Life and career
There are several good sources of information about the life and career of Louis Édouard Grimaux, particularly the one by his student Paul Adam (Adam, 1911) and the PhD thesis of Marie-Christine Journaux (Journaux, 1990). The material that follows is mostly taken from there (Adam, 1911; Bujeaud, 2005; Fournier, 2012; Gerber, 1900; Journaux & Viel, 1996; Journaux, 1990; Viel, Journaux, & Marché, 1997; Viel, 2005).
Louis Édouard Grimaux was born in Rochefort-sur-Mer on July 3, 1835, the son of Pierre-Chéri Grimaux (1785-1860), an officer-pharmacist of the Navy, and Veronique Cornélia Bouhet (1805-1852), Édouard received his basic education in his native village and then at Saintes; in 1852. After graduation he took courses at the École de Santé Navale, where his father taught botany. In 1853, after graduating as pharmacist of third class, he entered the health service of the French navy and served as pharmacist at the ports of Rochefort and Toulon (1853-1858). In 1857 he married Léontine Christine Boutet (1833-1925), from which he had two sons, Marcel (1860-1862) and Louis-George (1864-1943), and one daughter Jeanne (1859-1926). In 1858 he resigned his post at the navy and moved to Sainte-Hermine where his father-in-law, Eugène Boutet, lived. In 1860, he registered for studies in medicine and pharmacy and in 1861 he moved to Paris to obtain the diploma of pharmacist of first class, with the intention of returning to Sainte-Hermine to open a pharmacy. In 1863 he published his first memoir on the dry distillation of sulfonaphthalic acid (Grimaux, 1864a), and the next year, another one about the composition of gallic acid (Grimaux, 1864b). As the subject for his doctoral thesis in medicine, he chose the physiological effects of hashish (Grimaux, 1865). In received his medical degree in 1865 and in the next year he approved successfully the competition for agrégation , 1 with a thesis entitled Equivalents, Atomes et Molécules (Grimaux, 1866). In 1866 he was appointed agrégé de chimie at the Faculty of Medicine of Paris and joined the laboratory of Charles-Adolph Würtz (1817-1884). In 1869 he was charged with teaching a complementary course in chemistry, which he repeated in 1871 and 1873. In 1870 he received one-third of the Jecker Prize and in 1875 he received it again, this time completely. During the war with Germany he was employed in the production of bronze cannons, organized by the Société de Chimie. After the war he returned to work with Würtz and was appointed sub-director of the Laboratoire de Hautes-Etudes at the Sorbonne, directed by Paul Schützenberger (1829-1897). In 1876 Schützenberger left this position to replace Antoine-Jerôme Balard (1802-1876) at the Collège de France and was replaced by Joseph Riban (1838-1917). In 1876 the Institut Agronomique was reorganized and Grimaux won by competition the chair of General Chemistry. In 1877 Grimaux presented his thesis for the degree of docteur és-sciences, named Sur les Dérivés de la Série Urique (Grimaux, 1877). After 1879, Grimaux started working again with Würtz until 1881 when he was appointed to replace Auguste Cahours (1813-1891) as professor at the École Polytechnique, where he had already served as répétiteur1 since 1876. He continued in this position until 1898 when he was fired because his support of the innocence of Dreyfus (a detailed description of this issue appears in the paper of Viel, 1997). In 1898 Charles Friedel (1832-1899) took him as collaborator in his laboratory in the Sorbonne.
Grimaux received many awards and honors for his academic and public activities. In 1875 he was nominated Docteur Honoris Causa by the University of Leyde and in 1876 Docteur ès-Sciences by the Académie des Sciences of Belgium. He was also elected member of the Société de Biologie (1874), of the Comité Consultatif d'Hygiène Publique (1883), chevalier of the Légion d'Honneur (1880), and promoted to officer in 1895. In 1877 he was candidate to the Académie des Sciences, to replace Balard; he became a member of the Institute de France in 1894, replacing Edmund Frémy (1814-1894). He was President of the Société Chimique in three opportunities (1881, 1890, and 1900), President of the Association Française pour l'Avancement des Sciences (1898), and Vice-President of the League for the Defense of Citizens Rights, founded en 1898 (Adam, 1911).
Édouard Grimaux passed away on May 2, 1900, in Suresnes, near Paris, after a brain hemorrhage; his cinders were buried in the columbarium of the Père-Lachaise cemetery in Paris. In 1926, shortly after the death of his wife Léontine, his remains were removed from Paris and buried in her tomb, in the Protestant cemetery of Sainte Hermine On August 11, 1907, the City of Rochefort inaugurated a monument in his memory; general Marie-Georges Picquart (1854-1914), 2 Ministry of War, attended the ceremony (Adam, 1911).
Grimaux's research was mostly in the area of organic synthesis: He prepared, identified, and described the properties of a large number of derivatives of the uric series, of alkaloids, cyclic nitrogen compounds, aromatic aldehydes, etc. He also synthesized citric acid (Grimaux & Adam, 1880) and a series of colloids, albuminoids, and carbohydrates (e.g. Grimaux, 1884j, 1885).
