Artificial Photosynthesis-Bioinspired Technologies
Artificial photosynthesis is a promising strategy for moving from fossil fuels to a climate-neutral supply with green fuels and basic chemicals. Currently, different versions of artificial photosynthesis are investigated worldwide. They focus especially on the photocatalytic production of hydrogen from water and/or the reduction of carbon dioxide to energy carriers and chemicals such as alcohols and hydrocarbons [1, 2]. Due to the fact that both processes do not run without supplying great amounts of energy, the main challenge is to develop integrated apparatus and plants capable to use exclusively sunlight as energy source.
The chemical and biological evolution on our planet has developed the natural photosynthesis that combines both processes mentioned abovein an excellent way using solar light as unique energy source. Through a truly ingenious series of elementary processes, photons from visible light are absorbed and used for electrical charge separation in molecular species. In the photosystem I water gets oxidized via a series of electron transfer processes generating molecular oxygen O2 and hydrogen ions H+. Subsequently in the photosystem II, hydrogen ions H+ are reduced and bound in NADPH (nicotinamide adenine dinucleotide phosphate). This redox equivalent can be considered as a temporary hydrogen storage system because it contains hydrogen in a reduced state that formally can be considered as hydrogen atoms H or even as hydride ions H-. In the light-independent part of photosynthesis (Calvin cycle), reduced hydrogen atoms bound in NADPH are removed and transferred step by step to reduce carbon dioxide into different compounds, mainly glucose and other carbohydrates.
In order to realize artificial photosynthesis, mankind has to learn from nature trying to imitate the two sub-processes mentioned above. Consequently, the coupling of light-induced charge separation with catalytic redox processes should be raised as a central principle for the production of energy-rich compounds (Figure1).
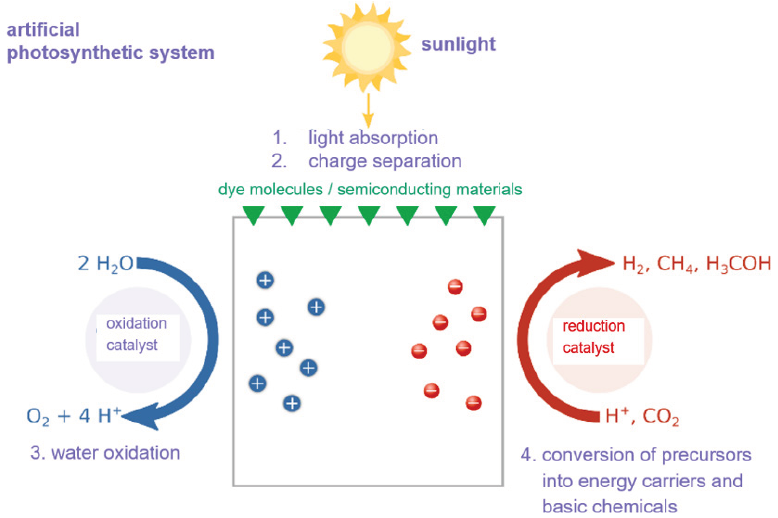
Figure 1: Sub-processes of artificial photosynthesis for the technical production of solar fuels and basic chemicals from CO2 with H2O as hydrogen source; simplified representation according to the report of German Academies of Sciences [3].
However, the imitation of central principles from natural photosynthesis as shown in Figure1 does not mean a 1:1 copy of processes in green plants. Instead, alternative materials and production methods must be researched and developed.
The German academies’ report [3] emphasizes that in addition to the scientific research concerning artificial photosynthesis, science journalism can and should contribute to increasing the social acceptance of research and development on this topic. We are deeply convinced that it also has to play a crucial role in education.
2. More Light! Already at School!
It is to be expected that light will advance to be the most important form of energy for sustainable technical processes within the 21th century. Therefore, light should get a key function in chemical education. Mainly for the growing young generation, that is for tomorrow´s scientists, engineers and technicians, teaching future-relevant contents and basic concepts of photochemistry is eminently necessary. Thereby, an outstanding topic should be the communication of the possibilities and opportunities as well as the problems still to be solved in connection with artificial photosynthesis.
