INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are emitted into the environment by natural processes or by anthropogenic emissions (Li et al. 2014, Duan et al. 2015). Once in the air, PAHs can attach to atmospheric particulate matter to be transported over long distances. Due to their acute toxicity and potential mutagenic, teratogenicity and carcinogenic effects on human health, sixteen PAHs have been considered as priority pollutants by both the United States Environmental Protection Agency (USEPA 1977) and the European Environmental Agency (EC 2001, Keith 2014). Over 90 % of total PAHs released to the environment accumulate in soils, which acts as a sink for these compounds (Eom et al. 2007). In Argentina, soil PAH contamination is mainly related to spills of petroleum products.
The Pampas Region is one of the largest temperate prairies of the world. It is located in the Southern Hemisphere, between 32º to 39ºS and 56 to 67ºW. This zone covers more than 52 Mha of agriculturally prime quality land, the remaining being either marginally suitable or unsuitable for cropping due to rainfall and slight differences in relief. Crude oil extraction began in the southwest of the Pampas region, Argentina, in 1969. The predominant soils nearby the petroleum extraction region are Entic Haplustolls and deep, coarse textured typic Hapludolls (Torri et al. 2011), with low nitrogen and phosphorus availability for plant growth (Díaz-Zorita and Buschiazzo 2006). The productivity of the zone is first related to soil water content, and then to nutrient availability (Díaz-Zorita et al. 1999). These agroecosystems are known to be more fragile, with longer time needed to recover from disturbances compared with other environments (Noy-Meir 1973, PROSAP/EPSA 2010). Until now, only one accident was officially informed in the southwest Pampas in 2015, due to the spill of 80 m3 of crude oil with a total affected area of 500 m2.
Anthracene and phenanthrene are usually found in high concentrations in PAHs-contaminated soils (Chirakkara and Reddy 2015, Dubrovskaya et al. 2016). Unlike other high-molecular-weight PAHs, these isomers do not pose a risk to human health. However, phenanthrene is mutagenic in bacterial and animal cells, and carcinogenic in rodents (Wilson and Jones 1993), whereas anthracene is highly toxic to wildlife (Cheung et al. 2008). Owing to their chemical structure that resembles certain carcinogenic PAHs, both compounds have been used as models for different environmental studies (Bouchez et al. 1995, Kanaly and Harayama 2000).
Indigenous soil microbial communities may have an adaptive response to the presence of PAHs (Margesin and Schinner 2001, Delille et al. 2004) if they are not limited by environmental conditions or low nutrient availability (Gavrilescu 2005, Nikolopoulou et al. 2013). In recent decades, different plant species began to be used in remediation technologies, mainly because PAHs dissipation in the rhizosphere may be significantly improved in comparison to the bulk soil (Bourceret et al. 2015). The release of root exudates enhances microbial biomass, activity and diversity (Vácha et al. 2010, Martin et al. 2014, Torri et al. 2014). But plant species differ in their root characteristics and exudates. For instance, grass species have a high root surface area compared to dicotyledonous plants, and possess an extensively branched, fibrous root system (Soleimani et al. 2010), which can interact with soil microorganisms (Dzantor et al. 2000). Besides, fine root death provides readily available nutrients, which may also increase the microbial degradation of PAHs (Olson et al. 2003). In the last years, much of the research on PAHs´ dissipation in contaminated soils has focused on using organic or inorganic amendments to immobilize pollutants or to increase their water solubility (Fernández-Luqueño et al. 2017, Han et al. 2017, Kong et al. 2018, among others). However, there is not available information concerning the long-term influence of these substances on the ecosystems (Fernández-Luqueño et al. 2017). Autochthonous adapted microorganisms have the advantage of being safe, eco-friendly and economical, apart from preserving soil natural structure and texture (Huang et al. 2004). Besides, they may be more adapted to the particular soil environment than non-indigenous commercial microbial inocula (Silva et al. 2009). However, there is a certain controversy about the capacity of indigenous microbial communities to degrade PAHs in contaminated soils. Some researchers indicated that competent degraders may be absent or present at too low abundances to perform remediation, especially if the site was not previously exposed to the contaminant (Sabaté et al. 2004, Couto 2010), while others reported that native hydrocarbon-utilizing microorganisms are present in most natural environments, but selectively enrich in contaminated soil (MacNaughton et al. 1999, Nandal et al. 2015, Baruah et al. 2017).
The objective of this study was to analyze the capability of endogenous soil microorganisms of a pristine sandy soil of the Pampas region, Argentina, not previously exposed to any kind of contaminants, to dissipate two isomers (anthracene or phenanthrene) in optimal temperature and water availability conditions, with or without plant (Lolium perenne L.). The influence of nutrient availability was also evaluated.
We hypothesized that the native soil microbial biomass of this pristine soil, not previously exposed to contaminants, was potentially capable of degrading anthracene or phenanthrene in optimal temperature and water conditions. PAHs removal would increase in the presence of L. perenne and adequate nutrient availability.