Scientific contribution
Grimaux wrote more than 120 papers and books (e.g. Grimaux, 1844-1845, 1872, 1874, 1877, 1884g, 1888, 1900) in different areas of organic chemistry. He wrote the first detailed biography of Antoine-Laurent Lavoisier (1743-1794) (Grimaux, 1888) and continued the compilation of Lavoisier's papers (volumes 5 and 6), initiated by Jean-Baptiste André Dumas (1800-1884) in 1863 (Lavoisier, 1864-1893). He also wrote a biography of Charles-Frédéric Gerhardt (1816-1856) (Grimaux and Gerhardt, 1900). As customary for a candidate to the Académie des Sciences, he published a booklet detailing his main research achievements (Grimaux, 1894).
Here we describe a few of his results.
Equivalents, atoms, and molecules
The contents of Grimaux's thesis for the aggregation are very interesting because it allows the reader of today to understand the concepts of equivalents, atoms, and molecules, as they were used during the second half of the 19th century, and the substantial differences between the formula and molecular mass of compounds as reported then, and the ones accepted today.
In his opening statement, Grimaux wrote that scientists were faced with the questions of understanding in which proportion bodies combined one with the other, which regulated the combinations, and how to determine the ratio of the elements, which formed a given compound. The terms equivalents, atoms, and molecules gave the answer to these questions; unfortunately scientists used them in a wide range of interpretations, leading to the corresponding confusion (Grimaux, 1866).
Grimaux wrote that the experimental data indicated that water contained 11.11% hydrogen and 88.89% oxygen weight, that is, the two components were in the ratio 1:8. Another way of expressing the composition was by volume: 2 volumes of water vapor contained 2 volumes of hydrogen and one volume of oxygen, that is, the volumetric ratio was also 1:8. Assuming that one volume of hydrogen weighed 1, then one volume of oxygen weighed 16 because the density of the latter was 16 times that of the former. Hence, 2 volumes of hydrogen weighed 2, one volume of oxygen weighed 16, and combined they gave water. In other words, the volumetric composition of water allowed us to determine the weighed ratio of its components.
For Jöns Jacob Berzelius (1779-1848) the term atom had the same value as the words particles, atoms, molecules, and chemical equivalents. Not only that, he admitted that atoms could be simple or composed atoms of first order, second order, etc. Jean-Baptiste André Dumas (1800-1884) distinguished between divisible atoms and non-divisible atoms, while Marcelin Berthelot (1827-1907) spoke about chemical atoms and physical atoms (Grimaux, 1866).
If we took an amount of silver nitrate containing 108 parts of the metal, experience indicated that the silver could be completely replaced from this combination by 31.5 parts of copper, 103.5 of lead, 28 of iron, or 39 of potassium, to yield the pertinent nitrates. We then said that these numbers were equivalent to 108 parts of silver. It was possible to use smaller numbers by assuming that the equivalent of hydrogen was 1 (or 8 for oxygen). It so happened that a given substance could have several equivalents; for example, in cuprous oxide a copper weight of 31.5 combined with an oxygen weight of 8; in cupric oxide the equivalent of copper increased to 63. Two combinations of hydrogen and oxygen were known, water containing 1 part of hydrogen and 8 of oxygen, and water peroxide, containing 1/2 part of hydrogen for 8 of oxygen. Due to these difficulties, the accepted convention was the following: whenever a body combined with different proportions of oxygen, then its equivalent was the smallest amount that combined with 8 of oxygen. Hence, for copper, its equivalent was 32.65. Charles-Frédéric Gerhardt (1816-1856) had rejected this convention, stating accepted that a given substance could have several equivalents and that for each value it was necessary to indicate to which properties or functions it referred (Gerhardt, 1844-1845) (The attentive reader will realize that the equivalent was actually related to the specific valence of the element) (Grimaux, 1866).
By similar calculations, based on sulfides, chlorides, and oxides, it was found that 16 parts of sulfur, or 35.5 of chlorine, or 8 of oxygen, combined with 103.5 parts of lead, 108 of silver, 39 of potassium, and one of hydrogen. This meant that the numbers 8 for oxygen, 16 for sulfur, and 35.5 for chlorine were the proportions by which these metalloids combined with the elements selected as examples. What happened when we had a compound instead of an element? Again, the experimental evidence indicated that 40 parts of sulfuric acid combined with 116 of silver oxide, 111.5 of lead oxide, 47 of potassium oxide, and 39.5 of copper oxide. Hence, these quantities of oxides were equivalent between themselves (all these figures were based on oxygen being 8 and hydrogen being 1). Grimaux summarized all these calculations in a table containing the values of the proportional numbers or equivalents for the 62 elements then known. Inspection of these quantities indicates that they are approximately equal to 50% or 100% of the present accepted values of the atomic mass. Particular important for the calculation of the molecular mass and global composition is the fact that the values of the equivalents of hydrogen oxygen, sulfur, and carbon, are 1, 8, 16, and 6, respectively (Grimaux, 1866).
