Life and career (F. Marié-Davy, 1910; Delaitre-Rélu 2006)
Edme-Hippolyte Davy was born in Clamecy, (France, Nièvre department) on April 28, 1820, the second of the four children of Claude-Paul Marié a cobbler, and Marie Moreau, member of a family of landowners. He took his basic education at the local municipal school and at the Lycée Moulins and then earned a scholarship to study at the Collège Rollins in Paris (1840). At the age of 20, after earning his baccalauréats ès lettres et ès sciences, he enrolled in the École Normale Supérieure from which he graduated in the first place of his class (1844); afterwards he was appointed professor at the Lycée de Saint-Étienne and at the Collège Royal de Rouen. In 1844 he won by competition a position of agrégé in physical and mathematical sciences (the door to an academic career) at the Faculty of Sciences of Montpellier, which allowed him to teach at the institution as well as follow doctoral studies (this act required a special approval because he was only 25 years old) (1845). He received his diploma of docteur ès sciences after successfully defending two theses, one about the molecular constitution of bodies (Marié-Davy, 1845a) and the other about the transmission of electricity by liquids (Marié-Davy, 1845b). The same year 1845 he was appointed with dispensation at the Faculty of Medicine of Montpellier, the oldest faculty of Europe, and in 1851 he received his degree of docteur ès médicine with a thesis about the climate of Montpellier (Marié-Davy, 1851). In 1844 he married Julie-Marie-Joséphine Davy de Lachevrie, the daughter of the physician Maurice Davy de Lachevrie. The revolution of 1848 and the 1851 coup of state of Louis Napoleon Bonaparte forced his father-in-law, a staunch republican, to exile to Paris. In order to help and protect him, Marié-Davy resigned his senior position at Montpellier and moved to a lower one at the Lycée Bonaparte (today Lycée Condorcet) in Paris. In 1862, his father in law, the physician, pressed him to change his name to Marie-Davy.
Marié-Davy passed away in Dornecy, on July 26, 1893.
Marié-Davy took an active part in French and international public and scientific activities and was recognized correspondingly. In 1859 he was appointed chevalier of the Légion d’Honneur. He was member of the Conseil de la Société des Agriculteurs de France, a founding member of the Société Centrale de Sauvetage des Naufragés, founding member and President of the Société Française d’Hygiène, member of the Société Française de Météorologie, Vice-président of the Société de Médicine Publique, corresponding member of the Institut and the Bureau des Longitudes, member of the Sanitary Institute of Great Britain, of the Société Royale de Médicine Publique of Belgium, etc. In 1861 he was appointed astronomer at the Observatory of Paris and in 1863, head of the International Meteorological Service. In 1873 he was promoted to director of the Observatoire Municipal de Montsouris, a position he kept until his retirement in 1886.
Davy wrote about 90 papers and books (e.g. Marié-Davy, 1852a, 1861c, 1866ab, 1867ab) on the subjects of electricity, electrochemistry, physiology, meteorology, astronomy, etc. In addition to the subject described below, he also studied the measurement of dynamic electricity (Marié-Davy, 1846, 1847a); the resistance opposed to homogenous conductors (Marié-Davy, 1847b); the laws of electrical attractions and repulsions and laws of voltaic currents (Davy, 1847bc); the physiological aspects of vision (Davy, 1849); the instruments used for measuring static electricity (Marié-Davy, 1850); the action of electricity upon the nervous system (Davy, 1852b, 1853b); the theory of electric motors (Marié-Davy, 1855); electromotive force as mechanical power (Marié-Davy, 1861b); the origin of the heat released during chemical reactions (Davy, 1859b); the heat of lunar thermal radiation (Davy, 1869ab); water in vegetation, (Marié-Davy, 1874); etc. As customary for candidates to the Académie des Sciences and the Académie Impériale de Médicine, he wrote a booklet describing his research achievements (Marié-Davy, 1868).
Marié-Davy was not only very prolific scientist; he also looked for a practical application of his knowledge. This was reflected in the numerous instruments he patented, for example, a naval periscope consisting in a vertical tube with two small mirrors fixed at each end at 45°, the Marié-Davy mercury bisulfate battery (see below), an electromagnetic motor, a submarine with an electrically driven propeller, a knitting machine, a procedure for isolating electrical cables, a hygrometer, etc.