MATERIALS AND METHODS
Chemicals
Analytical anthracene (~ 95 %) and phenanthrene (≥ 97 %) were purchased from Sigma Aldrich Co., Ltd, UK. All the other chemicals used in the study were of analytical purity.
Soil
The pristine soil selected was a typic Hapludoll (U.S. Soil Taxonomy) of the Pampas Region, Argentina. Sampling was performed near Carlos Casares Town (35º 37’ S - 61º 22’ W). Composite soil samples (10 sub samples, 0 - 15 cm depth) were collected from fields with no previous history of contamination, far from roads or urban areas in order to assure minimum concentrations of PAHs. Water holding capacity was determined according to the method proposed by Mizuno et al. (1978). Soil samples (10 sub samples) were thoroughly homogenized, air dried and passed through a stainless-steel sieve with 2-mm openings to remove stones and roots. Relevant soil properties are presented in table I.
TABLE I SOIL CHARACTERISTICS OF THE TYPIC HAPLUDOLL (A HORIZON, 0-15 CM) USED FOR THE POT EXPERIMENT
| Typic Hapludoll | |
| Clay (%) | 19.2 |
| Silt (%) | 23.2 |
| Sand (%) | 57.6 |
| pH | 5.12 |
| Organic carbon (g/kg) | 28.6 |
| Water holding capacity (%) | 19.3 |
| Total N (mg/g) | 2.62 |
| Electrical conductivity (dS/m) | 0.61 |
| Cation exchange capacity (cmol(c)/kg) | 22.3 |
| Exchangeable cations | |
| Ca2+ (cmolc/kg) | 10.2 |
| Mg2+ (cmolc/kg) | 2 |
| Na+ (cmolc/kg) | 0.3 |
| K+ (cmolc/kg) | 2.8 |
Soil spiking procedure
Soil was spiked with anthracene or phenanthrene. To maintain indigenous microbiota, the soil was not sterilized. Although PAHs are usually added to soils solubilized in different organic solvents (acetone, dichloromethane, hexane), in this work the compounds were added as a fine solid powder. This way was chosen in order to avoid any damage to the natural microflora by the organic solvent (Ruberto et al. 2006). The spiking technique used was stainless-steel spoon (Doick et al. 2003): 1 g of anthracene or phenanthrene crystals were finely ground in an agate mortar and added to a mixing vessel containing 200 g of dry soil, and gentle mixed using a sterile spatula for 5 min. The remainder 800 g soil was added in 200 g aliquots; blending was performed for 25 minutes. To ensure a uniform distribution of anthracene or phenanthrene, both spiked soils were each extended in a plastic tray, and replicated soil samples for analysis were taken from different parts of the bulk spiked soil.
Greenhouse experiment
A pot experiment was conducted in a greenhouse (23 ± 1 °C) sheltered from rain or direct sunlight. A disc of filter paper was placed in the bottom of each plastic pot (10 cm depth x 6 cm diameter) to avoid soil loss. The pots were filled with 250 g dry weight spiked or unspiked soils, and were afterwards covered with a layer of 5 mm of coarse sand to minimize PAHs volatilization (Zhou et al. 2013). Pots were left undisturbed and allowed to settle down over 10 days. During this period, and throughout all the experiment, soils were maintained at 80 % WHC using distilled water, preventing possible loss of PAHs due to leaching.
Ryegrass (Lolium perenne L.) seeds were surface sterilized by soaking in 30 % (v/v) H2O2 for 20 min and washed several times with distilled water. At day 10, twenty seeds were surface-sown in each pot according to the treatments detailed in table II. In all, 12 treatments [1 soil material x 3 (no pollutant, anthracene, phenanthrene) x 2 (plant, no plant) x 2 (non-fertilized, fertilized)] were each replicated four times. The pots were moved around at regular intervals to compensate for light differences.
TABLE II TREATMENTS
| Abbreviation | Description |
| C | non-spiked soil |
| CF | non-spiked soil with fertigation |
| CR | non-spiked soil with ryegrass |
| CRF | non-spiked soil with ryegrass and fertigation |
| A | anthracene spiked soil |
| AF | anthracene spiked soil with fertigation |
| AR | anthracene spiked soil with ryegrass |
| ARF | anthracene spiked soil with ryegrass and fertigation |
| P | phenanthrene spiked soil |
| PF | phenanthrene spiked soil with fertigation |
| PR | phenanthrene spiked soil with ryegrass |
| PRF | phenanthrene spiked soil with ryegrass and fertigation |
Germination was monitored closely between days 10-25. The number of germinated seeds in each pot was recorded and expressed as a percentage of the number of seeds added. Seedlings were thinned to ten at day 25.