After showing the numerous inconsistencies of these quantities, Grimaux concluded that the theory of the equivalents did not provide the proportions in which the elements combined; it only implied the equivalence in particular situations, when comparing compounds of similar functions (chlorides, sulfates, oxides, etc.) (Grimaux, 1866).
Molecule was considered to be the smallest quantity of a given body capable of existing in a free state, indivisible by physical means. For example, the molecule of HCl was composed of chlorine and hydrogen, which could be separated by chemical agents; one molecule of HCl yielded one particle of chlorine and one of hydrogen; these particles were known as atoms . Atoms then, represented the smallest quantity of an element that could exist in a molecule. The atomic mass could only be determined after determination of the molecular mass. After these definitions, Grimaux went on to explain the procedure for determining the molecular mass. The basic law for doing this was Gay-Lussac's law of volumes; (a) there exists a simple ratio between the volumes of two gases that combine, (b) there is also a simple relation between the volumes of gases that combine, and the volume occupied by the resulting combination. For example, 1 volumes of hydrogen and 1 of chlorine, combined to give 2 volumes of HCl; 2 volumes of hydrogen and 1 of oxygen combined to give 1 volumes of water vapor; and 3 volumes of hydrogen combined with 1 of nitrogen to give 2 volumes of ammonia. These considerations, combined with the hypothesis of Avogadro and Ampère, led to the conclusion that the molecular mass of a substance was proportional to the density of its gas. For example, if one volume of chlorine contained 50 molecules, then one volume of hydrogen also contained 50 molecules. Since the density of chlorine gas was 35.5 times that of hydrogen, it meant that the molecule of the former was 35.5 larger that of hydrogen. Then, fixing the atomic mass of hydrogen as 1 it was possible to compare the molecular mass of a compound, relative to that of hydrogen. The molecule of hydrogen was 2, thus it was only necessary to multiply by 2 the masses reported relative to the atomic mass of hydrogen, to obtain the molecular mass. Air was 14.4 denser than hydrogen, hence its molecular mass was 28.88, and, consequently, it was possible to obtain the molecular mass of a body by multiplying by 28.88 its density relative to that of air. For example, the density of water vapor relative to air was 0.622, hence its molecular mass was (0.0622)(28.88) = 18 (Grimaux, 1866).
Grimaux remarked that the hypothesis of Avogadro and Ampère failed when not considering that certain substances dissociated in the gas state, as occurred with ammonium chloride, phosphorus pentachloride, HCl, etc. (Grimaux, 1866).
Grimaux ended his thesis with a discussion of the Dulong-Petit rule, as an alternative procedure for determining atomic masses, and a discussion of the historical development of the notions of equivalent, atom, and molecule (Grimaux, 1866).
Solid-liquid equilibrium
The usual procedure for the volumetric estimation of acetic acid was titration with a standard solution of NaOH; this method was considered not very accurate because the change in color of litmus paper was not clear enough. For this reason, in 1870, Friedrich Rüdorff (1832-1902) proposed a new rapid and accurate method based in determining the freezing point of the acid (Rüdorff, 1870). Commercial glacial acetic acid solidified between 7° and 14 °C; in order to purify it, Rüdorff put it in a cold place until about three-quarters of it had solidified. The liquid phase was separated and the procedure repeated until a constant freezing point was attained. The resulting acid boiled at 117.8 °C and solidified at 16.7 °C (today, 118.05° and 16.45 °C, NIST database). Rüdorff used this acid determine the solidification points of synthetic mixtures of known composition. He reported his results for water contents between 0% (16.7 °C) and 19.354% weight (7.40 °C). The lowest temperature measured was −0.20 °C for water content of 13.043%. Rüdorff also reported that other substances also reduced the solidifying point of acetic acid; for example a mixture of 100 parts of acetic acid and 0.5 parts of sulfuric solidified at 16.40 °C; of 100 parts of acid and 1.8 parts of alcohol, at 15.25 °C, etc.
In 1873 Grimaux published a paper about the solid-liquid equilibrium of the system acetic acid-water, which shows the knowledge available about this type of equilibrium by the last quarter of the 19th century. According to Grimaux it was known that addition of water to crystallizable acetic acid (glacial acetic acid) resulted in a decrease of the solidification point. This fact was used to purify the acid. For concentrated solutions of acid, water was added and the solution cooled down until it broke into a solid and liquid phase. The latter, richer in water, was separated and the residue submitted repeatedly to the same process. In a second procedure, vinegar (a weak liquid solution of acetic acid) was cooled down until partially solidified. In this case the liquid phase was richer in acetic acid while the solid one was water almost pure. This procedure had a lower limit, beyond it (today: eutectic point) addition of water resulted in an increase in the temperature of solidification. Grimaux was intent in extending Rüdorff's data to determine the minimum crystallization temperature (Grimaux, 1873).
