Introduction
The copper cycle experiment has been widely used in Chemistry courses to study the mass conservation principle, and it is described by several authors (Condike, 1975; Umans & de Vos, 1982). Besides the quantitative approach, i.e., the mass conservation principle, the copper cycle experiment is also used to develop students’ knowledge about different types of chemical reactions. Thus, for students to fully understand the copper cycle, they must know not only the key concepts that it involves, and that have been taught in previous school years, but also their connections. However, students show some difficulties and misconceptions regarding previous central elementary chemistry concepts, and several studies describe these difficulties. For example, in a study performed by Ozmen and Ayas (2003) it is described that about half of the 150 participant students, attending the 10th grade, showed alternative conceptions about the principle of mass conservation. In a more recent study, carried out with 195 students from the 8th grade, Basheer et al. (2018) concluded that, even after teaching the principle of mass conservation, students still revealed alternative conceptions about this topic. Regarding chemical transformations, there are also several studies that describe students’ difficulties about this topic. For example, in what concerns oxidation-reduction reactions, Schmidt and Volke (2003) identified alternative concepts in a study carried out with 3074 high school students. In another study (Rosenthal & Sanger, 2012), with 55 students from an introductory course in Chemistry, the authors identified alternative conceptions about redox reactions. In relation to precipitation reactions, Ozmen and Ayas (2003) found that one of the most recurrent ideas of 10th grade students was that there was an increase in mass during a precipitation reaction, as consequence of the formation of a solid compound.
As described in the literature (Johnstone, 1982; Chandrasegaran, Treagust & Mocerino, 2008) the main reasons why students have difficulties in understanding chemical phenomena are related to the levels of representation (macro-, micro- and sub-microscopic) that are used to describe them. Additionally, and according to Treagust, Duit and Nieswandt (2000), there are many studies that describe that students’ preconceptions are crucial for learning new concepts. Taking into account that knowledge construction is an active process, and that new knowledge must be meaningfully anchored in correct scientific information, students’ previous experiences and ideas, as well as their interactions with the world, are crucial for understanding new concepts and their associations (Ausubel, 1968; Piaget, 1978; Vygostky, 1978). Thus, considering the importance of preconceptions, and in particular alternative conceptions, their identification is essential for the teaching-learning process.
Regarding the copper cycle and given the complexity and variety of the concepts involved in this activity, it can be a challenge for students. Thus, it is essential to know students’ conceptions in order to use teaching strategies that help them to overcome their difficulties. In this way, it is important to know students’ cognitive structures (CS) (Nakiboglu, 2008), to assess the conceptions they have, which can determine the construction of meaningful learning, and to use an inquiry-based learning activity (IBL) (Lederman, 2006) as an approach to promote the development of these CS.
Cognitive Structures
The constructivist perspective of the learning process (Piaget, 1978; Vygostky, 1978), makes teachers and researchers to be focused on trying to understand the cognitive structures (CS) of students. In this study it is considered that CS are the relationships that are established between concepts, terms and/or processes, stored in long-term memory in a hierarchical way (Taber, 2008). In this way, information about students’ CS can help teachers to understand and adequate the teaching-learning process and, thus, to help students to achieve a meaningful learning (Ausubel, 1968).
One of the methods used to disclose the CS is the Word Association Test (WAT), (Johnson, 1967), in which students are asked to write response words associated with the stimulus words provided by the teacher or researcher (Bahar, Johnstone & Sutcliffe, 1999). Through the analysis of the quantity and quality of the associated words, it is possible to build frequency maps and assess the understanding of a concept (Nakiboglu, 2008). However, in order to overcome the limitations of WAT, i.e., to clarify the nature of the associations established between concepts, the WAT must be complemented with interviews, free writing or concept maps (Gunstone, 1980; Bahar et al., 1999).
In the literature are described several studies that use WAT as a way of revealing students’ CS, specifically in the Chemistry area. As an example, Nakiboglu (2008) developed a study with 40 preservice teachers, whose objective was to understand the development of their CS about the atomic structure, through the application of a WAT. In another study, the authors (Derman & Eilks, 2016) applied a WAT related to the topic of dissolution, to 157 11th grade students. The results allowed the authors to conclude that students had a limited understanding about chemical solutions and the corpuscular nature of matter. It was also possible for Yildirir and Demirkol (2018) to investigate the cognitive structures of 153 students (6th grade) about physical and chemical transformations. The results of the WAT revealed that students did not understand a chemical reaction as a phenomenon that occurs at the sub-microscopic level and presented difficulties in differentiating physical and chemical transformations. In a more recent study (Baptista, Martins, Conceição & Reis, 2019), carried out with 12th grade students (N=68), a WAT about the saponification reaction and the results allowed the authors to confirm the development of students’ CS about this topic, as a result of a sequence of classes based on an IBL approach.