At day 25, 3 mL of an aqueous solution made up of 1 g/L containing N:P2O5:K2O 15:15:15 ratio (Xu et al. 2006) were added twice a week to each pot of the fertilized treatment. To ensure nutrient homogeneous distribution, nutrient solution was uniformly hand applied by drops on soil surface prior to watering. If weeds germinated, they were removed periodically by hand before they reached 0.5 cm in size. At day 90, the aerial parts of the plants were harvested. Aerial biomass was dried at 60 °C for 48 h, and then weighed (DW). The soil from each pot was collected and homogenized. Soil samples were stored at 4 °C before analysis. Since L. perenne´s roots explored all the pot´s volume, the recovered soils from planted pots were considered as rhizospheric soil, and the others as root-free soil (Chiapusio et al. 2007, Liu et al. 2013).
Anthracene or phenanthrene extraction and quantification
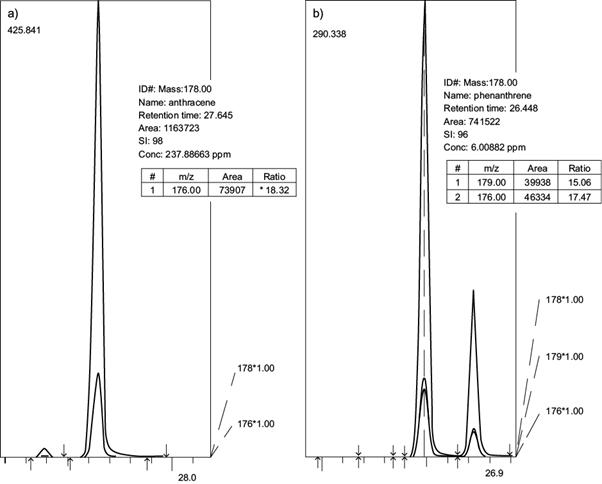
The procedure described by Torri and Alberti (2012) was used to determine anthracene and phenanthrene concentrations in soil samples. Briefly, 10 g of soil sample were sonicated 20 min with 20 mL of hexane:acetone (3:2, v/v) in an ultrasonic bath (frequency 35 kHz, Neytech 28H, USA), followed by centrifugation at 3000 rpm, reduced to 1 mL with rotary evaporator at 30 ºC and injected into the gas chromatography-mass spectrometry (GC/MS) system. The analysis was carried out with a GCMS-QP2010 equipment from Shimadzu (Shimadzu Corporation, Japan) equipped with a DB-1 fused silica capillary column (polydimethylsiloxane, 30 m long x 0.25 mm i.d. 0.25 μm film thickness, J&W Scientific, Folson, CA). The GC system was operated in splitless mode and 1 μL portions of the extracts were injected by using an autosampler. Both the injection liner, which contained deactivated glasswool for splitless injection (Agilent Technologies), and the transfer line were maintained at 280 °C. The oven temperature was programmed to rise from 70 °C (1 min hold) to 290 °C at a rate of 30 °C/min (22 min hold). Helium was used as the carrier gas at linear velocity 40 cm/s. The electron-impact (EI) ionization energy was 70 eV. The presence of the compounds was confirmed by means of the mass spectra obtained in full scan acquisition mode in the m/z range from 20 to 500. High purity analytical standards (> 98.5 %) of anthracene or phenanthrene were injected in triplicate to identify the retention time and mass spectra of each compound. Standard calibration curves were established by plotting peak areas (Fig. 1) against different concentrations of anthracene (range: 3.000-820.000 µg/mL) or phenanthrene (range: 0.441-66.216 µg/mL). Regressive equations for anthracene and phenanthrene were y = 54070x - 263065, R2 = 0.9962 and y = 140532x - 102291, R2 = 0.9986 respectively.
The system was controlled by an interface module and a computer. Mass spectra was compared with reference compounds. The peaks of the total components were integrated to obtain the total area. The area of each compound was divided by the total area and expressed as percentage. The concentration of those that produced a signal-to-noise of 3:1 in blank sample was defined as detection limit (DL). The DL for anthracene and phenanthrene were 2.0 ng/g (DW) and 2.3 ng/g (DW) respectively.
Counting of the total bacterial community
Ten grams soil samples were added to 100 mL sterile 0.85 % NaCl (w/v) solution, sonicated for 1 min and allowed to stand for 2 min. Ten-fold serial dilutions in the ranges 10-1 to 10-9 were prepared (Fawole and Oso 2007). Aliquots (0.01 mL) of these dilutions were seeded on sterile Petri dishes on tryptone soya agar medium in triplicate and incubated in the dark at 30 °C for 7 days. Uninoculated controls were included. Total heterotrophic bacterial count was determined by pour plate technique, and expressed as colony forming units per gram of dry soil (CFU/g).
Statistical analysis
The statistical analysis was performed with Statistix 7.0 (Analytical Software 2000), processing the data for analysis of variance (ANOVA) to test main and interactive effects. Normality assumption was tested by the Shapiro-Wilks test, and homogeneity of variance was tested using the Bartlett’s test. Significant effects and interactions between contaminant, plant and fertilizer were evaluated. Where significant F values were obtained, differences between individual means were tested using Tukey’s test. Statistical significance was defined as p < 0.05. All results reported are the mean of four replicates. The results were expressed as mean ± standard deviation.