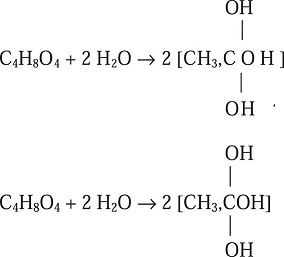
His experimental procedure was the following: A mixture of known composition was put in a small tube provided with an alcohol thermometer, which was calibrated before every measurement. The setup was introduced in a refrigeration mixture and cooled several degrees below the probable solidification temperature. It was then agitated to stop the sub-cooling and the increase in temperature up to the appearance of crystals was recorded. The acid used to prepare the synthetic mixtures solidified at 14.41 °C and contained 1.25 parts of acid per 100 of water (according to Rüdorff's tables). Grimaux remarked that his results should be considered relative and not absolute, because the solidification points observed were slightly higher than the fusion ones (!!). The experimental results indicated that the angular point (eutectic) occurred at a composition quite corresponding to the composition one molecule of acetic acid + two molecules of water, that is to say, one molecule of acetic acid hydrate combined with one mole of water [(36/96)(100) = 37.6%]. Grimaux justified this conclusion on the basis of a previous paper in which he had discussed the existence of hydrates of monobasic fatty acids. It was stated that lower fatty acids existed as di-acids (actually dimers), which dissociated as the temperature was increased. Using the theory of types Grimaux concluded that the hydrates of the lower fatty acids were unstable glycerin; at low temperatures they were present as a condensed anhydride of glycerin, while at higher temperatures they constituted direct anhydrides. Their solution in water was not only a dissolution, but hydration and splitting of a condensed anhydride, which he represented as follows (Grimaux, 1870):
According to Grimaux, the water-acetic acid eutectic contained between 37% and 38% water by weight and melted around −24 °C (Grimaux, 1873).
Alkaloids
According to Grimaux, inspection of the formulas of morphine (C17H19NO3) and codeine (C19H21NO3) showed that these two bases differed by a group CH2 and that codeine could be considered derived from morphine by substituting one hydrogen atom by a CH3 group. Work by other chemists had shown that the reaction of morphine with methyl iodide produced an isomer of codeine hydroiodide, which did not have the properties an alkaloid salt, was not precipitated by ammonia or KOH, and behaved like an iodide of quaternary ammonium. Heating morphine with HCl had resulted in the elimination of water and transformed it into apomorphine. The same reaction with codeine produced apomorphine and methyl chloride. These results indicated that morphine contained an alcoholic group (OH) and codeine an ether group (OCH3) but nothing about the possibility of transforming one base into the other. According to Grimaux, the properties of morphine suggested a similarity with phenols; it contained at least one phenolic hydroxyl so that codeine could be considered to be a methyl ether of morphine (Grimaux, 1881a).
In order to test this hypothesis, Grimaux heated one mole (Grimaux writes: one molecule) of morphine with an alcoholic solution containing one mole of NaOH and two mole of methyl iodide. The expected reaction was very fast, but accompanied by a second one. Instead of free codeine, it generated codeine iodomethylate, with a yield 85% of the theoretical value. Simultaneously with the double decomposition between methyl iodide and morphine, another part of methyl iodide combined directly with morphine. The latter was identical with the product of the reaction between codeine and methyl iodide. These results suggested that to obtain free codeine, it was necessary to reduce the initial amount of methyl iodide to one-half or less the original one. The experimental result indicated that free codeine was produced but with a very low yield (30%). Most of the methyl iodide participated in the formation of morphine iodomethylate. The codeine was extracted with ether and purified by standard procedures. The final products had all the properties of the same material extracted from ether (centesimal composition, fusing point, solubility in different solvents, the properties of its salts, and crystalline form) (Grimaux, 1881a).
Grimaux reported that substitution of methyl iodide by ethyl iodide yielded a new base, C19H23NO3, homolog with codeine, representing the ethyl ether of morphine, and which he named codethyline . It became crystallized into bright hard plates, less soluble in boiling water than codeine, very soluble in ether and alcohol, and melting at 83 °C. It was precipitated from its salts by KOH and alkaline carbonates, but not by ammonia. The results proved clearly that codeine was a methyl ether of morphine (Grimaux, 1881a).
Grimaux showed that this type of synthesis could also be carried using diverse chlorides, bromides, iodides, and similar compounds, such as allyl iodide, epichlorhydrin, and ethylene bromide, producing new bases which could easily be isolated (Grimaux, 1881c). He also showed that the rotatory power of synthetic codeine (α D = −130.34°) was basically the same to that of codeine extracted from opium (α D = −133.18°). To him, the difference was due to the small amount of synthetic product used (Grimaux, 1881b).
In a following publication Grimaux explained in more detail the preparation and properties of codeine iodomethylate, a compound having the properties of a quaternary ammonium iodide. This base was slightly soluble in water and very soluble in alcohol and ether. It crystallized as bright needles fusing at 118.5 °C. Boiled with aqueous KOH transformed it into methocodeine (methylmorphimethine), which acted on the body less energetically than codethyline. The reaction of codethyline iodomethylate with silver oxide and KOH and produced a tertiary crystallizable base, fusing at 132 °C, and similar to methocodeine, although less soluble in ether. Grimaux believed that this new reaction allowed the preparation of a whole new set of codeine homologues, containing alcoholic groups in a different position than the ones present in morphine (Grimaux, 1881e).