Inquiry-based Learning
Inquiry-based learning (IBL) is a student-centered educational approach that has a great potential, since it allows a deep learning of science and about science (Lederman, 2006). In IBL, students are involved in investigations, guided by a defined problem, and follow methods and practices, in order to find practical solutions and construct knowledge (Keselman, 2003). In this sense, IBL activities require and allow students to make observations, to identify the problem, to formulate hypotheses, to plan an experiment, to analyze and interpret data, to explore, to predict the answer to the problem and to communicate the results (NRC, 2000). One of the IBL instructional models is the BSCS 5 E’s Instructional Model (Bybee et al., 2006), that consists in a learning cycle that includes five phases: Engage, Explore, Explain, Elaborate and Evaluate.
There are several studies that describe the benefits of IBL. For example, Şimşek and Kabapinar (2010) investigated the effects of IBL activities on the conceptual understanding of the contents, on the development of scientific skills, on the attitude towards science and on the understanding of concepts about the nature of. Similar results were obtained by Vlassi and Karaliota (2013) with 174 students belonging to the 8th grade, involved in eight classes of IBL activities about the structure of the matter.
The main goal of this study was to evaluate the development of students’ cognitive structures about the copper cycle and in order to achieve that purpose, the following research question guided the investigation: What was the development of students’ cognitive structures regarding the copper cycle, after their involvement in an IBL activity?
Materials and Methods
This presented study is an exploratory study, that follows one group pretest-posttest design. In this sense, the design enables the comparison of students’ cognitive structures before and after an IBL activity about the copper cycle. Participants were 43 students (22 boys, 21 girls; 17-19 years old), attending the 12th grade from a Portuguese secondary school. The three lessons about the copper cycle (total of 270 minutes) were conducted by a Chemistry teacher with 26 years of professional experience. The IBL activity was constructed based on the 5 E’s model (Bybee et al., 2006) and it begun with the presentation of a text about the importance of copper recycling, which motived the students to perform an internet search to find the answer to the question “How can copper be recycled by using chemical processes?”. During this first lesson, students planned an activity about copper recycling and in the second lesson, performed that activity and registered their observations. In the last lesson, students answered the initial question, based on their observations, watched a video about the importance of the process of copper recycling, and reflected about the activity. The sequence of the activity is presented in Table 1. To develop the activity, students worked in the activity in groups of three or four.
Table 1 Sequence of the IBL activity about the copper cycle.
| Model’s Phase | Description |
|---|---|
| Engage | Students are motivated with a text about the importance of copper recycling. The text finishes with the question: How can copper be recycled by using chemical processes? |
| Explore | Students perform an internet search about the investigative question, plan an activity that allows them to answer the question, perform the planned activity and register their observations. |
| Explain | Students explain their observations and describe how copper can be recycled. |
| Elaborate | Students watch a video about the importance of recycling metallic objects and equipment, taking in account the limitation of natural resources. |
| Evaluate | Students reflect about the activity, giving answers to the following questions: What did you learn? What difficulties did you encounter? What did you like the most? What did you like the least? |
Data collection was done through a WAT, which aimed at evaluating the development of students’ CS about the copper cycle, after the implementation of the IBL activity. In this sense, WAT was applied at two distinct moments: three weeks before (M1, pretest) and three weeks after (M2, posttest) the implementation of the inquiry activity (M2, posttest). In each moment, five stimulus words were provided to the students: copper, copper nitrate, copper hydroxide, copper oxide and copper sulfate. These stimulus words were previously selected by the authors of these article, considering the Portuguese chemistry curriculum, and certified about their suitability by the participant Chemistry teacher. Students were asked to write, in 10 minutes, as many items (response words) as they could associate with each stimulus word, and to write a sentence including each one of the stimulus words and their response word (Bahar et al., 1999).