Electrochemistry
Marié-Davy wrote that the best procedure for measuring the intensity of a current was based on the chemical action it produced in a given time (Marié-Davy, 1846). Accordingly, he assigned the value 1000 to the current that was able to precipitate, within one hour, one equivalent of copper (32 g) or decompose one equivalent of water (9 g). He always used a compass to observe a current because of its rapid response; the use of compensated compasses was unacceptable. The needle had to be as simple as possible and outside the influence of magnetic influences. The results of his experiments indicated that the magnetic force of a current was proportional to its electrochemical force and the electrochemical force of a current was independent of the manner in which it was generated, provided that the voltmeters used were built with wires that were not corroded by the interpolated liquid (Marié-Davy, 1846).
The first step in the study of a battery should the determination of the losses it experimented when changing the conductor. The return of the needle of the compass should not be used for this purpose because it was a very complicated phenomenon. Instead, it was better to use the number of units of a well-defined resistance intercalated in the circuit, which produced the same effect on the needle. Marié-Davy found that the following formulas represented the results for a zinc diaphragm located in water acidulated with sulfuric, nitric, or HCl acids, respectively:
Where r is the total resistance and i the intensity of the current. Writing r = l + R, where l and R represent the additional variable resistance and the primitive one, respectively, leads to the following equivalent formulas (Marié-Davy, 1846):
According to Marié-Davy, the battery was an engine and should be considered as such.
Hence, the laws of mechanics ruled its operation: The motor work of any engine in
uniform movement was equal to the total resisting work. This principle was also
applicable to the battery (Marié-Davy,
1859a). In a steam engine, the motor work was created in the fireplace and
originated from the molecular work accomplished during the combustion of the fuel,
which was displayed as heat. In a battery, the motor work originated from the
chemical actions executed, positive or negative (positive for the dissolution of
zinc, negative for the reduction of hydrogen copper, or mercury, or the reduction of
nitric acid), and hence, it was proportional to the intensity of these actions
(neglecting the chemical actions taking place continuously, even when the battery
was inactive). This work was also proportional to the specific work originating from
each chemical reaction, in the same manner that the heat produced by the combustion
of several substances varied with the weight and nature of the fuel. Marié-Davy
illustrated this idea with the Smee battery (zinc plate and silver plate, coated
with platinum black, in sulfuric acid). Calling A the molecular work produced by the
substitution of one equivalent of zinc by one of hydrogen, the motor work in one
active Smee element would be proportional to A and to i, proportional to the product
Ai, and, consequently, equal to KAi, where the value of K depended on the units used
for measuring i (Marié-Davy, 1859a). The
resistant work appeared in the battery as an electrical movement, of unknown nature,
named current. It was known that any body moving through fluid with velocity V
presented a resistance proportional to V2 and a resistant work
proportional to V3. Similarly, the resistance of a solid slipping or
rolling over another solid was independent of V while the resistant work was
proportional to V. In the case of electrical movement, the resistant work was
proportional to i2. Assuming that a portion of the circuit of the battery
of section s and length l was traveled by a current i, the intensity of the current
traversing every unit of the section would be given by i/s and the resistant work y
would be proportional to i2/s2, and for the total section
(i2/s2)s = i2/s, that is, equal to K’
i2/s, where, again, the value of K’ would depend on the units used
for the current per unit section. The resistant work for the length l was (K’
i2/s)l and for a circuit composed of a number of different conductors
the corresponding equation would be
Marié-Davy added that the resistant work was not motor work lost but work transformed. The molecular work appeared in the conductor as heat released in every one of its points in an amount exactly equivalent to the pertinent resistant work. In the combustion of zinc in the battery, the amount of heat deposited in the circuit was the same that would be produced by the same chemical phenomenon occurring outside the battery (Marié-Davy, 1859a).
