward et al., 1998; Jager, 1999; Forward & Tankersley, 2001; Blanton et al., 2001). Calderon–Pérez & Poli (1987) considered that if postlarvae accumulation and immigration were merely passive phenomena, other penaeid larvae also present in the coastal area should be recorded inside the lagoon of their study area, and this has not been the case.
The results obtained here agree with previous findings, particularly with those of Rothlisberg et al. (1995) who studied the Nerang River estuary in Australia and found that the largest peaks of larvae appeared within 2 h of the start of the flood tide with both the new and the full Moon, and the abundance curves exhibited a rapid increase and a gradual decrease, similarly to the Santana inlet. Also, Young & Carpenter (1977) mentioned that from the onset of the flood, enormous numbers of postlarvae entered the estuary, and the immigration was complete within the first half of the flood tide while the velocity of the water was still increasing. They also observed that most of the immigrants entered within the first 3 h of the night time flood tide.
Results indicate that the largest larval densities occurred 3–4 hrs after the start of the flood tide. This longer time may be due to the tidal cycle being daily in our study area. It is possible to assume that the peaks in the Rothlisberg et al. (1995) study occurred during the period of greatest velocity.
This study also offers information for an adequate design of monitoring studies, considering that if the variations at the beginning and end of the high velocity period are not taken into account, the estimation of postlarvae densities that migrate into estuaries will be deficient. For example, the average densities of L. setiferus postlarvae caught in August during the first and last cast were 1409.7 and 193.4 per 100 m–3, respectively.
This immigration pattern, with the largest densities of larvae occurring at the beginning of the high flow period and declining at the end of the period, does not exclude the possibility of some shrimp larvae entering during the low current velocity period at the beginning or end of the tidal flood period. It is assumed that this may occur in a very low proportion as in other areas (Rothlisberg et al., 1995; Wenner et al., 1998; Blanton et al., 2001). Differences in this pattern may be expected from one area to another depending on several factors.
ACKNOWLEDGEMENTS
This study was financed by the Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México.
REFERENCES
Alarcón–Daowz, G. 1986. Estratificación de las postlarvas planctónicas de camarones peneidos durante la inmigración a través de la Boca de Puerto Real, Laguna de Términos. Tesis de Licenciatura (Biología), Facultad de Ciencias, UNAM, México. 78 p. [ Links ]
Blanton, J. O., F. E. Werner, A. Kapolnai, B. O. Blanton, D. Knott & E. L. Wenner. 1999. Wind–generated transport of fictitious passive larvae into shallow tidal estuaries. Fisheries Oceanography 8 (2): 210–223. [ Links ]
Blanton, J. O., P. G. Verity, J. Amft, E. L. wenner, C. A. Barans, D. M. B. Knott, W. Stender & S. B. Wilde. 2001. Key factors influencing transport of white shrimp postlarvae in southeastern U.S. estuaries. Final Report to Georgia Sea Grant Program and South Carolina Sea Grant Consortium. 42 p. [ Links ]
Boehlert, G. W. & B. C. Mundy. 1988. Roles of behavioral and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. In: Weinstein M. P. (Ed.). Larval fish and shellfish transport through inlets. American Fisheries Society Symposium 3. Bethesda, Maryland, pp. 51–67. [ Links ]