In order to promote the inclusion of photochemical processes in chemical education, we have published several experimental approaches to basic concepts of photochemistry in this journal [4-7] and in related journals dedicated to chemical education [8-10]. There are three reasons that determined us to develop and interconnect an internet platform with experiments, videos, movies, models, interactive animations and other digital materials available by the link in ref. [11]: i) we would like to offer a wide range of teaching and learning materials on the subject of chemistry with light, because this is indispensable in education for sustainable development; ii) we wanted to meet the challenge for distance learning as it was forced by the COVID-19 pandemic; iii) we want to support and accelerate curriculum modernization in face-to-face teaching with adequate digital media dedicated to topics relevant to the future. Most of our experiments include reactions powered either by sunlight or light from LED torches. This is also the case with the Photo-Blue-Bottle experiment (Figure 2).
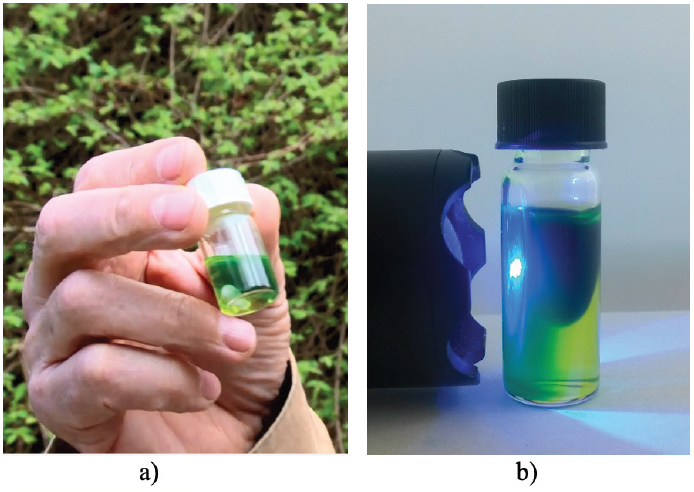
Figure 2: Basic version of the Photo-Blue-Bottle experiment; in these pictures it works as microscale version by irradiation with sunlight (a), and with blue light from an LED torch (b).
The basic version shown in Figure 2 and some further versions of the PBB experiment including teaching recommendations have been reported in ref. [8]. They emphasize basic analogies between the PBB experiment and the conversions of matter and energy in the natural cycle of photosynthesis and respiration. All this is illustrated and explained with the aid of film sequences and English-spoken commentaries in the tutorial entitled “Photosynthesis - A Case for Two (Part 1 of 2)“. The tutorial is accessible via the platform [11] in the section Movies & Videos.
Since the aqueous solution used in the PBB experiment enables the photocatalytic production of hydrogen, at least partially consistent with the concept of artificial photosynthesis shown in Figure 1, it plays also a key role in this article. Already at this point we present the results of our efforts in research and development of experimental setups for generation of hydrogen with the PBB solution as basic component (Figure 3).
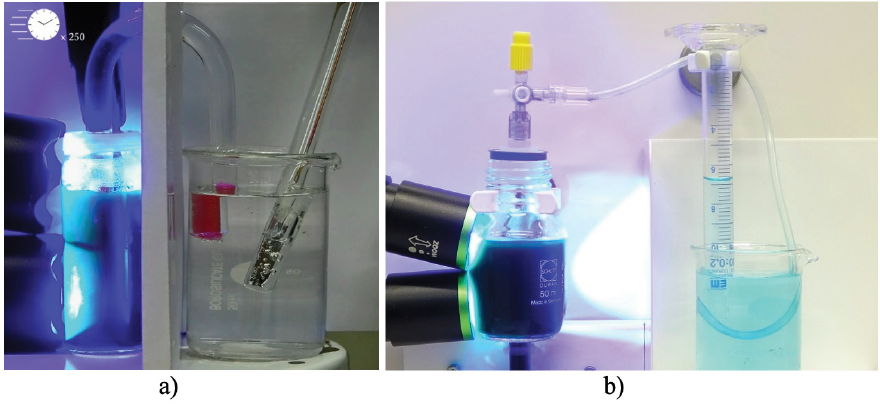
Figure 3: Photocatalytic generation of hydrogen in a two-pot cell (a) and in a one-pot cell (b) using the Photo-Blue-Bottle system as basic component.