RESULTS
Soils
Non-spiked soils had undetectable anthracene and phenanthrene concentrations. In the spiked soils, the initial levels of anthracene and phenanthrene (1000 ± 21 mg/kg and 1000 ± 29 mg/kg respectively) met the required coefficient of variance for spike-homogeneity for the mixing to be considered valid and statistically sound (Hakanson 1984). Therefore, mean anthracene and phenanthrene concentration measured in the subsamples was assumed to be representative of all the spiked soil (Northcott and Jones 2000). These initial levels (1000 ± 21 mg anthracene/kg and 1000 ± 29 mg phenanthrene/kg) represent the mean concentration of both PAHs at day 0 in the greenhouse trial, and may well be its soil concentration after an accidental discharge into the environment (Alvaro et al. 2017).
Effect of PAHs treatments on L. perenne growth
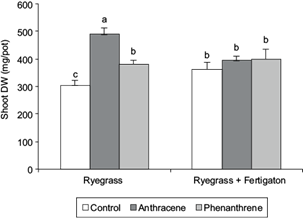
Percentage seed emergence of L. perenne (93-95 %) did not significantly vary (p < 0.05) between spiked and non-spiked soils. Plants did not exhibit apparent signs of stress or toxicity along the growing period under PAHs treatment. Soil addition of anthracene (AR) or phenanthrene (PR) resulted in a statistically higher production of aerial biomass compared to the unfertilized control (CR; Table III) in soils (CRF), biomass yield significantly increased with fertigation. However, the aerial biomass yield of the fertilized anthracene spiked soil (ARF) was significantly lower (p < 0.05) than the unfertilized treatment (AR), whereas no statistical differences were observed between fertilized and non-fertilized phenantrene spiked soils (PR vs. PRF). All fertilized treatments (CRF, ARF and PRF) showed no statistical differences in relation to aerial biomass (Fig. 2).
TABLE III INITIAL CONCENTRATION OF ANTHRACENE OR PHENANTHRENE (DAY 0), RECOVERIES AND PERCENT DISSIPATED AFTER 100 DAYS IN EACH TREATMENT
| Treatment* | mg pollutant/kg soil day 0 | mg pollutant/kg soil day 100 | % dissipated | |||||
| A | 1000 | ± | 21 | 331.45 | ± | 4.68 | a | 66.90 |
| AF | 1000 | ± | 21 | 348.60 | ± | 49.26 | a | 65.14 |
| AR | 1000 | ± | 21 | 167.51 | ± | 33.34 | b | 83.25 |
| ARF | 1000 | ± | 21 | 112.92 | ± | 6.74 | b | 88.71 |
| P | 1000 | ± | 29 | 88.40 | ± | 9.96 | bc | 91.20 |
| PF | 1000 | ± | 29 | 103.65 | ± | 9.36 | b | 89.60 |
| PR | 1000 | ± | 29 | 2.74 | ± | 0.81 | c | 99.70 |
| PRF | 1000 | ± | 29 | 0.50 | ± | 0.11 | c | 99.95 |
*Treatments: A = anthracene spiked soil; AF = A with fertigation; AR = A with ryegrass; ARF = A with ryegrass and fertigation; P = phenanthrene spiked soil; PF = P with fertigation; PR = P with ryegrass; PRF = P with ryegrass and fertigation. Distinctive groups are marked with different letters (p < 0.05).
Dissipation of anthracene and phenanthrene in soil
GC-MS chromatograms of anthracene and phenanthrene remaining in the soil at the end of the experimental period are shown in figures 3 and 4; their residual concentrations are shown in table III. After 100 days, the mean concentration of both PAHs was significantly reduced in all treatments. Initial anthracene (1000 mg/kg soil) and phenanthrene (1000 mg/kg soil) were significantly reduced to 331.5 mg anthracene/kg soil (A) and 88.4 mg phenanthrene/kg soil (P) in unplanted treatments (p < 0.05). This represents a removal efficiency of 66.9 % and 91.2 % respectively.