Burke, J. S., M. Ueno, Y. Tanaka, H. Walsh, T. Maeda, I. Kinoshita, T. Seikai, D. E. Hoss & M. Tanaka. 1998. The influence of environmental factors on early life history patterns of flounders. Journal of Sea Research 40 (1): 19–32. [ Links ]
Calderón–Pérez, J. A. & C. R. Poll 1987. A physical approach to the postlarval Penaeus immigration mechanism in a Mexican coastal lagoon (Crustacea: Decapoda: Penaeidae). Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 14 (1): 147–156. [ Links ]
Comisión Nacional Del Agua (CNA). 1998. Boletín de datos hidrométricos. México D.F. [ Links ]
Condie, S. A., N. R. Lonerang & D. J. Die. 1999. Modeling the recruitment of tiger prawns Penaeus esculentus and P. semisulcatus to nursery grounds in the Gulf of Carpentaria, northern Australia: Implications for assessing stock–recruitment relationships. Marine Ecology Progress Series 178: 55–68. [ Links ]
DeLancey, L. B., J. E. Jenkins & J. D. Whitaker. 1994. Result of long–term, seasonal sampling for Penaeus postlarvae at Breach inlet, South Carolina. Fishery Bulletin 92: 633–640. [ Links ]
Flores–Coto, C. & F. Zavala–García. 1994. Un nuevo sistema para muestreo simultáneo de zooplancton en varios niveles, para zonas someras. Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 21 (1–2):149–153. [ Links ]
Forward, Jr., R. B., R. A Tankersley & K. A. Reinsel. 1998. Selective tidal stream transport of spot (Leiostomus xanthurus Lacepede) and pinfish [Lagodon rhomboides (Linnaeus)] larvae: contributions of circatidal rhythms in activity. Journal of Experimental Marine Biology and Ecology 226 (1): 19–32. [ Links ]
Forward, R. B., K. A. Reinsel, D. Peters, R. A. Tankersley, J. H. Churchill, L. B. Crowder, W. F. Hettler, S. Warlen & M. M. Green. 1999. Transport of fish larvae through a tidal inlet. Fisheries Oceanography 8 (2):153–172. [ Links ]
Forward, Jr., R. B. & R. A Tankersley. 2001. Selective tidal stream transport of marine animals. Oceanography and Marine Biology. An Annual Review 39: 305–353. [ Links ]
García, S. & L. Lareste. 1981. Life cycles, dynamics, exploration and management of coastal penaeid shrimp stocks. FAO (Food and Agricultural Organization of the United Nations) Fisheries Technical paper 203. 215 p. [ Links ]
Gracia, A. 1989. Ecología y biología del camarón blanco Penaeus setiferus (Linnaeus, 1767J en la Laguna de Términos–Sonda de Campeche. Tesis de Doctorado en Ciencias (Biología), Facultad de Ciencias, UNAM, México. 127 p. [ Links ]
Gracia, A. 1997. Pesquería artesanal del camarón. In: D. Flores–Hernández, P. Sánchez–Gil, J. C. Seijo & F. Arreguín–Sánchez (Eds). Análisis y Diagnóstico de los Recursos Pesqueros Críticos del Golfo de México. Universidad Autónoma de Campeche. EPOMEX. Campeche, pp. 173–184. [ Links ]
Gracia, A., A. Vázquez–Bader, F. Arreguin–Sánchez, L. E. Schultz–Rulz & J. A. Sánchez. 1997. Ecología de camarones peneidos. In: D. Flores–Hernández, P. Sánchez–Gil, J. C. Seijo & F. Arreguín–Sánchez (Eds). Análisis y Diagnóstico de los Recursos Pesqueros Críticos del Golfo de México. Universidad Autónoma de Campeche. EPOMEX. Campeche, pp. 127–144. [ Links ]
Gutiérrez–Estrada, M. & A. Galaviz–Solis. 1983. Morfología y sedimentos recientes de las lagunas El Carmen, Pajonal y La Machona, Tabasco, México. Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 10: 249–268. [ Links ]
Jager, Z. 1999. Selective tidal stream transport of flounder larvae (Platichthys flesus L.) in the Dollard (Ems Estuary). Estuarine, Coastal and Shelf Science 49 (3): 347–362. [ Links ]
Jager, Z. & H. P. J Mulder. 1999. Transport velocity of flounder larvae (Platichthys flesus L.) in the Dollard (Ems Estuary). Estuarine, Coastal and Shelf Science 49 (3): 327–346. [ Links ]