However, we would like to mention à priori that the efficiency of hydrogen production in the two setups from Figure3 is very different. In the one-pot cell b) 10 mL of hydrogen can be produced within 30 minutes, whereas about 90 minutes are needed for finally generating 0.6 mL of hydrogen in the two-pot cell a). The reason for this huge difference in efficiency is explained in the following sections of this article, when the theoretical background and experimental details are elucidated. Nevertheless, we will finally bring forward some arguments in favor of the two-pot cell from the didactical point of view.
3. Photocatalytic Generation of Hydrogen - Theoretical Background
Hydrogen is the fuel with the highest mass-related energy storage density. It amounts to 33 kWh/kg and is thus twice as high as that of the fossil fuels petrol (12,5 kWh/kg), diesel (12 kWh/kg) and methane (14 kWh/kg). Thanks to the diverse, climate-neutral and resource-saving uses of hydrogen as an energy carrier and basic chemical, hydrogen technology is being promoted as a promising future scenario. However, its success depends on the extent to which “green hydrogen” can be produced in industrial quantities not at the expense of fossil fuels, as it is the case today, but exclusively using sustainable energy sources. A promising option is the direct photocatalytic production of hydrogen from water using solar light without the bypass via photovoltaics and electrolysis. This is possible according to the sub-processes of artificial photosynthesis outlined in Figure1. However, most of the reported visible-light-responsive photocatalysts are only active for either hydrogen evolution or oxygen production. If the production of hydrogen is intended, in a preliminary step hydrogen ions H+ have tobe generated. Up to now, this occurs on the cost of a sacrificial donor that gets oxidized instead of water [1].
That’s also the case in the crucial photocatalytic system used in our Photo-Blue-Bottle experiment. Actually, the PBB experiment works in more than 10 different versions, always using a diluted aqueous solution containing proflavine PF+ as photocatalyst, ethylviologene EV2+ as redox mediator, and EDTA as sacrificial donor. The formulas of the three chemicals as well as of the oxidized and the reduced species from the following equations (1) - (5) are shown in Figure 7. The aqueous PBB solution (see preparation in section 4) contains an excess of sacrificial donor, while the concentration of the redox mediator is in the range of c= 10-3 mol/L and the concentration of the photocatalyst about 100 times smaller.
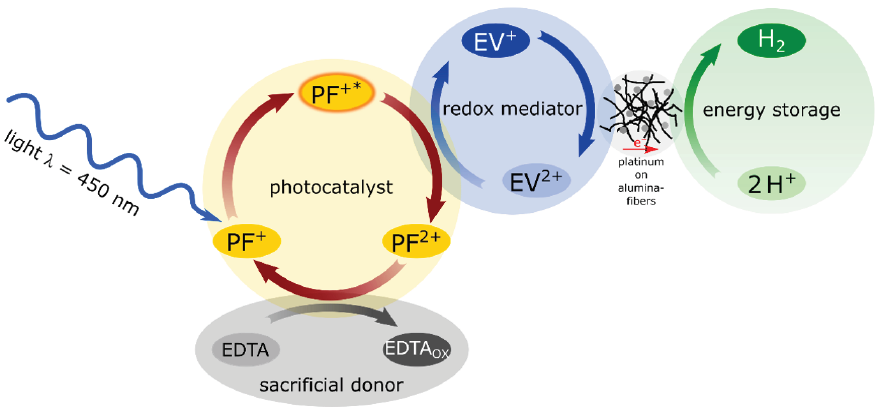
Figure 4: Coupled reaction cycles in the photocatalytic production of hydrogen with PBB solution and 5% platinum on alumina as reduction catalyst in the one-pot cell.