The collected data were first used to ascertain inter-judge reliability: each one of the authors analyzed independently the data and their analysis were compared, using as criteria the counting of the total of different response words. Following Miles and Huberman (1994) method, the consensus among the authors/judges was considered satisfactory-higher than 90%.
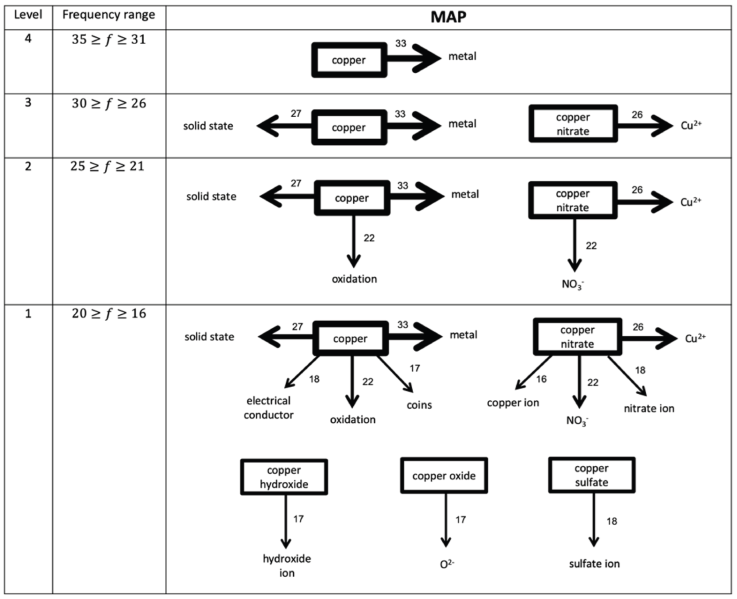
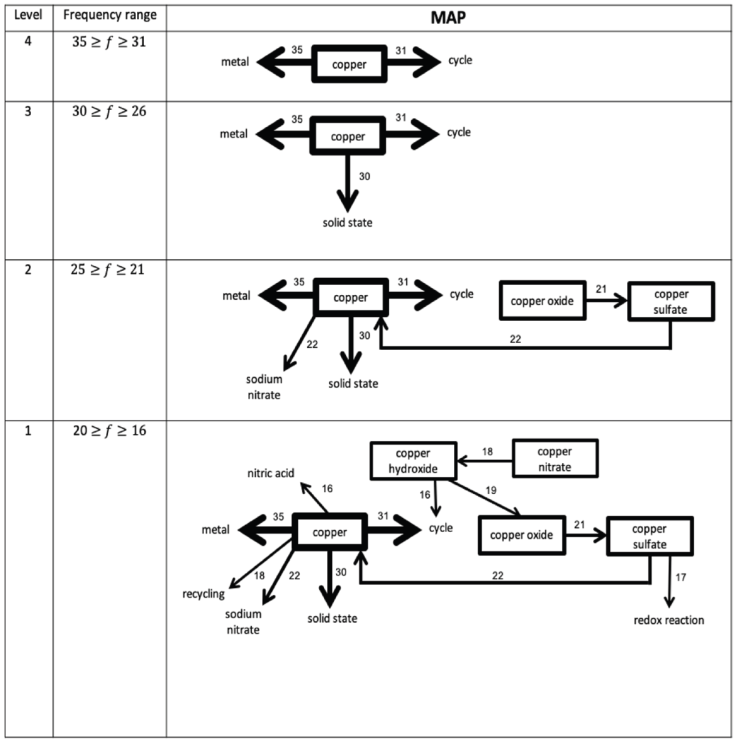
The information gathered from the WATs was analyzed through the response frequency map method (Nakiboglu, 2008). A frequency table was constructed (Table 2) and students’ cognitive maps were assembled for M1 and M2 (Figures 1 and 2). The highest frequency interval, which corresponds to the strongest level of association, was established as 35 ≥ f ≥ 31 and the lowest frequency level was set as 20 ≥ f ≥ 16 for both moments, because all the stimulus words appeared in the maps at this frequency range (Nakiboglu, 2008). The construction of CS maps was done by placing the stimulus words in a box and drawing arrows from the stimulus word to the response word, considering the established frequency levels. The width of the frames and arrows is an indication of the strength of the associations: the thicker the arrow, the greater the frequency and stronger the association (Nakiboglu, 2008; Derman & Eilks, 2016). In order to overcome the limitations pointed out in the literature (Gunstone, 1980; Bahar et al., 1999) the nature of the associations between concepts established by the students was investigated through a qualitative analysis of the sentences written by the students.