Joyeux, J. C 1999. The abundance of fish larvae in estuaries: Within–tide variability at inlet and immigration. Estuaries 22 (44): 889–904. [ Links ]
McCleave, J. D. & R. C. Kleckner. 1982. Selective tidal stream transport in the estuarine migration of glass eels of the American eel (Anguilla rostrata). Journal du Conseil International Pour L' Explotation de la Mer. 40: 262–271. [ Links ]
McCleave, J. D., J. J. M. Bedaux, P. G. Doucet, J. C. Jager, J. T. L Jong, W. J. Van Der Steen, & B. Voorzanger. 1987. Statistical methods for analysis of plankton and nekton distribution, with application to selective tidal stream transport of juvenile American eels (Anguilla rostrata). Journal du Conseil International Pour L' Explotation de la Mer. 44: 90–103. [ Links ]
McHugh, J. L. 1975. Estuarine Fisheries: are they doomed? In: M. Willey (Ed.). Uses, Stresses and Adaptation to the Estuary. New York Academic Press, pp. 15–27. [ Links ]
Pietrafesa, L. J. & G. S. Janowitz. 1988. Physical oceanographic processes affecting larval transport around and through North Carolina inlets. In: M. P Weinstein (Ed.). Larval fish and shellfish transport through inlets. Bethesda Maryland: American Fisheries Society Symposium 3. pp. 34–50. [ Links ]
Poli, C. R. & J. A. Calderón–Pérez. 1987. Efecto de los cambios hidrológicos en la boca del río Baluarte sobre la inmigración de postlarvas de Penaeus vannamei (Boone) y P. stylirostris (Stimpson) al sistema lagunar Huizache–Caimanero, Sinaloa, México (Crustacea: Decapoda, Penaeidae). Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 14 (1): 29–44. [ Links ]
Rothlisberg, P. C., J. A. Church & B. Fandry. 1995. A mechanism for near–shore concentration and estuarine recruitment of post–larval Penaeus plebejus Hess (Decapoda, Penaeidae). Estuarine, Coastal and Shelf Science 40 (2): 115–138. [ Links ]
Soto, L. A. & A. Gracia. 1987. Evaluación de los efectos de hidrocarburos fósiles sobre las poblaciones de camarones peneidos en el Banco de Campeche. Anales del Instituto de Ciencias del Mar y Limnología. Universidad Nacional Autónoma de México 14 (1): 133–146. [ Links ]
Vázquez–Gutiérrez, F. 1994. El sistema lagunar El Carmen–Pajonal–la Machona del estado de Tabasco: Su hidrodinámica, la estabilidad de sus bocas y de su línea de costa. Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, México. 132 p. [ Links ]
Warlen, S. M. & J. S. Burke. 1990. Immigration of larvae of fall/winter spawning marine fishes into a North Carolina estuary. Estuaries 13 (4): 453–461. [ Links ]
Wenner, E., D. Knott, J. Blanton, C. Barans & J. Amft . 1998. Roles of tidal and wind–generated currents in transporting white shrimp (Penaeus setiferus) postlarvae through a South Carolina (USA) inlet. Journal of Plankton Research 20: 2333–2356. [ Links ]
Yap, W. G., F. D. Apud & J. H. Primavera. 1987. Manual de cultivo de camarón. Traducción de Publicaciones Dirección General de Comunicación Social de la Secretaria de Pesca, México. 49 p. [ Links ]
Young, P. C. & S. M. carpenter. 1977. Recruitment of postlarval penaeid prawns to nursery areas in the Moreton Bay, Queensland. Australian Journal of Marine and Freshwater Research 28: 745–773. [ Links ]
Zavala–Hidalgo, J., S. L. Morey & J. J.O. Brien. 2003. Seasonal circulation on the western shelf of the Gulf of Mexico using a high–resolution numerical model. Journal of Geophysical Research 108 (C12): 3389, doi: 10.1029/2003JC001879. [ Links ]
Zijlstra, J. J. 1988. Fish migrations between coastal and offshore areas. In: Jansson B.O. (Ed.). Coastal–Offshore Ecosystem Interactions, Lecture Notes on Coastal and Estuarine Studies. Germany, Springer–Verlag, pp. 257–272. [ Links ]














