INTRODUCTION
Whitefly Bemisia tabaci (Gennadius) is one of the main pests of Capsicum annuum in both greenhouse and field (Gangwar and Gangwar, 2018; Padhi et al., 2017). Direct damages caused by feeding include stomatal closure, formation of chlorotic spots on leaves, formation of anthocyanin pigment and inhibition of plant growth (Hilje and Stansly, 2018). The most important damage by B. tabaci is the transmission of geminiviruses, begomoviruses and criminiviruses in a wide range of horticultural crops (De Marchi et al., 2017; Hernández-Espinal, 2018; Polston et al., 2014).
Resistance to B. tabaci in landrace, semi-wild and commercial genotypes has been evaluated in various vegetable crop species, such as soybean (Glycine max) (Cruz and Baldin, 2017), tomato (Solanum lycopersicum) (Oriani et al., 2011; Rakha et al., 2017), eggplant (Solanum melongena) (Hasanuzzaman et al., 2016), melon (Cucumis melo) (McCreight et al., 2017), cassava (Manihot esculenta) (Mwila et al., 2017) and pepper (Capsicum annuum) (Ballina- Gomez et al., 2013; Firdaus et al., 2011; Jeevanandham et al., 2018). In genotypes of C. annuum different degrees of resistance to B. tabaci have been observed (Ballina-Gomez et al., 2013; Firdaus et al., 2011; Latournerie-Moreno et al., 2015). The wide genetic variation of C. annuum offers an opportunity to explore host plant resistance and to study the plant traits that influence such resistance. In this sense, the genetic and morphological variability of wild populations and landrace genotypes of C. annuum from Southern Mexico provides an important scenario to study the role of host plant on the interaction B. tabaci-C. annuum (Aguilar-Meléndez et al., 2009; Castañón-Nájera et al., 2008).
Resistance to B. tabaci in tomato genotypes has been clearly associated with the presence of glandular trichomes and acyl sugar content (Baldin et al., 2005; Leckie et al., 2016; Smeda et al., 2016). In pepper, host plant resistance to B. tabaci has been scarcely studied. A study by Firdaus et al. (2011) found strong negative association of oviposition rate with cuticle thickness and density of glandular trichomes. No additional studies have undertaken the leaf traits on the resistance of pepper to B. tabaci; thus, the objective of this study was to evaluate the resistance of C. annuum landrace genotypes to B. tabaci and to determine whether this response is correlated with specific physical or chemical leaf traits.
MATERIALS AND METHODS
Pepper genotypes
Fifteen landrace genotypes of C. annuum, including the cultivated Huero, Dulce, X´catic, Verde and the semiwilds Maax, Blanco, Pozol, Picopaloma, Miraparriba, Pijadegato, Crespo, Chawa, Sucurre, Amaxito and Simojovel were collected in Southern Mexico (Chiapas, Campeche, Yucatan). Seeds were extracted manually from dried fruits collected in the field, home gardens or local rural markets. The commercial genotype Jalapeño was included as a control for susceptibility to B. tabaci.
Planting and seedling management
Seeds were immersed for 48 h in 250 mg L−1 gibberellic acid (Plant Health Care, Mexico) under constant oxygenation by using an aquarium air pump (Elite®, USA) to homogenize germination. Seeds were sown in 200 square plug polystyrene trays (45 × 70 cm) in a sphagnum peat moss substrate (Cosmocel, Mexico). The substrate was maintained at 60 % moisture. The seedlings were fertilized with 19N:19P:19K (Poly-feed triple 19, Haifa, Mexico) at 2 g L-1 dissolved in the irrigation water. Thirty-five days after sowing, the seedlings were individually transplanted in 2 L pots containing substrate composed of red soil, peat moss and fine gravel in a weight proportion of 50, 30 and 20 %, respectively. Plants in pots were fertilized with 4.5 g L-1 of the previously described fertilizer. Plants were kept into a greenhouse at the Instituto Tecnológico de Conkal, where the experiments were carried out, for 10 days after transplanting plants (45 days after sowing).
Colony of Bemisia tabaci
Adult whiteflies (B. tabaci) were originally collected from habanero pepper (Capsicum chinense Jacq.) agroecosystems in Conkal, Yucatan, Mexico. The stock colony was reared on habanero pepper into a greenhouse at the Instituto Tecnológico de Conkal, Yucatan, Mexico. To have homogeneous cohorts of B. tabaci for the experiments,a separated colony was maintained and reproduced on healthy plants of habanero pepper in entomological cages (1.2 × 1.2 × 1.2 m) made of aluminum and anti- aphid mesh and placed under the same greenhouse as the stock colony (Ballina-Gomez et al., 2013). Adults from homogeneous cohorts (1-2 days old) were collected with a small mouth operated aspirator made of a 50 mL glass jar with two openings on the screwable cap, one opening was connected to 1-cm diameter rubber tube and the other one was connected to a 5-mm diameter rigid plastic tube.
The free ending of the rigid plastic tube was placed on leaves where B. tabaci adults were settled, while a gently suction was applied with the mouth to the free ending of the rubber tube. Adults were kept in the glass jar for no more than 5 min before being transferred to the entomological cages for the bioassay on oviposition preference or being transferred to a fridge at 4-5 ºC where they were briefly chilled (1-2 minutes) to immobilize. Immobilized adults were observed under the stereomicroscope. Females were separated based on body size and abdomen shape. Adult females were then immediately transferred to the clip cages with the aid of a fine camel-hair brush for the experiments on nymphal mortality.
