Introduction
This research arises from the need to know the mental models that 1st year university students have around the atom. Lecturers of the University’s Chemistry Degree (blinded to review) requested collaboration from the Department of Didactics (blinded to review) to analyze these mental models. The main need was to study the atomic model of the students of that first year of university, but based on the results obtained, this research was extended to the high-school stage (15 to 18 years´ students) in a total sample of 454 students. Idenng difficulties and to plan the teaching (Talanquer, 2009). In this context, over the past few years, it has been observed that when first year students of our university are asked to define and represent an atom, they show important conceptual errors and have problems to do it according to the atomic model they should have. Therefore, the first step was to review the bibliography published to date in relation to the mental models that students have about the atom. In some international reviewed research, authors like Papageorgiou, Markos and Zarkadis (2016) and Fitriza and Gazali (2018), studied the atomic mental model of students from grade-12 to 16. And in the Spanish framework, these models had been investigated by authors such as Solbes et al. (1987); Quesada, Valcárcel & Sánchez (2005); García-Carmona (2006); Solaz-Portolés and Sanjosé (2008). All this bibliography has served to guide our research, however, the objective of the work has been to update and expand the knowledge about the atomic model of the students in the context of the new high-school educational law in Spain. The educational law in Spain changed in 2014, and this change introduced modifications in the contents worked on in each course.
In addition to bibliography on atomic models in students, we also reviewed research on atomic models present in Spanish textbooks (García-Carmona, 2004; 2011; Cid and Dasilva, 2012; Domenech, Savall and Martínez-Torregrosa, 2013). All of them included research contextualized in texts born of the previous educational law. For this reason, we consider as necessary to update the bibliography on the models that students should have according the current curricula and compare them with those they really have, based on an investigation with students who have completed all of high school levels, in the context of the current Education law in Spain.
The curriculum in Spain around the concept of atom has been studied to know what models, and what evolution of these models, students should be taught. Therefore, this research has been developed in order to answer the following research questions:
Theoretical framework
Atomic mental models
There are several definitions of what can be understood by mental model; it is generally assumed that this term encompasses both the pictorial-virtual representation and the explanation of phenomena by an individual (Wang and Barrow, 2013). A model can be viewed as an intermediary between the abstraction of the theory and the concrete actions of experiments (Dori and Barak, 2001). Thus, once the student builds his or her own model on something, it would be used to explain phenomena and to analyse information in order to make predictions (Kozma et al., 2000). Mental models are unstable; this means that they evolve during student learning. In this regard, it has been shown that the evolution of a mental model will be easier the greater the number of tests that the individual has successfully submitted to his model (Coll and Treagust, 2001). The less they test their models, the harder it will be to evaluate them and therefore more difficult it will be for them to evolve. Therefore, it is important for teachers to know the mental models of the students and test them so that students can perceive the weaknesses of their models and build more evolved models (Erduran and Duschl, 2004).
Students´ difficulties in their atomic mental model construction
There are several studies that describe the difficulties for students to develop an ageappropriate mental atomic model. Therefore, given that the different models that students must acquire are well established, a question arises: which are the real difficulties for students to move from a simple atomic model to a more evolved one?
The main aspect that hinders the construction of the atomic model is its abstraction (Dori and Barak, 2001; Toomey et al., 2001; Cokelez and Dumon, 2005; García-Carmona, 2006; Cokelez, 2012). Most students do not have enough level of abstraction or spatial vision to be able to build a mental atomic model consistent with atomic theories. Toomey et al. (2001) concluded that the age of the students influences the understanding of the concept of atom. Therefore, this concept should be introduced at another stage of the educational process and from a perspective that could allow them to link it with observable facts in a way that facilitates the construction of their mental model. Another possible difficulty is that established by Cid Manzano and Dasilva Alonso (2012) who wrote that sometimes the textbooks themselves favour alternative ideas in students. In order to avoid these alternative conceptions, authors like Harrison and Treagust (2000), García-Carmona (2004) or Stevens et al. (2010) came to suggest potential teaching sequences to the atomic concept.
A key moment in the difficult task of building the mental model on the atom is when the model students have faces new situations, therefore, it is put to the test and consequently new models are established. According to Justi and Gilbert (2002), students might be confused when a new model, which combines attributes from different models, is introduced to them. Therefore, to build a more complex mental model, it must be based in some underpinning knowledge and connected to ideas of other related topics (Stevens et al., 2010).