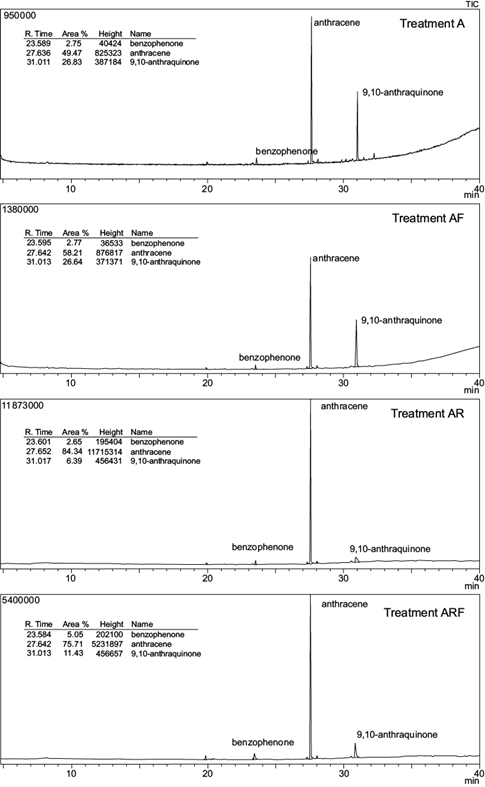
Fig. 3 Total ion chromatogram (TIC) from the gas chromatography-mass spectrometer analysis of anthracene spiked soils at the end of the experimental period. Treatments: A = anthracene spiked soil; AF = A with fertigation; AR = A with ryegrass; ARF = A with ryegrass and fertigation
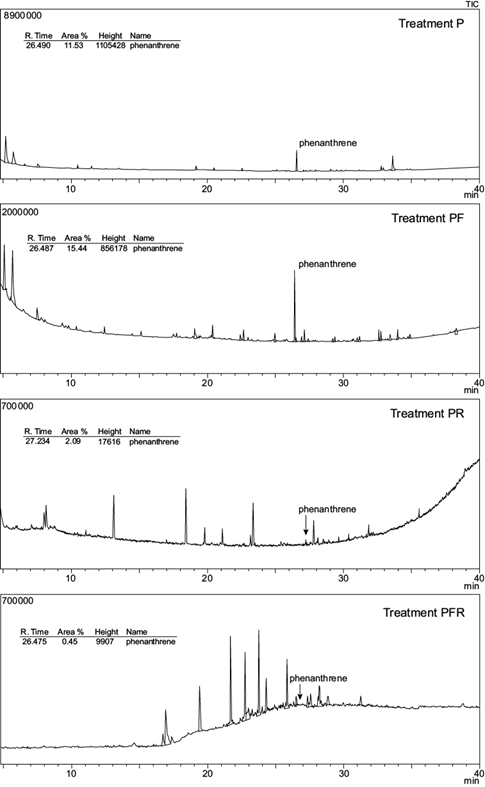
Fig. 4 Total ion chromatogram (TIC) from the gas chromatography-mass spectrometer analysis of phenanthrene spiked soils at the end of the experimental period. Treatments: P = phenanthrene spiked soil; PF = P with fertigation; PR = P with ryegrass; PRF = P with ryegrass and fertigation
In planted spiked soils, anthracene and phenanthrene were reduced to 167.5 and 2.74 mg/kg respectively (treatments AR and PR), representing 83.25 % and 99.7 % depletion. Moreover, the comparison of mean values based on the AOV model statement indicated a significant interaction (p < 0.01) between two individual factors: plant and contaminant (Table IV). No other interactions were observed. The addition of nutrients had no effect on both PAHs dissipation: no significant differences in anthracene or phenanthrene soil concentration were observed between fertilized vs non-fertilized treatments (A and AF, AR and ARF, P and PF or PR and PRF).
TABLE IV ANALYSIS OF VARIANCE OF STUDIED FACTORS (POLLUTANT, PLANT, FERTIGATION) AND PARTITION OF THE TREATMENT SUM OF SQUARES INTO MAIN EFFECT AND INTERACTION
| SOURCE | DF | Sum of squares | Mean square | F | P |
| FERTIGATION (A) | 1 | 1220.88 | 1220.88 | 0.82 | 0.3740 |
| PLANT (B) | 1 | 158727 | 158727 | 106.67 | 0.0000 |
| SPIKED (C) | 1 | 273945 | 273945 | 184.10 | 0.0000 |
| AxB | 1 | 2062.97 | 2062.97 | 1.39 | 0.2506 |
| AxC | 1 | 2846.63 | 2846.63 | 1.91 | 0.1794 |
| BxC | 1 | 17262.9 | 17262.9 | 11.60 | 0.0023 |
| AxBxC | 1 | 427.584 | 427.584 | 0.29 | 0.5969 |
| RESIDUAL | 24 | 35711.8 | 1487.99 | ||
| TOTAL | 31 | 492204 |
DF = degrees of freedom; F = F-statistic; P = P-value
The overall extent of PAHs dissipation was clearly compound-dependent: after 100 days, the concentration of anthracene was significantly higher (p < 0.05) compared to that of phenanthrene for the same treatment in all spiked soils (Table III).
Total bacterial count
Figure 5 shows the mean total bacterial counts in all treatments after the 100 days pot experiment. In the non-spiked soil, bacterial counts significantly increased (Tukey, p < 0.05) with nutrient addition (CF) or L. perenne planting (CR) as compared to control (C), although no significant differences (p > 0.05) between CF, CR and CRF were observed.