In 1873 Adolph von Baeyer (1835-1917) showed that aldehydes combined with two moles of aromatic hydrocarbons and phenols, in the presence of sulfuric acid, with elimination of water. Thus, formaldehyde reacted with two moles of benzene to form diphenylmethane and water (Baeyer, 1873). Grimaux thought that since morphine behaved like an aromatic phenol, it could be possible to use Baeyer's synthesis to produce a whole series of new derivatives. He mixed powdered morphine, or its solution in glacial acetic acid, with a few drops of methylene acetochlorhydrin, and an excess of sulfuric acid, and noticed that the resulting solution became immediately colored rose, and then changed to the color of concentrated potassium permanganate. Addition of water intensified the color and then destroyed it. Addition of ammonia resulted in the precipitation of a new yellow amorphous base, very soluble in alcohol, slightly soluble in ether, insoluble in benzene, and having the property of becoming immediately tinted violet purple in the presence of sulfuric acid. Grimaux believed that this new base had the formula CH2(C17H18NO3)2. Additional experiences showed that all the morphine ethers reacted in the same manner (i.e. codeine, codethyline, dicodethine, etc.) (Grimaux, 1881d).
Grimaux summarized all the above results in another paper published in 1882, where he added more details about the method of preparation and elemental analysis of codeine iodomethylate, codethyline (ethylmorphine) and its iodomethylate, dicodethine (ethylene morphine), and methocodeine (Grimaux, 1882a).
It was known that cinchonine, quinine, and other alkaloids could be transformed into quinoline (C9H7N) and its homologues, as well as into bases of the pyridine (C5H5N) series, but it was not known if the alkaloids contained the nucleus of these compounds, modified only by substitution, or in a hydrogenated state. In order to answer this question, Grimaux decided to prepare the products of the addition of bromine to quinoline and pyridine, and compare them with the corresponding products of cinchonine (Grimaux, 1882b).
For this purpose he added bromine drop-wise to an aqueous solution of quinoline. A red crystalline bromine smelling matter separated immediately, which was then dissolved in warm chloroform. On cooling, the solution deposited fine red needles of a very unstable compound, which in contact with air rapidly lost bromine and hydrogen bromide. Analysis of the needles indicated that the action of bromine on quinoline led to the formation of the tetrabromide, C9H7NBr4, which under the action of KOH or H2S regenerated pure quinoline. Boiling a chloroform solution of the tetrabromide transformed it into the hydrobromide of quinoline dibromide, C9H7NBr2,HBr, a stable compound, which separated as red brown crystals, very soluble in alcohol and ether, insoluble in water and chloroform, and melting at 86 °C (Grimaux, 1882b).
The reaction of bromine with pyridine was very similar; the corresponding addition product was very unstable and could be isolated by dissolving it in chloroform and immersing the solution in a refrigeration mixture. The resulting dark red needles, melting at 126 °C, were totally comparable to hydrobromide of quinoline dibromide, except in the amount of HBr it contained [(C5H5Br2)HBr]. It was soluble in water, alcohol, and ether, and treated with cold KOH it regenerated pyridine (Grimaux, 1882b).
In 1881, Zdenko Hanns Skraub (1860-1910) reported the synthesis of quinoline and some of its derivatives by reacting glycerin with a mixture of aniline and nitrobenzene in the presence of sulfuric acid (Skraub, 1881). Since in this reaction glycerin transformed first into acrolein, Grimaux thought that it was possible to use Skraup's reaction to synthesize quinoline bases by replacing glycerin by phenylacrolein (cinnamaldehyde) (Grimaux, 1883). Experience proved him correct; heating to 170-180 °C a mixture of aniline, nitrobenzene, sulfuric acid, and essence of canella, he obtained a black mass from he could separate fine needle crystals, melting at 84 °C, and having an elemental analysis corresponding to C15H11N, corresponding to a phenyl quinoline, C9H8(C6H5)N. The compound was very soluble in ether, slightly soluble in cold alcohol, more soluble in boiling alcohol, and yielded a crystallizable chlorydrate, sulfate, and chlorplatinate. According to Grimaux it contained the phenyl group in the pyridine side of quinoline, in the position para relative to nitrogen (Grimaux, 1883). Grimaux also used Skraup's method to synthesize the iodomethylates of quinine. In this work he also proved that in the basic salts of quinine the acid was not attached to the nitrogen of the quinoleic group but to the nitrogen of the other group, probably of piperidine nature. Equally, or more important, he found that the action of alkalis upon the di-iodomethylate resulted in a yellow powder that in concentrated alcoholic or acetic solution possessed a very intense fluorescent property, similar to that of fluorescein (Grimaux, 1892a). The findings of this paper led to the synthesis of several double salts of quinine (Grimaux, 1892b).
Skraup also used his method to synthesize para -methoxyquinoline (quinanisol) by reacting glycerin with anisole (methoxybenzene) in the presence of sulfuric acid. Grimaux went on to synthesize quino-phenetole (quinethole) by reacting glycerin with phenetole (phenyl ethyl ether) in the presence of sulfuric acid. He described quinethole as an oily liquid, which distilled without decomposition between 290° and 292 °C, and reacted with mineral acids to produce crystallizable salts. Acid solutions of the latter showed the fluorescence of quinine salts (Grimaux, 1895).