Mental model assessment
To determine the mental model of atom of the students, teachers can ask them to draw and explain the structure of an atom and concrete elements such as name and location of the parts of the atom or number of orbitals (Wang and Barrow, 2013). In the drawing, students must translate their information into an image that represents spatial relationships between the functional elements. The content of this picture show deep cognitive and metacognitive processing and thus indicates the understanding of the material. Therefore, the analysis of the representations and the arguments used in the explanation of the drawings is a valuable tool to evaluate the mental model of the student (Park and Light, 2009; Rau, 2015). In fact, according to Rau (2015), these tests can be used to evaluate the learning success by means of the graphical representations the students make.
Materials and method
Subjects and procedure
The mandatory education in Spain ends when students turn 16 years old, and they begin their university studies at 18 years old. Some of them continue studying chemistry during these years while others do not. In the present investigation, we are interested in knowing the mental model about the atom of students who begin the first chemistry university course, but after analysing these results, we extended the research to know the models the students of the last two courses of compulsory education have acquired. Therefore, the sample of this study consisted of 454 students aged from 15 years to 18 years who, voluntarily, participated in the investigation. Of the total number of participants: 86 were 15-year-old students (named Group 1 onwards), 165 were 16-year-old students (named Group 2 onwards), and 203 were students in the first 18-year university course (named Group 3 onwards). The number of girls and boys was balanced in all cases. The total sample includes varied socioeconomic profiles. To make the sample as representative as possible, the students were selected from both public and private centres. Their own instructor collected the data anonymously, during the first semester of each course, before any explanation about the topic, so that the outcomes of this study represent the knowledge on the subject acquired until the end of the previous course.
Instruments and data collect
A specific questionnaire (ad hoc) was designed for the purposes of the present study (see Figure 1). The questionnaire had been validated by 12 experts before the data collection.
According authors such as Harrison and Treagust (2000), in order to know the mental model of the students the most accurate way is to ask them through tools (like interviews or questionnaires) designed specifically. Attending to the bibliography, to assess the mental model a variety of questions must be asked in order to obtain several types of information. Thus, a semi-structured questionnaire with four questions (including both open and closed) where the students had to: construct explanations, define a concept, argue differences and make representations, was laid out. The students were asked to draw a graphic representation of two different atoms, Helium (He) and Lithium (Li). They had to draw these atoms according to their mental model and to explain their drawings describing the parts they had drawn, establishing the differences between these two atoms and finally, they were asked about what an electron is and where it is located within an atom.
Data analysis
In order to know the atomic models taught in every educational level, the Spanish curriculum was studied in depth. Based on it, Table 1 shows the expected atomic models corresponding to each group of students, and the characteristics, which define them.
Table 1: Expected atomic model of every Group of students.
| Group of students | Expected atomic model |
|---|---|
| 1 | Rutherford´s model • Nuclear atom with circular orbits • Minuscule atomic nucleus with positive charges and the main atomic mass • Negative charges (electron) around the nucleus. Only one electron in each orbit • Orbits not well defined • No references to energy |
| 2 | Bohr´s model • Defined nucleus with negative charges around • Valence electrons • Energy orbitals • Electrons could move between orbitals (energy modifying) |
| 3 | Quantum model • Orbital instead of orbit • Electron position as a probabilistic position • Idea of the electron cloud • Energy sub-levels • Dual wave-particle character |
Due to the qualitative nature of the research questions, the analysis of the data collected was mainly qualitative. However, a quantitative description of the sample relating to the appropriated mental model about the topic analysed in this study was also carried out. Several categories were defined as Cokelez and Dumon (2005) were proposed. In the case of the present study the number of categories is different in each group of students.
And, also other emerging categories were found during the analysis of the results.
Results
As well as the expected outcomes described in the Table 1, other emerging ones were found during the results analysis and they are all commented in the present section.
Outcomes for Group 1 (15-years-old students)
Before the analysis of the questionnaires, some categories of analysis were defined. The aim of establishing these categories was to conclude if the atomic model belonging to group 1 was the appropriate according to their educational level or not. However, other categories emerged during the analysis. All of these are shown in Table 2.