Marié-Davy wrote that no matter how carefully the units of resistance and of current were defined, inasmuch that these units were arbitrarily chosen, it could be expected that in estimating the electromotive force of a battery they would directly furnish the heat effect of the special work performed by the chemical actions of this battery. It was necessary to determine the value of a constant numerical coefficient, which, like the units of resistance and of current, could be easily done by an experimenter. The Smee battery was the simplest one. The only chemical action produced normally was the dissolution of zinc in acid with disengagement of hydrogen. Pierre Antoine Favre (1813-1880) had determined that the heat released was 18,444 units for ordinary zinc and 18,791 units for amalgamated zinc (Favre, 1843). Marié-Davy used these values to determine the numerical coefficient of this battery. After measuring 125 times its electromotive force he obtained values of the coefficient varying between 16,886 and 20,604; the difference of about 20% indicated the presence of the following possible error sources (1) air dissolved in the acidulated water. The electromotive force of the Smee battery with aerated acid water was found to decrease gradually as the battery worked, and stayed fixed when operating under vacuum. The oxygen of the air attacked the zinc and the corresponding quantity of water escaped decomposition; (2) sulfate dissolved in the solution. The water, acid, and sulfate were separately conductors; each conducted and each was decomposed. Water and acid produced hydrogen while the sulfate was reduced; according to Marié-Davy, neglecting the influence of air, the presence of zinc sulfate caused the gradual weakening of the battery; (3) concentration of the acid. The electromotive force of the battery remained constant when the solution contained more than 25 equivalents of water for one of acid; higher concentrations of acid led to an increase of the electromotive force and higher concentrations of acid led to the formation of SO2; (4) influence of the zinc. It was known that amalgamated zinc produced stronger piles than non-amalgamated zinc; (5) purity of the acid. Commercial acids contained traces of nitrogen compounds that increased the electromotive force of the Smee battery; (6) influence of the water. Distilled water produced different results than normal potable water; and (7) the operating temperature (Marié-Davy, 1861a).
A detailed analysis of the above factors led Marié-Davy to operate a Smee battery composed of a platinum strip submerged vertically in sulfuric acid diluted in 8 to 20 times its weight in distilled water degassed by vacuum. The solution was contained in a vertical glass column containing at its bottom a liquid amalgam of pure zinc dissolved in mercury. The negative pole was a platinum wire traversing the bottom of the tube. The pile was submerged inside large vase holding water at constant temperature. The electromotive force of the pile was assumed to be 18,510 units (Marié-Davy, 1861b).
In 1858 Marié-Davy and Louis Joseph Troost (1825-1911) published three papers explaining the use of a battery for determining the heat and work effects of some chemical reactions (Marié-Davy and Troost, 1858abc). Marié-Davy had already done fundamental theoretical and experimental work about the electromagnetic motor and found, among other things: (1) the resistance encountered by an electric movement of any nature, at any point in an electric circuit, was proportional to its intensity at that point; (2) at every point of the circuit, the work done by the resistance was proportional to the square of the intensity of the movement; and (3) in an active cell, the total work of the resistances of the circuit was proportional to the electromotive force of the cell and to its useful expenditure of zinc (Marié-Davy, 1855). In addition, he had also found that the battery functioned according to the laws of mechanics and that the intensity of the current, i’, in battery with connected poles, increased gradually with time (t) according to the formula:
Where i was the final value. At the equilibrium stage, the work of the resistances of the circuit was identical to the electromotive work of the battery, as shown before: A, the electromotive force of the battery, was the work performed per equivalent, by the chemical reactions. The electromotive work equilibrated by the resistant work was not destroyed, it appeared as the heat effect (Marié-Davy, 1855).
James Prescott Joule (1818-1889) had shown that the heat developed by a chemical reaction was due to the resistance that the media opposed to the establishment of an electric movement, and that the electromotive force of a cell was proportional to the algebraic sum of the heat effects of all the chemical reactions that took place in the cell. Using different arguments, Rudolf Julius Emanuel Clausius (1822-1888) had shown that the electromotive force of a cell could be used to measure the amounts of heat released during the chemical reactions taking place in the cell.