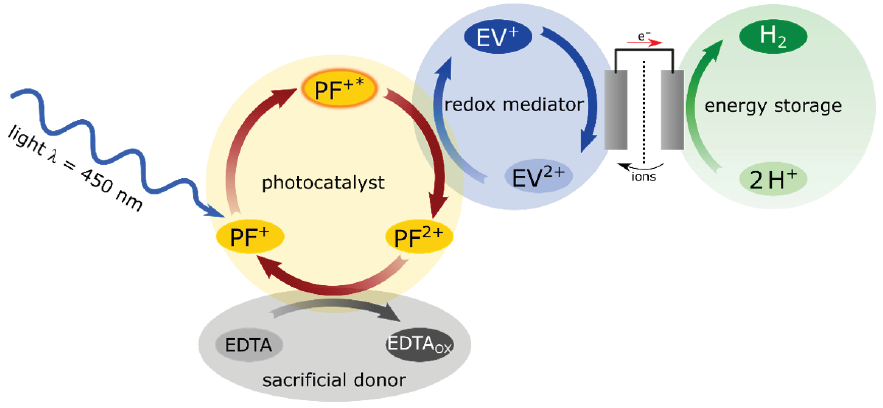
Figure 5: Functional scheme for the photocatalytic production of hydrogen with PBB solution in the two-pot cell.
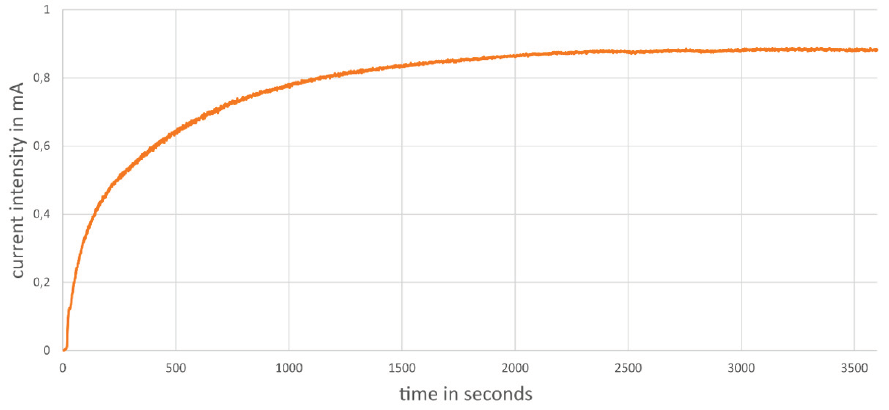
Figure 6: Temporal change of the current intensity in the photocatalytic two-pot cell during the irradiation of the PBB solution.
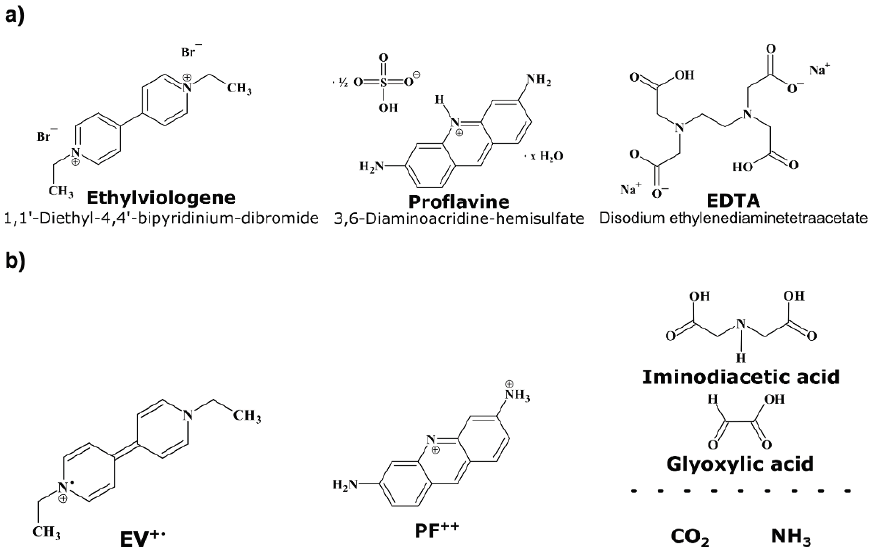
Figure 7: a) Chemicals for the Photo-Blue- Bottle solution: redox mediator ethylviologen, photocatalyst proflavine und sacrificial donor EDTA; b) formula of reduced and oxidized species in equations (1) - (5): ethylviologen monocation radical EV+., proflavine dication PF++, several oxidized products of EDTA.