Grimaux and Albert Léon Arnaud (1853-1915) carried a series of investigations related to quinine. They proved that the quinine was the methyl ether of cupreine, a soluble base extracted from the bark of Quina cuprea (Grimaux & Arnaud, 1891a); they synthesized quinethyline, the ethyl ether of cupreine (Grimaux & Arnaud, 1891b); transformed cupreine into quinine di-iodomethylate (Grimaux & Arnaud, 1892a); they prepared the propyl, isopropyl, and amyl derivatives of cupreine (Grimaux & Arnaud, 1892b), and summarized their results in a long memoir (Grimaux & Arnaud, 1892c).
Grimaux, in collaboration with the physicians Jean Baptiste Vicent Laborde (1830-1903) and Henri Bourru (1840-1914), published some papers on the physiological and therapeutic action of quinine chlorhydro-sulfate (Grimaux & Laborde, 1893), and the homologues of quinine cupreine and methyl, ethyl, propyl, and amylcupreine (Grimaux, Laborde, and Bourru, 1894).
Colloids and albumins
According to Grimaux, albuminoid substances constituted in science a separate category, for which no analogs were known. The complexity of their molecules, the impossibility of determining their molecular mass, their easy coagulation under weak influences, their reciprocal transformations, etc. gave them a mysterious character. The first publications, which had provided a more precise knowledge about their nature, were those of Paul Schützenberger (1829-1897), who had split the complex building of albuminoid substances and shown that by hydration they converted into acid amides, ammonia, and urea (Schützenberger, 1864). Based on this property. Grimaux defined an albuminoid substance as a nitrogenous substance that on hydration split into CO2, ammonia, and acid amides. One of the first projects he carried on the subject was the synthesis of a colloid by the reaction between urea and aspartic anhydride (C32H26N8O17). The anhydride was prepared by heating to 200 °C aspartic acid hydrochloride in a stream of CO2. The product was a white powder, insoluble in boiling water. Heated with one-half its weight of urea it transformed into a thick mass, completely soluble in boiling water. The resulting solution was gummy, hardly filterable, and having all the properties of colloids. It was coagulated by acids, alkaline salts (potassium sulfate and nitrate, sodium sulfate, ammonia, and sodium acetate), by magnesium sulfate, aluminum sulfate, the salts of iron, mercury, and copper, and by tannin. All the respective precipitates were gelatinous and thick. The precipitates formed by HCl and nitric acid were soluble in a large excess of acid; addition of water separated again the viscous substance. The jelly produced by acetic acid dissolved in cupric sulfate produced the blue violet coloration observed under the same conditions with albuminoids, but was not coagulated by heat. Elemental analysis of the colloid led to the global formula C34H40N10O25, representing the union of eight molecules of aspartic acid with two molecules of urea, and elimination of two molecules of ammonia and nine of water (Grimaux, 1881f).
Grimaux believed that if it would be possible to prepare the mixed anhydrides of the residues of aspartic acid, leucine, tyrosine, etc., then it would be likely to transform them into amides approaching the nitrogen colloids present in living organisms (Grimaux, 1881f).
A further paper dealt with the preparation of another nitrogen derivative, which had the property of coagulating when heated. Grimaux treated amidobenzoic acid with phosphorus pentachloride and obtained a white powder, which he believed was an anhydride originating from the union, after dehydration, of several molecules of the acid. This powder was dissolved in ammonia and the resulting solution evaporated under vacuum, at room temperature. Initially, the amidobenzoic colloid formed a thick jelly, and afterwards dried into transparent yellow plaques, resembling serum albumin. The plaques swelled in cold water and were very soluble in hot water. Evaporation of the solution in a water bath left a residue resembling the original material, but completely insoluble in water, and soluble in ammonia, sodium phosphate, and alkalis. Solutions of this colloid behaved like diluted solutions of albumin. Addition of small amounts of different salts led to coagulation at a temperature, which depended on the quantity of salt added. Diluted solutions of albumin and the amidobenzoic colloid behaved equally under the influence of heat and salts. At sufficiently high dilution they were precipitated by a cold stream of CO2; this precipitate redissolved in a current of air. This result had been observed only with globulins (Grimaux, 1884d).
Grimaux showed that diluted solutions of albumin became coagulable under the same conditions as his amidobenzoic colloid, for example, after addition of small amounts of sodium chloride, calcium sulfate, magnesium sulfate, ammonium chloride, etc. Carbon dioxide exhibited the same action as these salts: bubbling it through a dilute solution of albumin resulted in coagulation, even upon slight heating. Addition of diluted acetic acid resulted in the precipitation of a jelly quite soluble in an excess of acid, and reappearing upon neutralization with an excess of alkali (Grimaux, 1884e).
In another memoir, Grimaux reported that reacting an alcoholic solution of ferric chloride with sodium ethylate led to the precipitation of sodium chloride and formation of a transparent dark red solution of ferric ethylate. Distillation of this solution left a black pasty mass, soluble in absolute alcohol, benzene, chloroform, ether, petroleum ether, and methanol. Heating the pasty mass under vacuum to eliminate the residual alcohol left a brown powder of ferric hydrate (Grimaux, 1884a).