Table 2: Categories established from the results of the Group 1, and % of responses.
| Nº | Expected | % of responses |
|---|---|---|
| 1 | Draw a nuclear atom | 92 |
| 2 | Define or draw a minuscule nucleus | 1 |
| 3 | Describe or draw negative charges around the nucleus and the positive and neutral charges inside it | 77.9 |
| 4 | Mention orbits | 24.4 |
| 5 | Talk about differences in mass between He and Li atoms | 0 |
| 6 | Represent the model in 2Dimensions | 72.1 |
| 7 | Draw electrons around the nucleus with no references to orbits | 75.6 |
| Emerging | ||
| 8 | Talk about the insignificant mass of electron within the atom | 7 |
| 9 | Specify the movement of the electron | 22.1 |
| 10 | More than one electron represented in an orbit | 73.3 |
| 11 | Talk about orbitals of electrons | 26.7 |
| 12 | Talk about valences | 13 |
| 13 | References to energy | 3.5 |
| 14 | Draw particles within a non-defined nucleus | 53 |
| 15 | Represent the model in 3Dimensions | 5.8 |
| 16 | Relate the atomic structure with gas or metal properties | 2.1 |
| 17 | Talk or draw about cloud of electrons | 1.2 |
| 18 | Relate the movement of the electron with the energy of the atom | 1.2 |
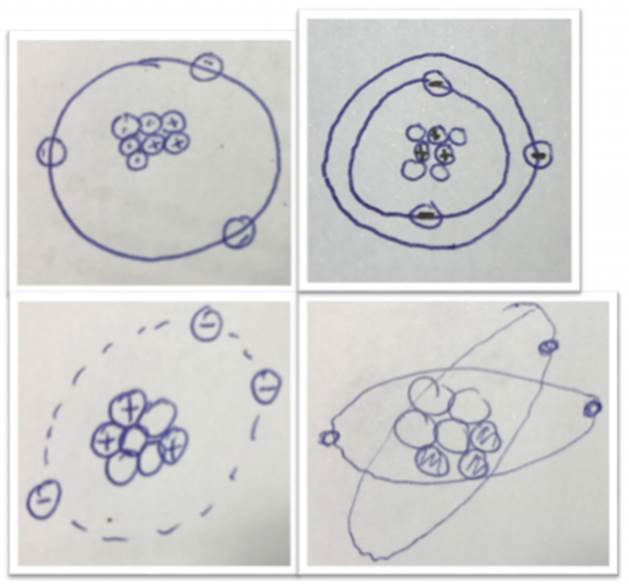
The main part of the atomic structures were 2Dimensions representations (72.1%) but also a small part of the students drawn a 3Dimensions structure for the atomic model (5.8%) as it can be observed in Figure 2. The results show that almost all the students presented a nuclear model for the atom, but barely 1% defined the nucleus as a small part within the atom structure and, surprisingly, 53% of the nucleus drawn had non-defined limit which might imply an advanced model. A total of 7% of the responses explained that the electron has an insignificant mass compared with the whole mass of the atom. What seems to be clear is that negative charges are located outside the nucleus and positive and neutral charges are inside it (77.9% of the responses). Connected with the previous response, a percentage of 22.1% explained that these electrons are in a continuous movement around the nucleus, which is an advanced concept for this group of students, and 1.2% even talked about a cloud of electrons around the nucleus.
When the students described the position of electrons, only 24.4% made any reference to orbits. A percentage of 73.3% of the students had drawn more than one electron in every orbit (in the Li atomic representation), which also implies an advanced mental model of the expected one.
The concept of orbitals of electrons was named by 26.7% of the students but there were no references to the movement of electrons between orbitals.
Another surprising results are that more than 13% of the students mentioned the concept of valences which is corresponded with Bohr´s atomic model instead of Rutherford´s atomic one, and 3.5% of the responses talked about the energy of the atoms, which is advanced in the same way as the previous result.
Other answers, which imply advanced atomic model, are that some responses (1.2%) related the movement of the electron with the energy of the atom. And, on the other hand, at 2.1% of the questionnaires the students established relationships between the atomic structure with the nature of the element (differenced if the elements were gas or metal).
The analysis of the 86 questionnaires allowed to find important differences depending on the educational centre the students came from. Therefore, it was found that the atomic model of the students is more a function of the educational centre than of the curricula content, which is the same for the whole sample of students.
Outcomes for Group 2 (16-years-old students)
The expected atomic model from the students of this group should be the Bohr´s model, but it was considered that these students had previous models according to the educational curriculum, therefore an initial list of the expected responses were done. Seven preliminary categories were defined where the general model described was considered. Also the questionnaires with no responses and with responses without sense were accounted within these categories.
The results show that the same part of the students owned a correct atomic model (24% described the Bohr´s model), as the part of students, which presented indistinguishable models, models not like any of the ones taught in the curriculum. On the other hand, an important percentage (19%) displayed previous atomic models (as Rutherford´s model or Thomson´s model) and 14% of the responses showed mixed models of the previous ones and the Bohr´s model. This could be understood as being in the process of the construction of the Bohr´s model.