From the energetic point of view, chemistry with light in all experiments based on the PBB solution begins with the light absorption and charge separation following the principle shown in Figure1 for artificial photosynthesis in general. As it is well-known, colored substances are necessary for light absorption in the visible range,. In the PBB experiment the tasks of the colored chlorophylls and carotenoids from green plants are performed by the yellow-green photocatalyst proflavine PF+. The elementary processes running in the one-pot cell shown in Figure3b are expressed by the simplified equations (1) - (4), summarized in the Figure 4, and explained in the text below.
Initially, in the step corresponding to eq. (1), the absorption of a photon from blue light (λ = 450 nm), leads to the electronically excited state of the proflavine monocation PF+*. In general, the excited state, the “heart of all photo processes” [12], exhibits a dramatically changed redox potential compared to that of the ground state. In the case of proflavine the redox potential shifts from about +1.05 V to about -0.6 V vs. NHE (see a detailed scheme with redox potentials of the PBB components in ref. [8]).
The next step expressed by eq. (2), consists of an electron transfer from the excited proflavine monocation PF+* to the ethylviologen dication EV2+. In this redox reaction the excited photocatalyst, the monocation PF+* gets oxidized and deactivates to the dication PF2+, and the dication EV2+ is reduced to the monocation EV+. This reduced form of the redox mediator ethylviologen is the species that causes the blue coloration of the PBB solution because of its planar, quinoid structure (Figure 7b). It is stable in absence of oxygen.
Additional note: In the basic version of the PBB experiment shown in Figure 2, the blue color of the solution generated by irradiation, gets quenched when oxygen is introduced into the solution, for instance by shaking the vial. In this case EV+ is re-oxidized to EV2+, and molecular oxygen works as electron acceptor that is reduced according to eq. (5).
Basically, the whole triad proflavine/EDTA/ethylviologen works as photocatalytic system in both photocatalytic cells shown in Figure 3. Due to the buffer effect of EDTA (pH ≈ 4.5), the PBB solution even offers H+ ions in a sufficient concentration as electron acceptors for the production of molecular hydrogen in the one-pot cells shown in Fig 3b. At pH = 4.5, the redox potential of the system H2/2H+ is E° = - 0.265 V. Since the redox pair EV+/EV2+ has a pH-independent redox potential of E° = - 0.449V [13], the redox reaction according to eq. (4) is electrochemically possible. However, for obtaining a satisfactory yield of hydrogen, a reduction catalyst which facilitates the electron transfer and the gas evolution has to be added into the PBB solution. Commercially available products composed of 5% platinum on alumina have proven to be suitable for this purpose. Details are given in section 4.
The two-pot cell shown in Figure 3a does not need a reduction catalyst, because a much higher concentration of H+ ions is offered as electron acceptor. However, this is realized in a separate half-cell, containing hydrochloric acid, c = 1 mol/L. This half-cell is connected to the half-cell containing the PBB solution by an external electron conductor of metal cable and an internal ion conductor of potassium chloride solution (Figure 5). We do not use HCl in the salt bridge, as this would cause a considerable decrease of the pH in the PBB solution. The pH must not be lower than 4.5, otherwise the catalytic activity of PF would be greatly reduced.
Differently than in the 1-pot cell, a foil of graphite immersed as electrode in the PBB solution works as electron acceptor collecting electrons from the ethylviologen monocations EV+. Thus a large area graphite foil is used as excellent and low-cost electron acceptor. The negative charges are conducted from the graphite electrode to the platinum electrode via the external metallic conductor. On the platinum electrode H+ ions are reduced, molecular hydrogen is generated and escapes as gas.