Marié Davy and Troost carried their experiments along two lines: whenever possible, they built a cell with the substances they wanted to combine and measured the electromotive force generated. Although this seemed to be to most direct approach, it was applicable only to a limited number of cases. The second method, much more fertile, consisted in passing the current of a cell through the substances to be combined or decomposed, and thus force reactions that otherwise would not take place. The electromotive force of the cell was measured before and after the current flowed through the pertinent substances and the difference between both values gave the result desired (Marie-Davy and Troost, 1858ab). Basically, the thing to do was to measure the electromotive forces. This they did by the standard procedure of intercalating in the circuit well-calibrated resistances and measuring by means of a compass the corresponding current intensity. In their calculations they used the fact that the solution of one gram of zinc in dilute sulfuric acid released 567.9 calories, or that one equivalent of zinc (32.5 g) released 18,457 calories (Favre, 1843). Marié-Davy and Troost reported the heat released during the combination of one part of alkali (KOH, NaOH, ammonia, and zinc oxide) in a diluted solution, with one part of acid (10 inorganic and 5 organic) in diluted solution, which agreed completely with those reported by Favre and Jean-Thiébault Silbermann (1806-1865) using standard calorimetric procedures (Marie-Davy and Troost, 1858ac, Favre, 1843).
In a following work, Marié-Davy and Troost used their electrochemical procedure to determine the heat of reaction on one equivalent of chlorine with one equivalent of thirty-three metals, to produce the pertinent chloride (among them, the chlorides of sodium, potassium, lithium, zinc, cobalt, nickel, lead, mercury, copper, and gold) (Marié-Davy and Troost, 1858b).
According to Auguste de la Rive (1801-1873), it was known that putting in contact manganese dioxide with platinum resulted in positive electricity passing through the platinum and negative through the finger or any wet body touching the dioxide (de La Rive, 1836). Studying this phenomenon, de la Rive understood that the production of electricity was due to a chemical action exerted on the dioxide, probably a slight deoxidization accompanied by the formation of a hydrate. It was very weak with distilled water and stronger, although in different degrees, with acid or alkaline solutions, or with the finger where the humidity was always slightly acid or alkaline. It was nil when contacting the dioxide with the platinum. The same phenomenon occurred when replacing the platinum with a very dry strip of wood. De la Rive found that placing the strip of wood over the plate of a capacitor and then touching the dioxide with a finger or a piece of paper soaked with an alkaline or acid solution, produced in the electroscope a clear signal of positive electricity. To observe the negative electricity, he conducted the opposite experiment: a platinum strip was mounted over the plate of the capacitor, a piece of soaked paper over the platinum, and the dioxide over the paper. Touching the latter with a dry finger charged negatively the capacitor. The results of these experiments indicated that the action of wet bodies over the manganese dioxide resulted in the generation of electricity. The law of this release was that the negative electricity passed into the attacking moist body, while the positive electricity remained in the dioxide, from which it passed into the non-attacking bodies in contact with it. De la Rive succeeded in verifying these conclusions with a galvanometer. The intensity of the current depended essentially on the nature of the liquid interposed between the elements of the couple; it was very strong with HCl and nitric acid. It was known that the action of HCl on manganese dioxide resulted in a strong deoxidization of the dioxide and that the action of nitric acid resulted in the release of oxygen bubbles due to the formation of a nitrate. The action of water over the dioxide and the formation of a hydroxide justified very well the assumption that the current was produced by deoxidization. Furthermore, replacing the manganese dioxide by potassium chromate resulted in a very strong current, particularly when employing HCl or nitric acid as the liquid interposed between the chromate and the platinum. Similar results were obtained with lead dioxide or trioxide. In every case, the substance attacked performed as negative in relation to platinum (de la Rive, 1836).
Marié-Davy commented that manganese dioxide was a poor conductor of electricity giving batteries a very strong internal resistance and generating very weak currents. Lead dioxide did not have this negative characteristic but left as residue an insoluble and poor conductor product, and was a very expensive material. Both dioxides required the use of a free acid. For these reasons none of these oxides had found a practical use in electrochemistry (Marié-Davy, 1859b). He reported that he had built a battery composed of zinc, very pure water, and silver nitrate, which worked perfectly. Its initial internal resistance was very large, but decreased as the zinc chloride formed dissolved in water. This problem could be solved by using instead a solution of zinc chloride prepared beforehand. The silver chloride was reduced completely in its internal parts, while keeping exactly its shape. The insolubility of the reducible salt was clearly an advantage because it allowed eliminating the porous vases, which always offered a strong resistance to the current (Marié-Davy, 1859b).