Table 2 WAT frequency table.
| Response Words | Stimulus Words | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Copper | Copper nitrate | Copper hydroxide | Copper oxide | Copper sulfate | ||||||
| M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | |
| Copper | - | - | 15 | 10 | - | 8 | 3 | 7 | - | 22 |
| Copper nitrate | - | 4 | - | - | - | - | - | - | - | - |
| Copper hydroxide | - | 9 | - | 18 | - | - | - | - | - | - |
| Copper oxide | - | 3 | - | - | - | 19 | - | - | - | - |
| Copper sulfate | - | 4 | - | 3 | - | - | - | 21 | - | - |
| Metal | 33 | 35 | - | - | - | - | - | - | - | - |
| Solid state | 27 | 30 | - | - | - | 7 | 12 | - | 8 | 8 |
| Cycle | - | 31 | - | 12 | - | 16 | - | 11 | - | 7 |
| Recycling | - | 18 | - | 5 | - | 4 | - | 6 | - | 3 |
| Electrical conductor | 18 | - | - | - | - | - | - | - | - | - |
| Oxidation | 22 | - | - | - | - | - | 7 | - | 4 | - |
| Cu2+ | 8 | - | 26 | - | - | - | 7 | - | 5 | - |
| NO3 - | - | - | 22 | - | - | - | - | - | - | - |
| Copper ion | 12 | - | 16 | - | - | - | - | - | 7 | - |
| Nitrate ion | - | - | 18 | - | - | - | - | - | - | - |
| Redox reaction | 2 | 13 | - | 7 | - | - | 2 | - | 9 | 17 |
| Coins | 17 | 7 | - | - | - | - | - | - | - | - |
| Hydroxide ion | - | - | - | - | 17 | - | - | - | - | - |
| Sulfate ion | - | - | - | - | - | - | - | - | 18 | - |
| O2 - | - | - | - | - | - | - | 17 | - | - | - |
| Nitric acid | - | 16 | - | - | - | - | - | - | - | - |
| Sodium nitrate | - | 22 | - | 6 | - | - | - | - | - | - |
Some response words with f < 16: Air, Cation, Anion, Wires and Cables, Reduction, Brass, Bronze, Silver, Gold, Minerals, Acid-Base Reaction, Precipitation Reaction, Oxidation State, Zinc Sulfate, Heating, Sodium Hydroxide, OH-, Base, SO4 2-, Zinc, Sulfuric Acid.
Results and Discussion
The development of students’ CS was evaluated through the construction of a frequency table (Table 2), in which is specified the number of response words per stimulus word for both moments.
Data from Table 2 were used to draw the cognitive maps at M1 (Figure 1) and M2 (Figure 2) and students’ sentences were analyzed in order to disclose the nature of the associations. Besides the words (stimulus and response-words) some of the sentences written by the students are presented in the following paragraphs, as being representative of students’ answers, i.e., we only present the sentences that illustrate the most common associations between words.
At M1, students’ CS (Figure 1) is composed of four levels of association and characterized by the presence of isolated islands, i.e., there are no connections between the stimulus words. At this moment, and at Level 4, students only associated the stimulus word “copper” to “metal”, and in Level 3, a new response word appeared (“solid state”) as being associated with the word “copper”. One example of sentence written by the students discloses the connections made: “copper is a metal that is in the solid state at room temperature” Also, in this level, a new stimulus word (“copper nitrate”) appears, and students recognized it as having Cu2+ ions in its composition: “copper nitrate is the result of the combination of Cu2+ with another ion”. At Level 2, a new response word (“oxidation”) is associated with the stimulus word “copper” and, according to the students, “copper undergoes oxidation and that is why we see green objects, like the Statue of Liberty”. Also, at this level, students associate the stimulus word “copper nitrate” to “Cu2+” and “NO3 -” and state that “copper nitrate is composed of Cu2+ and NO3 -”. Finally, at the weakest level of association the three remaining stimulus words appear, each one associated with one response word. Examples of the associations made by the students are: “copper sulfate has sulfate ion” and “O2- is in the copper oxide”.