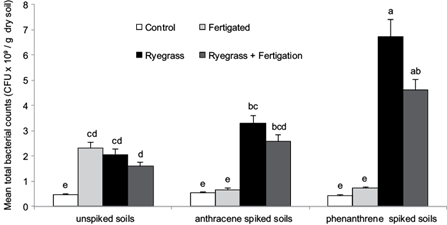
Fig. 5 Mean total bacterial counts (x109 CFU/g) at the end of the experimental period. Distinctive groups are marked with different letters (p < 0.05)
Soil spiking with anthracene (A) or phenanthrene (P) produced no significant differences (p > 0.05) with respect to bacterial counts relative to the unspiked control. No effect due to nutrients addition was observed in AF or PF compared to A or P respectively. However, bacterial counts in the anthracene or phenanthrene spiked soils were significantly higher (p < 0.05) in plant treatments (AR or ARF; PR or PRF) as compared to unplanted treatments (A, AF or P, PF respectively).
DISCUSSION
Ryegrass (Lolium perenne L.) was chosen as the test plant for phytoremediation to reflect typical species found in the Pampas region. Aerial biomass was measured at the end of the experimental period to explore the ability of L. perenne to grow in anthracene or phenanthrene spiked soils.
Toxic effects of both PAHs on the growth of this species have been previously described (Günther et al. 1996, Cheema et al. 2010, Acosta-Santoyo et al. 2017). These authors found that root and shoot yields of L. perenne were significantly reduced in PAHs-polluted soils compared to control soils. Low-molecular-weight volatile hydrocarbons are soluble in hydrophobic plant materials, and can penetrate root´s cell membranes (Salanitro et al. 1997). Phytotoxicity may be exerted in part by PAHs ability to damage cell membranes, reducing nutrient or metabolite transport (Chouychai et al. 2007) or water utilization efficiency (Ma et al. 2010, Nakata et al. 2011). Photosynthetic activity and electron transport may also be inhibited (Mallakin et al. 2002, Torri et al. 2009). These effects are nonspecific, and depend on PAHs water solubility. Reilley et al. (1996) suggested that PAHs might reduce the ability of contaminated soil to provide water and nutrients to plants, leading to a decline in biomass production. PAHs may also induce retard growth, genetic mutation, and increase plant sensitivity to other stresses (Maliszewska-Kordybach and Smreczak 2000).
Contrary to the results reported by other researchers, the presence of anthracene or phenanthrene did not affect seedling emergence of L perenne. Several studies reported that seed germination might be insensitive to bioavailable toxic chemicals because seedlings obtain nutrients from internal materials (Smith et al. 2006, Eom et al. 2007, Torri et al. 2009, Anyanwu and Semple 2015). Surprisingly, plant aboveground biomass (DW) was significantly higher in spiked treatments compared to non-spiked soils, despite the high spiking-dose used. Growth promoting effects of PAHs were first described by Gräf and Nowak (1966). Low levels (below 10 mg/kg) of soil PAHs concentrations were reported to stimulate rather than inhibit plants growth at the early stages of plant development (Maliszewska-Kordybach and Smreczak 2000). Other studies reported that higher phenanthrene levels (200 mg/kg) produced no significant differences in ryegrass biomass (DW) before 60 days of seedling emergence (Xu et al. 2006, Liu et al. 2013) although, afterwards, a restrain in growth due to toxicity stress was observed, which resulted in a significantly lower biomass than control at the end of the experiment (Liu et al. 2013). Similarly, Binet et al. (2000) reported that shoot and root biomass were significantly lower than control in a 40 days pot experiment using 200 mg/kg anthracene and phenanthrene spiked soil. But Fismes et al. (2002) observed no detrimental effect on plant growth in soil PAHs concentrations even up to 2526 mg/kg. But herein, a statistically higher production of aerial biomass as the result of soil addition of anthracene or phenanthrene compared to unfertilized control was observed (Table III). An explanation to this may be a positive priming effect (PE) originated as a consequence of anthracene and phenanthrene soil inputs, which released bioavailable nutrients to the soil solution (Joner et al. 2002). This positive PE was reported to decrease in high nutrient availability conditions, as microorganisms may fulfill their nutrient demand by utilizing the added nutrients rather than mineralizing them from native SOM (Dimassi et al. 2014, Liu et al. 2017). Therefore, the PE, which may have occurred in non-fertilized pots, would have masked a fertigation effect on L. perenne biomass and, as a result of this, no significant increase in aerial biomass was observed in fertilized compared to non-fertilized plant treatments.
Alternatively, a rapid initial mineralization of both contaminants cannot be ruled out. Numerous studies have shown that the availability, and therefore, the biodegradation of anthracene and phenanthrene in spiked soils is related to the degree of sorption onto soil organic matter (SOM) (Ahmad et al. 2001, Ahangar et al. 2008), for sorbed substrates are more resistant to biodegradation than non-sorbed ones (Wszolek and Alexander 1979). Soils´ capacity to absorb PAHs is positively related to the aromatic constituents of SOM (Ahmad et al. 2001). In the Pampas region, the SOM of coarse textured soils is more aliphatic, less aromatic and less rich in carboxylic acid groups compared to that of fine-textured soils (Galantini et al. 2004). In line with this, anthracene or phenanthrene may have been weakly adsorbed onto the SOM of the typic Hapludoll (Ran et al. 2007), and, therefore, potentially available for microbial degradation.