After studying the results above, a deeper analysis was needed in our opinion. The extended list of categories, resulting from this analysis indeepth, is commented as following. To establish these categories, both the expected and the emerging ones were considered (see Table 3) and, on the other hand, the 14% of the students who did not answer were not considered for this analysis.
Table 3: Categories established from the results of the Group 2, and % of responses.
| Nº | Expected | % of responses |
|---|---|---|
| 1 | Bohr´s model with a defined nucleus, orbitals and with negative charges in its orbitals | 33 |
| 2 | Rutherford’s model | 20 |
| 3 | Incoherent drawings (which do not correspond to any specific model) | 24 |
| 4 | Nucleus contains positive and neutral particles | 58 |
| 5 | Electron is located outside of the nucleus | 70 |
| 6 | Electron is smaller than nucleus | 15 |
| 7 | Electron has negative charge | 70 |
| Emerging | ||
| 8 | orbitals are drawn, incorrect number of particles | 15 |
| 9 | All electrons located in the same orbital | 11 |
| 10 | Each electron in a single orbital | 5 |
| 11 | Advanced drawing, notions of superior model | 6 |
| 12 | Rutherford´s model, two electrons in one orbit, one in other | 19 |
| 13 | Thomson’s model | |
| 14 | Bohr´s model is explained correctly | 31 |
| 15 | Only particle’s location is described | 69 |
| 16 | Incorrect explanation | 24 |
| 17 | Advanced notions of probabilistic and/or inaccuracy of electron position | 6 |
These categories emerged from quotations such as: “The electrons are located in the atomic crust, structured in orbitals according to their energy, so that those with lower energy are closer to the nucleus, since they are attracted to it” (category 14, Table 3). “The electrons are spinning around the nucleus in a probability cloud in which we know where we think the electron is with a mathematical calculation. Actually these orbitals, according to quantum mechanics, are nothing more than clouds of probabilities, in which it is more possible to find the electron.” (category 17, Table 3).
More than half of the students drawn a nucleus containing positive and neutral particles (58%) and 70% of these students located the electron (with negative charge) outside of the nucleus. 33% of the drawings presented a Bohr´s model with a defined nucleus and orbitals with negative charges in these orbitals and almost all of them (31% of the total) were able to explain this model in a correct way. In 15% of the drawings, it was identified more than the correct number of particles per orbital, which is a common mistake in the representation of models according to bibliography (García-Carmona, 2011). In 11% of the questionnaires all the electrons of the atom were in the same orbitals, and in the 5% of the responses every electron was drawn in a different orbital. In relation to the electron, only 15% mentioned its small size in comparison to the atomic size. It was not surprising that 20% of the models presented were the Rutherford´s atomic model because as Seok Oh and Jin Oh (2011) said, it is really difficult to modify an acquired model. It takes time and it needs developing experiences to contrast the model (Clement, 2000) so it is usual that an important part of the students keeps the model they acquired in their previous academic years. Up to 6% of the responses or drawings mentioned advanced notions as probabilistic or inaccurate concepts related to the electron position.
In the same way as it was commented in the results from the group 1, also from this analysis it can be concluded that important differences were found, depending on the educational centre of the students.
Outcomes for Group 3 (18-years-old students)
A total of 203 students were surveyed at the beginning of their first University year. Thus, the knowledge they expressed in the questionnaires was the knowledge acquired along the last academic years before University. It can be seen that an important part of the students answered in a correct way all the questions (33%), which means they did not mix models in their explanations of the atomic model, however, a total of 95.5% of these students described the Bohr model instead of the quantum model they should have. Only 3 students (4.5%) showed the expected quantum model. The main part of the answers (37.4%) was indicative of the confusion between models. The most usual mistake in this case was they used the Bohr model for both explanation and representation, but they described it using the orbital concept of the quantum model instead of the orbit concept (88.2%). A small part of the students mixed concepts both in the explanation and in the drawing (11.8%).
28.6% of the responses were completely incorrect. The reasons where they mixed models and their answers were wrong (48.3%), they drew the atomic structure in a wrong way (43.1%) or although they drew correctly, they explained it wrong (8.6%).