Note that in the 2-pot cell under irradiation, a constant current intensity gets established and can be measured in the external circuit over a longer period of time. (Figure 6).
This corresponds to the setting of a photostationary state (photosteady state) in the photocatalytic system from the irradiated half-cell. In the case under discussion, it is the state, in which the formation and disappearance of the reduced form of the redox mediator EV+ proceed at the same rate. The constant current intensity over time means that all electrons generated per time interval by light absorption and charge separation flow through the external circuit. As long as the photostationary state persists, the formation of hydrogen also proceeds at a constant rate. Our measurements indicate that the limiting step for the current in the whole process is the charge transport through the salt bridge.
Contrary, one can conclude that in the two-pot cell as well as in the one-pot cellthe formation of hydrogen at a constant rate is always due to a state of photostationary state in the cell. This is analogously the case with the photosynthesizing green leaves under constant irradiation by sunlight. Furthermore, the stratospheric oxygen-ozone equilibrium in the polar summer is also a case of photostationary state.
4. One-Pot & Two-Pot Cell - Experimental Details & Results
The Photo-Blue-Bottle solution is prepared by dissolving the following three chemicals (see formula in Figure7) in 500 mL of distilled water which is stirred continuously:
1 g of EDTA (Ethylene-diaminetetraacetic acid disodium salt, Merck/Sigma-Aldrich, CAS No. 6381-92-6),
561 mg of ethylviologen (1,1’-Diethyl-4,4’-bipyridinium dibromide, Merck/Sigma-Aldrich, CAS No. 53721-12-3), and
12 mg of proflavine (3,6-Diaminoacridine-hemisulfate, Merck/Sigma-Aldrich, CAS No. 1811-28-5)
The solution should be stored in a closed brown glass flask. In this case, it remains stable for several months and can be used repeatedly in the one-pot as well in the two-pot cell.
For school experiments in the one-pot cell, suitable reduction catalysts were selected from commercially available products. For this purpose, at least 3 series of measurements were carried out with each tested reduction catalyst that was added to 50 mL of PBB solution and irradiated with the LED-module type Sahlmann specified below. The results are shown in Figure 8.
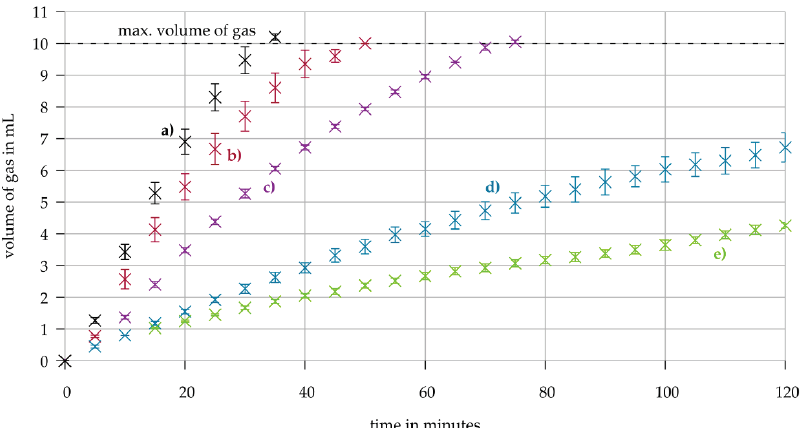
Figure 8: Kinetic measurements on the activity of platinum-containing reduction catalysts in photocatalytic hydrogen production.
The following reduction catalysts have been tested: (a) 100 mg of alumina fibers containing 5% (mass %) platinum, (b) 100 mg of alumina powder containing 5% (mass %) platinum; (c) 5 mg of pure platinum powder, diameter ≤ 20 μm, (d) 100 mg of alumina spheres, containing 0.15%(mass %) platinum and palladium, (e) 100 mg of alumina spheres containing 1% (mass %) platinum.