Both PAHs concentration in root-free soils significantly decreased at the end of the experiment compared to initial concentrations. Previous studies have shown that the number of PAHs-degrading microorganisms and their proportion in the heterotrophic community increased upon previous exposure of soils at PAHs concentrations greater than background (van der Meer et al. 1992). But the pristine soil used in this study was not previously exposed to PAHs pollution. In fact, the Pampas is recognized as a non-polluted region (Torri 2014), with very low background concentration levels of PAHs, in the range of 1.8-34 ng/g (Wilcke et al. 2014). Conversely, other authors indicated that soil autochthonous microbial communities can rapidly degrade low molecular weight PAHs because these ubiquitous compounds are known to be present in all soils at very low concentrations (Stroud et al. 2007). The dissipation of anthracene or phenanthrene in root-free spiked soils at the end of the experiment revealed the catabolic capability of the autochthonous soil microbiota. These results are in good agreement with a previous experiment with the same root-free treatments, where we measured the production of C-CO2 along 60 days as an indirect estimation of microbial activity (Torri et al. 2018). The production of C-CO2 in all incubated soils increased from day 0 to day 10, with no significant differences between treatments. But from day 10 onwards, the average respiration in unspiked soils decreased to a minimum on day 24, whereas C-CO2 emission in anthracene or phenanthrene spiked soils was significantly higher than controls (p < 0.01), with a maximum between days 10-18, related to PAHs degradation. Apparently, a compatible microflora existed or rapidly established in the Hapludoll soil, which resulted in anthracene or phenanthrene degradation. Therefore, we cannot determine whether PAH degradation in the pristine Hapludoll is a characteristic of indigenous soil microbial communities or an acquired ability induced by exposure to undetectable levels of PAHs. In any case, 77.3 % of anthracene and 91.2 % of phenanthrene were removed from the unplanted spiked soils at the end of the experimental period. Similar results for spiked soils were reported by Binet et al. (2000), Xu et al. (2006) and Cennerazzo et al. (2017)) in pot experiments. Nonetheless, the results obtained in this study may differ from those obtained in field conditions, because organic compounds that have aged in contaminated soils are less bioavailable than in freshly spiked soils and therefore, their removal rate may be reported to occur relatively slow (Fu et al. 2012). Besides, adverse environmental conditions in natural soils in comparison with laboratory conditions usually cause less efficient biodegradation of organic pollutants.
As expected, L. perenne favored to decrease the concentration of anthracene and phenanthrene in spiked soils compared to non-vegetated spiked soils (Table III). The removal of PAHs from vegetated soils may occur by three processes: abiotic dissipation, plant uptake or degradation by soil microorganisms. In this pot experiment, abiotic dissipation (leaching or volatilization) was prevented by the experimental conditions chosen. On the other hand, plant uptake of phenanthrene or anthracene has been reported to be very low in many phytoremediation studies. Reilley et al. (1994) found that total accumulation of anthracene in roots and shoots of different plant species accounted for less than 0.03 % of total added compound, Cheema et al. (2010) indicated that only 1.1 % of the spiked phenanthrene was absorbed by L. perenne roots after a 65 days pot trial, while Cennerazzo et al. (2017) reported that less than 1 % of total phenanthrene carbon was taken up by ryegrass roots after 21 days. Similar results were reported by Fu et al. (2012). An explanation to this is the low water solubility of PAHs (Binet et al. 2000), together with the non-polar organic composition of root tissue, such as lipid contents (Chiou et al. 2001, Gao and Zhu 2004) that might prevent significant uptake by plant roots. In the light of this, biodegradation by native soil microorganisms is likely to be the dominant mechanism for the dissipation of anthracene and phenanthrene in the rhizosphere of L. perenne treatments. Some researchers speculated that plants may respond to the presence of a chemical stress in soil by increasing or changing exudation, modifying rhizospheric microflora composition or activity (Walton et al. 1994). The synergistic effect of bacteria and root exudates on the selective growth of PAHs degraders in contaminated soils has been previously reported (Khan et al. 2013, Yang et al. 2014, Guo et al. 2017). In addition, roots possess wall-bound and soluble oxidative enzymes that may be directly implicated in the degradation of PAHs (Rezek et al. 2008). These results are consistent with the findings of previous studies, which showed that anthracene and phenanthrene degradation in spiked soil was significantly higher in rhizospheric than in non-rhizospheric soils (Günther et al. 1996, Binet et al. 2000, Korade and Fulekar 2009).