The alcoholic solution of ferric ethylate did not react with dry ammonia but with CO2 it produced a brown ochre precipitate and with H2S, another of ferrous sulfide. Leaving the solution in contact with atmospheric air, or adding to it a small quantity of water, resulted in the coagulation of ferric hydrate. Addition of a larger amount of water produced a transparent liquid having the properties of the solutions of colloidal ferric hydrate described by Thomas Graham (1805-1869) (Graham, 1864): it coagulated spontaneously after a long period of time, or rapidly under the action of heat; it precipitated after addition of CO2, sulfuric acid, tartaric acid, or salts of potassium, sodium, and barium. Acetic acid, nitric acid, HCl, and ammonia, did not affect the solution. With H2S it produced a black precipitate. It was also observed that the coagulation time increased with dilution and lowering of the temperature. According to Grimaux, the coagulum was a thick transparent jelly, which retained enough water to fill the vase. Eventually the jelly contracted while releasing part of the water. All these characteristics showed inorganic colloids and the nitrogenous colloids of living organisms behaved in a similar manner (Grimaux, 1884a).
Another memoir described the preparation of a series of colloidal compounds derived from ferric hydrate. The alkali-ferric derivatives of polyalcohols such as glycerin, mannite, sugar, and glucose, were prepared by reacting an aqueous solution of ferric chloride with the polyalcohol and KOH. This solution coagulated under different conditions, depending on the relative proportions of water, alcohol, and KOH. In the absence of water the solution did not coagulate even on boiling, but did upon addition of water. All the solutions coagulable by heat were promptly precipitated by CO2; the precipitate was stable and did not disappear under any condition. Other ferric colloids prepared were ferric-potassium tartrate, ferric arseniate, ferric arsenite, borax, sodium phosphate, and soluble silica (Grimaux, 1884b).
Grimaux showed that the reaction of leucine with phosphorus oxychloride (phosphoryl chloride) produced an amorphous product, which after washed with boiling water, dissolved in alkalis. Addition of HCl resulted in the precipitation of a gelatinous material, soluble in ammonia. Evaporation of the excess ammonia yielded a liquor having all the properties of a colloidal solution; for example, if treated with cupric sulfate and KOH it produced the biuret reaction. Similar results were obtained when using a mixture of leucine and tyrosine (Grimaux, 1884c).
In 1857 Eduard Mathias Schweitzer (1818-1860) reported that a solution of copper hyposulfate or of cupric sulfate dissolved in an excess of ammonia (Schweitzer's reagent or cuprammonium) had the property of dissolving large amounts of cellulose (Schweitzer, 1857). It had also been established that the cupric oxide in ammonia was the agent that dissolved cellulose, and that the presence of foreign salts diminished substantially this particular activity. Eugène Melchior Péligot (1811-1890) studied the action of air on copper in the presence of ammonia, and recognized that the resulting liquor contained nitrous acid, originating from the oxidation of ammonia by atmospheric air. Peligot found that the hydrate of blue turquoise copper oxide (blue cinder) that precipitated when adding enough water to the reagent, could be redissolved in ammonia yielding an even better solvent for cellulose, silk, and other organic substances that resisted the action of common solvents. This solution contained a copper salt, which Peligot believed to be a nitrate. Later work showed that the nascent acid was nitrous acid because addition of silver nitrate precipitated silver nitrite (Peligot, 1861).
Grimaux found that dialysis of the above solution in a porous vessel showed that the ammonium cupric oxide was a colloidal substance. During the first few days a large amount of blue salt was found in the external part of the apparatus; after six or seven days it was found that the excess ammonium contained no copper. The porous vessel retained a blue liquid composed of ammonium cupric oxide, which totally resisted dialysis. When treated with distilled water, the liquid precipitated gelatinous flakes of copper hydrate; the amount of decomposition depended on the amount of water added. The solution was precipitated by small amounts of the sulfates of aluminum, calcium, or copper, and diluted acetic acid. Sodium chloride and potassium sulfate had no effect (Grimaux, 1884f).
Grimaux also studied the characteristics of the colloids prepared from condensed pyruvic ureides, from amido aspartic acid, and from soluble silica (Grimaux, 1884f).
In a following paper Grimaux wrote that all the experience he had accumulated allowed him to establish a theory of coagulation that explained all the phenomena observed during the act of coagulation, spontaneous or induced. In certain situations, the phenomenon was retarded by dilution, in others it was accelerated. The first situation took place with substances such as ferric chloride and soluble silica; here the molecules joined together, with elimination of water, by a slow reaction similar to a slow esterification. All the conditions that favored esterification, such as heat and salts (which behaved as dehydration agents) also favored coagulation; the presence of water retarded it. Coagulation could then be attributed to molecular condensation accompanied by water loss. For colloids, where water favored coagulation (e.g. ferric glicerate and ferric potassium tartrate), a slow decomposition (similar to a saponification) took place, accompanied by elimination of a substance different from water (e.g. the coagulation of ferric chloroarseniate was accompanied by the elimination of HCl). The progressive contraction of the coagulum was also due to slow and progressive condensation of the molecules (Grimaux, 1884h).