The results shown in Figure 8 suggest that the two catalysts (b) and (a) with the best activities are to be recommended for school experiments. They have the same composition of 5% platinum on inert alumina, and can be purchased from different companies, e.g. via https://bit.ly/3fTGHpd respectively. The prices of catalysts (a) and (b) per gram are about the same. For school experiments, (a) is recommended because it is available in small packages of 1 gram. Considering that in additional experiments we found that 50 mg of the catalyst (a) were enough for producing 10 mL of hydrogen from 50 mL of PBB solution within 30 minutes, the price of about 15 Euro per gram of 5%Pt/Al2O3 is fairly low.
The scanning electron micrographs in Figure 9 provide an explanation for the slight difference between the two catalysts (a) and (b). It can be seen that the platinum nanoparticles (luminous spots), are evenly distributed on the alumina fibers (a), while they are partially aggregated to larger clusters on the alumina grains in the powder (b). However, the activity of the two catalysts is approximately the same, so that both are well-suited to be used in the one-pot cell for photocatalytic hydrogen production.
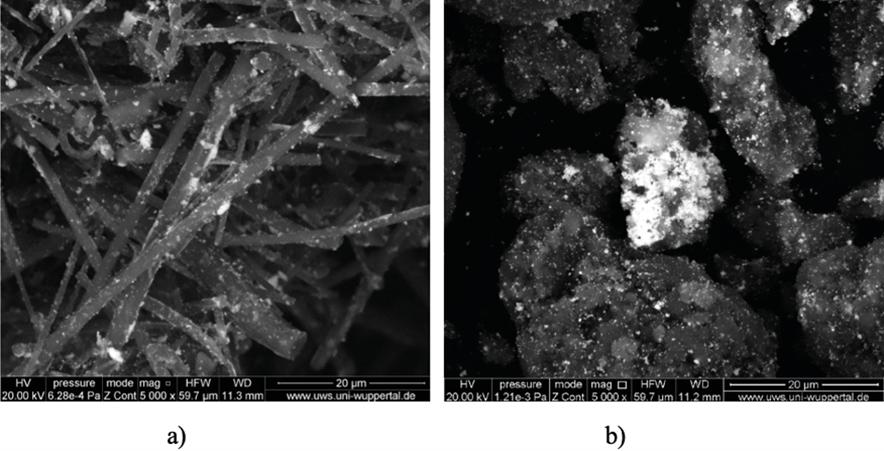
Figure 9: SEM-photographs of platinized alumina-fibers 5%Pt/Al2O3 (a), and platinized alumina-powder 5%Pt/Al2O3 (b); both in 5000 times magnification
Both reduction catalysts 5%Pt/Al2O3 (a) and (b) can be regenerated and reused. Regeneration has been carried out by filtering off the PBB solution, treating with sulfuric acid, c = 1 mol/L and hydrogen peroxide solution, w = 30%. After renewed filtration and rinsing with water, the catalyst can be used for a further reaction batch in the one-pot cell.
In all experiments in the one-pot cell as well as in the two-pot cell, the following blue light emitting light sources were used as:
LED torches with rechargeable battery, type: LE CREE Multicolor Rechargeable, 6 colors: violet (400 nm), blue (450 nm), green (524 nm), yellow (580 nm) red (623 nm), white; battery lifetime for blue light: max. 5 hours, max. power (blue light): 3 W;
LED modules with mains operation, type: Sahlmann Photochemical Solutions, 3x LXML PR01, λmax = 447 nm, power LED: 3 x 910 mW; price on request from the manufacturer; e-mail: bs@sahlmann-ps.de.
The two LED lamp types were measured with an Ocean Optics Spectrometer of the type Ocean-HDX-XR. A maximum of 33,000 counts were obtained for the LED torches and a maximum of 50,000 counts for the LED modules with an integration time of 20 ms. These signals are proportional to the light intensity of the respective lamp. The number of counts results from the number of photons impinging on a certain surface per time interval (constant quantities during measurement).
In order to ensure constant light intensities during the entire irradiation period when investigating the activity of various reduction catalysts (see curves in Figure 8), the mains-powered LED modules were used for these experiments. This type of LEDs is also suitable for experiments in bachelor’s theses, master’s theses, and in youth research projects. For experiments in teacher traineeships and in school lessons, the cheaper LED torches are perfectly adequate. Such lamps can be found on the web using the search terms “LED multicolor torches”. They are currently priced between USD 15 and USD 50, depending on the number of colors and the wavelengths of the emitted light, the power of the LEDs, the runtime of the batteries and other parameters. The performance parameters of the LED torches available for purchase are tending to improve, while the prices are declining.