Marié-Davy used these results to search for a cheap commercial substance that could be used advantageously in the building of batteries. His search indicated that lead sulfate and lead chloride were appropriate for this purpose. Lead sulfate was available as the residue of the reaction between lead acetate and alum to produce aluminum chloride for textile uses. It was recommended to wash it before use because it might contain an excess of soluble lead acetate, which produced a flaky deposit over the zinc. The electromotive force was slightly lower than that of the Daniel battery. Melted lead chloride did not present the inconveniences of the sulfate, although it was more expensive. The insolubility of both lead sulfate and chloride allowed eliminating the use of porous vases (Marié-Davy, 1859b).
Marié-Davy wrote that his batteries were made of tinned iron plates manufactured for domestic use. The bottom of these vases was internally doubled with a washer in the same dimensions. Each of them was lined with a layer of lead sulfate a few millimeters thick, filled with pure or salty water, or holding dissolved zinc salt. These vases were placed parallel in a vertical column above the others so that the zinc of an element plunged into the water of the lower element. Lead chloride was used in the same way, only it was advantageous to pour it in advance into thin plates which were broken into fragments, which made it possible to fill and empty the elements more easily (Marié-Davy, 1859b). The development of this battery was the prelude to one of the many practical inventions of Marié-Davy.
Many batteries were proposed as a modification of the Daniell battery (copper, copper sulfate, sulfuric acid, zinc), where a metal and its sulfate replaced the copper and its sulfate. The most interesting modification was proposed by Marié-Davy where cupric sulfate was replaced by mercuric sulfate and zinc by carbon (Niaudet, 1878). Actually, to be an exact replacement of the Daniell cell, the copper had to be replaced by mercury metal, but the high price of the metal and its liquid state forced the substitution by carbon. Nevertheless, the action of this battery resulted in the reduction of the sulfate and the deposition of liquid mercury on the negative or conducting pole. With time, this process resulted in a piece of carbon being submerged in an electrode of mercury. Experience showed that the electromotive force was not changed by elimination of the carbon and the use of mercury, as long as this metal was pure enough (Niaudet, 1878).
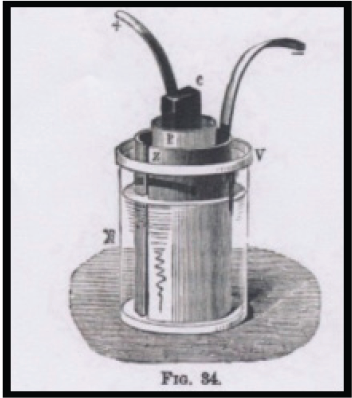
Niaudet wrote that the battery was built as follows (Figure 2): The zinc, z, is a hollow cylinder placed in a glass jar, v, suspended by a natural rubber cover and surrounded by a porous jar containing the carbon electrode c surrounded by a liquid paste of mercury sulfate. The carbon is crowned with an electrochemical deposit of copper (or lead) to which is soldered a strip of the same metal connected with the zinc of the adjoining cell. The operation of the cell is similar to the one of Daniell: the sulfate is reduced and an equivalent quantity of zinc sulfate formed. The zinc is dissolved and metallic mercury is deposited upon the surface of the carbon electrode or in the mass of mercury sulfate. Replacing by mercury bisulfate (Hg2SO4) or trisulfate [3(HgO)SO3] the sulfate was not found to alter the value of the electromotive force (Niaudet, 1878).
According to Niaudet, the Marié-Davy battery presented the following advantages: (1) its electromotive force was substantially higher than that of the Daniell cell; (2) the sparingly solubility of the sulfate resulted in a very slow diffusion in the outer liquid, producing reduced local actions and lost work (a significant problem of the Daniell cell); and (3) the deposition of mercury on the zinc by local action amalgamated the zinc, preventing the waste of the sulfuric acid if it was used in the outside jar (amalgamated zinc produced stronger piles than non-amalgamated zinc). Anyhow, consideration had to be given to the fact that mercuric sulfate was a powerful poison and a very expensive raw material (Niaudet, 1878).
His son Ferdinand Marié-Davy wrote that that Marié-Davy patented his battery and offered it free of use to the French government. It was tested, adopted, and used for many years by the Post Office administration, and, particularly, by the French Army during the Crimean war (fought during by 1953-1956 by the Ottoman Empire, France, and Sardinia against the Russia empire) (F. Marié-Davy, 1910).











 nueva página del texto (beta)
nueva página del texto (beta)