According to Grimaux, his theory allowed explaining certain phenomena that occurred during the dialysis of colloids, which Graham had attributed to the decomposing force of diffusion. The fact that dialysis of basic ferric chloride decomposed it into soluble ferric hydrate could be explained to the fact that water decomposed the salt into ferric hydrate and dialyzable HCl, which was eliminated by the porous membrane (Grimaux, 1884i).
Carbohydrates
The information available about dextrin indicated that it was a defined chemical species; several substances were known to have the general properties of the dextrin discovered by Payen and Jean-François Persoz (1805-1868) (Payen & Persoz, 1833), but possessing a different rotatory power and reducing ability. In 1886, Grimaux and L. Lefèvre reported that they had succeeded in transforming glucose into dextrin by distilling under vacuum, a solution of one part of glucose, and eight parts of HCl. The residue of the process was a dark amber syrupy mass. The latter was dissolved in its weight of water and treated with distilled alcohol until precipitation ended. The soft gummy precipitate was dissolved in water; the solution was then bleached by boiling in the presence of animal carbon, and left to cool. The white pulverulent precipitate was separated and dried and found to have the same appearance as commercial dextrin. It was very hydroscopic and produced an aqueous solution. Its rotatory and reducing powers were found to vary according to the number of time its was precipitated and redissolved in water. In addition, it was found to contain a certain amount of a fermenting sugar. The latter was eliminated by the action of beer yeast, leaving a dextrin having a reducing power of 17.8 (against glucose) and a rotatory power of +97.48° (Grimaux & Lefèvre, 1886).
According to Grimaux and Lefèvre, this synthetic dextrin seemed to belong to the achrodextrins family because it was not colored by iodine and was not affected by a malt infusion. It was slowly hydrated by diluted acids; boiled with sulfuric acid in partly transformed into glucose (70%) only after 20 h of contact. The regenerated glucose was easily fermented and converted into alcohol (Grimaux & Lefèvre, 1886).
Grimaux and Lefèvre found that their procedure transformed galactose (obtained from the splitting of milk), into galacto-dextrin (Grimaux & Lefèvre, 1886).
In 1861, Eugen Franz von Gorup-Besanez (1817-1878) reported the transformation of mannite into glucose and mannitic acid, under the action of moistened platinum black (Gorup-Besanez, 1861). Grimaux and Lefèvre thought that it might be possible to use the Gorup-Besanez procedure to oxidize glycerin to glyceraldehyde, a compound having the same elemental composition as glucose and being simultaneously an alcohol and an aldehyde, (Grimaux & Lefèvre, 1887). In their first experiments they treated dry glycerin with platinum black, not very active, and noted that glycerin acquired a strong reducing power as well as an acid character. The oxidation was very slow; after 960 h the reducing power reached a maximum value of 30-35 per 100 parts of glucose. Using a more active platinum black resulted in a very energetic reaction, forcing to carry the reaction with a 33% aqueous solution of glycerin. Under these conditions, the maximum reducing power was attained after 4-8 h. The product of the reaction was diluted with water and then concentrated by distillation under vacuum. The resulting mass reduced energetically Fehling's liquor, and gave a metallic mirror with ammonia silver nitrate; it also turned yellow when boiled with alkalis, limewater, and barite. Treated with a mixture of sodium acetate and phenylhydrazine hydrochloride it produced a colored precipitate, which could be separated by fractional crystallization into several hydrazine derivatives, some soluble in KOH and others insoluble in KOH. The production of hydrazine derivatives soluble in water suggested that the oxidation of glycerin with platinum black also produced an acid aldehyde (glyceric acid). According to Grimaux and Lefèvre, the most important characteristic of their product was its ability to ferment in the presence of beer yeast, releasing CO2 and forming alcohol (Grimaux & Lefèvre, 1887).
In a following work, Grimaux and Lefèvre reported that they had been unable to convert glyceraldehyde into glucose, using HCl as the aldolization reagent. Anyhow, they concluded that they had been the first to synthesize a fermentable aldehyde (Grimaux & Lefèvre, 1888a).
In a last publication, Grimaux and Lefèvre, wrote that in theory there were two isomers, which would be obtained by eliminating a hydrogen atom from glycerin: glyceraldehyde and dihydroxyacetone. This time, they were intent in synthesizing the latter, believing that it should also have the properties of a fermentable sugar. They were unable to so; instead of the target compound, they were able to synthesize its dioxethyl derivative:
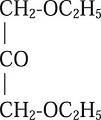
which they described as a colorless liquid, with aromatic odor and a cold sweet taste, having density 0.980 at 17.980 °C, soluble in alcohol and ether, and slightly soluble in water. It strongly reduced Fehling's solution and ammonia silver nitrate, with formation of a mirror deposit (Grimaux & Lefèvre, 1888b).











 nueva página del texto (beta)
nueva página del texto (beta)