Discussion and educational implications
These results show that most students have a mental model that does not correspond to the atomic models that appear in the curriculum according to their educational courses. Indeed, their responses show mixed atomic models. Probably this mixture of models reflects the evolution of their own mental model of atom. As Justi and Gilbert (2002) explain, the evolution of a mental model is somewhat slow and hard and needs an assimilation time that will be longer or shorter depending on the times that the model has been tested and evaluated. At the same time, it must be taking account that to build a correct atomic model, the students should have abstract and spatial knowledge which is more difficult to achieve in young people than for adults (Talanquer, 2009; Fitriza and Gazali, 2018). On the other hand, it has been found that teachers offer to students few situations to check their mental models, so they are not able to assess their atom´s model and thus, it takes so much time to find the strengths and weaknesses of their models (Justi and Gilbert, 2002; Park and Light, 2009). In the case of the Group 1 (15-year-old students), the atomic model they should have shown is a model based on Rutherford´s one, where the atom is represented as a tiny nucleus, surrounded by other negatively charged particles located in diffuse orbits. These results show that most students from this Group 1 do not perceive (or at least not draw) the atom as something spatial, but in 2D dimensions, so it might be interpreted that the movement of electrons is also 2D. In fact, only 22.1% of students made some reference to the movement of electrons. This fact indicates the difficulty of using a complex spatial representation for students of that age to interpret something they do not see (the atom) (Dori and Barak, 2001; Toomey et al., 2001; Cokelez and Dumon, 2005; Cokelez, 2012). Other students, despite drawing and explaining the orbits as something diffuse, were not able to represent the atom in three-dimensions and, even less, to explain the movement of electrons around the nucleus. These results evidence that the mental model of atom that they had constructed is not representative of what they should have (Rutherford) but is a mixture of this with an earlier planetary model. There are also a few students who mix Rutherford´s model and the immediately advanced one, the Bohr´s model. Surprisingly there are even students who establish relationships between the structure of the atom and the nature of the element it represents. In this sense, it should be mentioned that, according to Shane and Bodner (2006), when students can establish relationships between the structure and the function of the atom they are showing advanced atomic mental models.
As more advanced models were introduced (Group 2), it was observed that many students maintain the mental model of the Rutherford´s atom (19%). Others were in the process of testing their model and moving to Bohr (14%) and 24% of the students had evolved their mental model to the expected one and use the Bohr´s model correctly. It should be noted that some students (4%) had more complex knowledge about the atom than would be expected for their age.
The most surprising results for us has been to find that, both in Group 1, and in Group 2 constituted by 15-16-year-old students of high school level, the results analysed had an important variability depending on the centre from which they were collected. That is, the centre in which the students course their secondary education, determines the mental model of the atom they build. This may be due to several reasons such as: the knowledge of the teachers in each centre, the methodology they use in their teaching process, the teaching objectives, the textbook they use, the type of teaching resources applied, etc (Quesada, Valcárcel and Sánchez, 2005; Domenech, Savall and Martínez-Torregrosa, 2013; Fitriza and Gazali, 2018). These variables were not analysed in this work and will constitute a line of future research that could be of great educational interest.
In relation to Group 3, formed by students who were starting their university studies, most of them had mixed models and only 33% of the students described the atom using correct models. From them, the vast majority used the Bohr´s model. This indicates that their mental models, despite showing coherence, had not evolved to the quantum model they should have according to the curriculum. The reasons can be many, in this context Park and Light (2009) marked as a fundamental step to achieve that evolution, is that the models should be tested with activities or situations designed specifically for it.
Conclusions
The research developed in the present study arises from the need to know the mental model about the atom´s concept that the students possess. After analysing an extensive and representative sample, the main conclusion obtained from this research is that students understand the atom as a mix of several models. In general, the models they mixed are the models they studied in their last educational courses. A small part of the students possesses a unique mental model about the atom, and a really few of these students described the atom as it could be expected according their educational level. That means that, the students who show a correct model (not mixed) usually showed the previous model studied instead of the expected one according the curriculum.
The reasons behind these observations could be several. The first possible one is the hard and slow pace/time of construction of a mental model, even more in the case of the atom, which is an invisible particle, thus it needs abstraction and the use of spatial knowledge. Other reason could be that we are requiring this abstract and spatial knowledge to very young students, which is more difficult to achieve that for adults (Talanquer, 2009; Fitriza and Gazali, 2018).
Bearing in mind that most of the students who have participated in our research have used the Bohr´s model and only a few of them used the quantum model, a question arises: is this fact due to the time dedicated to work about the model of atom is fundamentally focused in primitive and simple models and the quantum model is only briefly introduced during the last stage of high school education? or could be related to the higher level of abstraction and knowledge of mathematics required to understand the quantum model? We leave open the possibility to investigate about the reasons for these results.











 nueva página del texto (beta)
nueva página del texto (beta)