As in M1, the map of students’ CS at M2 (Figure 2) is also composed of four levels of association but, while the map at M1 is characterized by the presence of isolated islands, in the posttest all the stimulus words are incorporated in a network.
As it can be seen, at Level 4, students, in addition to associating the stimulus word “copper” with the response word “metal”, as happened in the pretest, they also associate it with the word “cycle”. The association made between these three words, reflects the main idea of the copper cycle, i.e., that “copper is a metal that can be recycled through the copper cycle”. At the next level, a new response word is associated with the stimulus word “copper” (“solid state”). The Level 2 of the map of students’ cognitive structures in the posttest is characterized by the appearance of two stimulus words: “copper oxide” and “copper sulfate”. These words, in addition to being associated with each other, are also associated with the stimulus word “copper”, through one of them (“copper sulfate”). The sentences written by the students, reveal the understanding of some of the stages of the copper cycle, such as: “copper oxide reacts with sulfuric acid, through an acid-base reaction, and copper sulfate is formed” and “in the last stage of the copper cycle, copper sulfate reacts with zinc and originates copper in the solid state” Through these examples, it is possible to see that the associations made by students in this posttest are different from those identified in the pretest, in which students only associated their constituent ions with these compounds. Also, at this level, students associated a new response word (“sodium nitrate”) to the stimulus word “copper” as follows: “in the second stage of the copper cycle, a precipitation reaction occurs in which copper hydroxide is formed (precipitate) and sodium nitrate”. In the last level, with lower frequencies, the remaining stimulus words - “copper hydroxide” and “copper nitrate” - are correctly associated with the concepts about the copper cycle, as shown in the following sentences: “solid copper hydroxide is formed from copper nitrate” and “copper hydroxide, which precipitates when heated, decomposes to copper oxide and water”.
The maps of the students’ CS (Figures 1 and 2) are characterized by having four levels, which correspond to different levels of association. Although the frequency intervals of these levels are equal at both moments, islands of isolated words are seen in M1. On the contrary, at M2, all words are associated, directly or indirectly with each other, thus forming a network of concepts interconnected in the students’ CS. According to the literature (Derman & Eilks, 2016), this is illustrative of a more structured and organized conceptual map, which facilitates the learning of new content, properly anchored in students’ prior knowledge (Ausubel, 1968). The results obtained in the pretest (Figure 1) are illustrative of the knowledge and associations between concepts that students had before carrying out the activity. At this time, students were not aware of the copper cycle, so the associations are essentially based on their daily experiences or on previously learned concepts. However, at M2 (Figure 2), the associations made were consistent with the copper cycle and that was clear in the sentences written, which reveal the understanding of the role played by each of the reagents in the various reactions. In this sense, the connections that students established reflect the incorporation of new concepts in their cognitive structures. In this sense, these results indicate that the IBL activity allowed students to recall the taught concepts three weeks after performing the activity. According to some authors (Solsona, Izquierdo & de Jong, 2003), one of the difficulties that students have in relation to laboratory activities is to remember them, so that, through the results described, IBL activities can contribute for students to build knowledge in a meaningful and lasting way.
Conclusions
The copper cycle experiment allows students to understand and deepen several central concepts of Chemistry, such as the principle of mass conservation and various types of chemical reactions. As such, it is important that students can carry out their learning in a way that allows them to incorporate it into their cognitive structures. Inquiry-based learning activities have been identified as teaching-learning strategies that allow the development of various procedural skills, as well as building knowledge, according to a constructivist perspective. About the achieved learning, WAT proved to be a suitable instrument to investigate the relationships that students established between concepts, that is, to assess their CS and to detect their development, as a result of the development of the inquiry-based learning activity. In this sense, this study contributes to the knowledge of the students’ CS about the copper cycle, thus reinforcing the research developed in the area of Chemistry instruction, by identifying some of the students’ difficulties on this topic and describing a pedagogical approach that allows students to achieve the learning goals. Although the WAT, as applied in the present study, doesn´t allow the assessment of individual cognitive structures, it allows the determination of the most prevalent associations and directions of such associations. Thus, this instrument can be a suitable method to elicit about prior knowledge or conceptions that students have and can be used by teachers to identify students’ difficulties and select proper pedagogical experiences that help students to learn. Moreover, it can be used to evaluate the accomplishment of students’ learning objectives.











 nueva página del texto (beta)
nueva página del texto (beta)