Soil removal of phenanthrene and anthracene was different, although they both contain three fused aromatic rings. In all cases, the degradation of phenanthrene was significantly higher than anthracene (Table III). This result may be related to the higher water solubility of phenanthrene (1.1 mg/L), as compared with anthracene (0.045 mg/L) (Bianche et al. 2014). Many microorganisms are known to degrade PAHs only when they are dissolved in an aqueous media (Johnsen et al. 2005). In fact, water solubility of many PAHs is the rate-limiting factor for biodegradation since microbial biodegradation is considerably slower from sorbed sites than from the soil solution (Gordon and Millero 1985, Semple et al. 2003). Therefore, a large labile pool of phenanthrene may have been present in the soil solution of the spiked typic Hapludoll, readily available for soil microorganisms’ degradation. This process lead, in turn, to the desorption of more phenanthrene from the solid phase to the aqueous phase (Mueller and Shann 2006), increasing phenanthrene degradation respect to anthracene degradation.
The addition of inorganic nutrients is a strategy to enhance PAHs microbial biodegradation rate in contaminated soils (Kalantary et al. 2014). But this was not the case here. Contrary to what we expected, no significant differences in anthracene or phenanthrene dissipation were observed between fertilized and non-fertilized treatments. Soils in the Pampas region are moderately acid, low in available P, and have high organic carbon content (Torri and Lavado 2002). Therefore, nutrient availability seemed to be adequate in non-fertilized treatments along the studied period, for no significant differences in terms of anthracene or phenanthrene degradation were observed between fertilized and non-fertilized treatments at the end of the experimental period.
Soil microbial biomass is closely related to soil fertility (Zhong and Cai, 2007). Total bacterial counts in the unspiked soil was similar to other soils of the Pampas region (Merini et al. 2007). Although the effects of nutrients on microbial biomass have been investigated intensively, the results are inconsistent. Some studies showed that chemical fertilizers increased microbial biomass (Geisseler and Scow 2014), but other researchers reported that soil P and N contents had no significant effects on soil microbial populations (Zhong and Cai 2007). Some other evidence suggests the use of nitrogen fertilizers may cause ammonia or nitrite toxicity to microorganisms (Tibbett et al. 2011), which may be particularly severe in sandy soils with limited buffering and water holding capacity (Ferguson et al. 2003). In our study, the chemical fertigation (NPK) of the pristine soil improved nutrient availability, increasing total bacteria counts as compared to the control at the end of the experimental period.
As expected, the growth of L. perenne promoted the degradation of anthracene and phenanthrene, and total bacterial counts were significantly stimulated. The bacterial abundances in rhizosphere soils were higher than those in root-free soils, indicating that L. perenne roots significantly stimulated the growth of the bacteria in spiked soils. At the end of the experiment, the highest value of total bacteria counts was observed in phenanthrene spiked soils with plant treatment (PR). This treatment exhibited the highest removal efficiency (99.7 %). The increase of bacterial counts in the rhizosphere has already been observed in other studies (Shahsavari et al. 2015, Thomas and Cébron 2016, Guo et al. 2017). Although the growth of hydrocarbon-degrading bacteria may be strongly enhanced by fertigation with inorganic N and P (Nikolopoulou and Kalogerakis 2010), this was not observed here. These results suggest that nutrient availability was adequate in non-fertilized spiked soils, or ammonia or nitrite toxicity to microorganisms as a result of N addition, as indicated above. Nonetheless, further investigation is needed to identify the microbial communities responsible for anthracene and phenanthrene dissipation in this pristine soil.
CONCLUSIONS
The major finding of the present study was the natural capacity of a pristine soil of the Pampas region, which was not previously exposed to PAH pollution, to degrade anthracene or phenanthrene. No phytotoxic effects of both contaminants on L. perenne growth were observed; on the contrary, plant aboveground biomass significantly increased as a result of treatments. On the other hand, ryegrass significantly enhanced soil dissipation of both contaminants. The addition of inorganic nutrients did not produce a biostimulation effect. In all cases, the dissipation of phenanthrene was significantly higher than anthracene, and may be related to the higher water solubility of the former.
Results suggest that microbial degradation was largely responsible for PAHs dissipation, suggesting that indigenous PAHs degrading microorganisms might exist in the pristine Hapludoll of the Pampas region, and exert a degrading function. At the end of the experimental period, total bacteria counts in rhizosphere soils were higher than those in non-rhizosphere soils, revealing that L. perenne´s roots significantly stimulated the growth of bacteria in spiked soil.
Nevertheless, freshly applied PAHs may not behave in the same way as aged pollutants in contaminated soils. Moreover, the rate of PAHs biodegradation in natural environments may be different compared to those observed in this experiment. This is because environmental factors, which determine the success of bioremediation, may not be maintained at optimal range in contaminated environments. Provision of oxygen, moisture, nutrient availability, pH and temperature are amongst the most important environmental factors that need to be kept at optimal range for autochthonous (indigenous) microorganisms´ growth and metabolism, and for plant survival and growth. The present findings are based on a pot experiment. Therefore, this remediation strategy needs to be applied and validated in the field, to ensure the safe and cost-effective restoration of PAHs contaminated soils.











 nueva página del texto (beta)
nueva página del texto (beta)