5. Videos as Digital Assistants
As an additional service for experiment-based teaching and learning, we offer a 4-minute video for each of the two cell types [14, 15]. Each video shows the experimental procedure and the results in detail, partly in real time and partly in time-lapse.
The videos can be used equally in face-to-face, distance and hybrid teaching. They provide exact working instructions for carrying out the real experiments in visual rather than written form. To explain the phenomena and to develop or apply the technical concepts, it is useful to verbalise, write down and discuss selected scenes from the video. Doing so, appropriate technical terms such as catalyst, photocatalyst, redox couple, redox mediator, sacrificial donor, coupled reactions, photochemical reaction, “green” hydrogen, and artificial photosynthesis should be used.
The reflexion on the experiments shown in the videos should be conducted in such a way that it also stimulates the development of ideas for alternative experimental versions. These may include a modified experimental set-up (e.g. number and placement of torches, solar light instead of light from LED, shape and size of the reaction vessel containing the PBB solution, collection device for the hydrogen) and/or a modified mode of operation (e.g. flow-reactor with continuous removal of the hydrogen). Such alternatives of the video experiments can be the subject of research work on the topic of artificial photosynthesis in the secondary education or in teacher training.
6. One-Pot vs. Two-Pot Cell - Didactic Recommendation
Which of the two types of cells is best for teaching photocatalytic generation of hydrogen with respect to artificial photosynthesis? Simply spoken: neither one alone, only both together.
The teaching concepts with two-pot- and one-pot cells (Figure 11) is well-applicable not only for photocatalytic cells described in this article, but also for electrochemical and photogalvanic cells [16-18].

Figure 11: Teaching concept for electrochemical, photogalvanic and photocatalytic cell types in in education of sustainable applications in technology.
In electrochemical, photogalvanic and photocatalytic two-pot cells, redox reactions take place in separate reaction chambers: anodic oxidation in one half-cell and cathodic reduction in the other. The half-cells are connected to each other via an outer circuit of metallic conductors and an inner circuit of electrolyte solutions. Electron transfer takes place at the electrodes of the two half-cells, where ions from the respective solution are oxidized or reduced. The charge balance between the half-cells is achieved by electron flow in the outer circuit and ion movement in the inner circuit.
Compared to two-pot cells, one-pot cells are more compact experimental devices with better performance parameters and didactic bridging function to the respective functional units in technology. The disadvantage is that the observable phenomena cannot be assigned to the processes at the particle level as clearly and directly as in the case of the respective two-pot cell. This disadvantage is eliminated if the respective two-pot cell is investigated and clarified before dealing with the one-pot cell.
Consequently, the experimental and conceptual investigation in an inquiry-based approach [19] should follow the scheme in Figure 11, when technical innovations concerning photogalvanic, photovoltaic and photocatalytic processes are targeted.
7. Photocatalytic Hydrogen Production - Ready for Teaching
Although there are not yet any technical plants for the photocatalytic production of hydrogen, in chemical education it is appropriate and necessary to focus on the photocatalytic production of “green” hydrogen. The model experiments presented in this paper, realize three of the four sub-processes of artificial photosynthesis indicated in Figure 1. Only the sub-process no. 3, the oxidation of water to molecular oxygen and hydrogen ions H+, is not realized. Instead of water, the sacrificial donor EDTA is oxidized in all experiments working with the PBB solution as photocatalytic system. In this respect, the model experiments in this paper correspond to the state of the art in scientific research, where different sacrificial donors are also used [1]. Furthermore, the problem of the sacrificial donor motivates the learners to think about the possibilities of complete water splitting with light and to inform themselves about the current state of research [20, 21]











 nueva página del texto (beta)
nueva página del texto (beta)