Bioassay of oviposition preference
For oviposition preference, free-choice bioassay was performed as described by Ballina-Gómez et al. (2013). Healthy plants (45 days after sowing) were kept in entomological cages as previously described for whitefly rearing. Ten cages were set up for this experiment. A single cage contained 32 randomly distributed plants (two plants of each genotype). In total, 20 plants per pepper genotype were evaluated. A plant represented a replicate. Infestation of plants set in the entomological cages was carried out with unsexed adults. Individuals were released from the glass jar placed in the center of the cage. Each cage received 640 adults (average rate of 20 individuals per plant). Oviposition was evaluated 2 d after adult release in two leaves of the upper third section of plants. The number of eggs per leaf was recorded using a stereoscopic microscope at 40X (Leica Co., Jalisco, Mexico). After egg counting, leaf area (cm2) was individually measured with an area meter (LI3100, LiCOR, Lincoln, Nebraska, USA). Environmental conditions inside the cages were 18-35 °C of temperature, 65-95 % of relative humidity and natural photoperiod of 13:11 h (light:dark).
Evaluation of nymphal mortality
To evaluate nymphal mortality, the clip cage procedure was used to obtain a homogeneous cohort of nymphs as described by Ballina-Gómez et al. (2013) and Firdaus et al. (2011). Briefly, 20 adult females were confined in a section (1 cm2) of a fully expanded young leaf of a plant, after 24 h, the adults were removed. Some eggs were also removed to left groups of 25 eggs per leaf section. The leaf section with the eggs was enclosed again with the clip cage to prevent migration of first-instar nymphs. After 15 d after the eggs were laid, leaves were cut and nymphal mortality was evaluated by observing the leaf sections with nymphs in a stereoscopic microscope at 40X (BME; Leica, Jalisco, Mexico). Nymphs were considered dead if they were blackish, deformed or dry (Ballina-Gomez et al., 2013). Twenty leaves (one per plant) were evaluated per genotype. A leaf represented a replicate. A second assay of nymphal mortality was conducted with only four genotypes, including two that produced high mortality and two that produced low mortality of B. tabaci nymphs. The environmental conditions for the assays of nymphal mortality were the same as described for the oviposition preference study.
Evaluation of morphological leaf traits
Evaluations were performed in fully expanded young leaves of the upper stratum of plants. For all traits, a leaf represented a replicate. Each trait was assessed in a separated group of plants. Trichome density was evaluated in two leaves per plant. Forty leaves were assessed per genotype. The classification system consisted of five levels or classes of trichome density (Matos et al., 2011), where the approximate average of trichomes in 5 cm2 of leaf section was 0 for class 1, 8.7 for class 2, 73.6 for class 3, 211.7 for class 4 and 900.2 for class 5. Leaf area was measured with a leaf area meter (LI3100C, LI-COR, Lincoln, NE, USA) in two leaves per plant. Twenty plants (40 leaves) were assessed per genotype. Leaf toughness was determined using a TA Plus Penetrometer (Texture Technologies, Hamilton, MA, USA). Leaf toughness was reported as the force required to punch a 4- cm2 leaf section; the values were given in kg cm-2 s-1. One leaf per plant was used. Twenty plants were assessed per genotype.
Chemical composition of leaves
After performing the second assay of nymphal mortality on four pepper genotypes with differential responses on nymphal mortality, four leaves of the middle and upper strata per plant were cut and pooled, which made a group of 80 leaves in total per genotype. Then, four groups of 20 leaves were formed, washed with distilled water containing 5 % neutral detergent (Extran MA02 5 %) and rinsed with distilled water. Each group of 20 leaves was placed in a paper bag and dried at 45 ºC for 72 h. Four samples of 3g of dried leaves were taken per genotype. Each sample represented a replicate. The nitrogen content of samples was determined by the Kjeldahl method. The content of P and K was determined by the wet digestion method. Quantification of mineral elements was carried out by atomic absorption spectroscopy. Total phenolic content (g equivalent of gallic acid per g of dry mass) was determined by the Folin-Ciocalteau method and total flavonoid content (g equivalent of quercetin per g of dry mass) by the aluminum chloride colorimetric method as described by Hossain et al. (2011).
Experimental design and data analysis
The experiment was set under a completely randomized design. The pepper genotypes represented the treatments. All data were checked for normality and homoscedasticity. Results for oviposition, nymphal mortality, leaf area and leaf toughness in the 16 genotypes were analyzed by one-way analysis of variance and the Scott-Knott multiple comparison test. This test consists of a cluster analysis when a large amount of means is compared (Scott and Knott, 1974). Results of trichome density were analyzed by the nonparametric Kruskal-Wallis test. To determine the relationship between physical leaf traits with oviposition preference or nymphal mortality, an analysis of correlation was performed.
Data of the second assay of nymphal mortality and chemical composition of leaves were analyzed by analysis of variance and Tukey´s multiple comparison tests. All data analyses were carried out in InfoStat software (InfoStat, Cordoba, Argentina). Differences were considered significant if P ≤ 0.05.
RESULTS
Oviposition preference and nymphal mortality
Table 1 Response of Capsicum annuum genotypes to Bemisia tabaci and physical characteristics of leaves
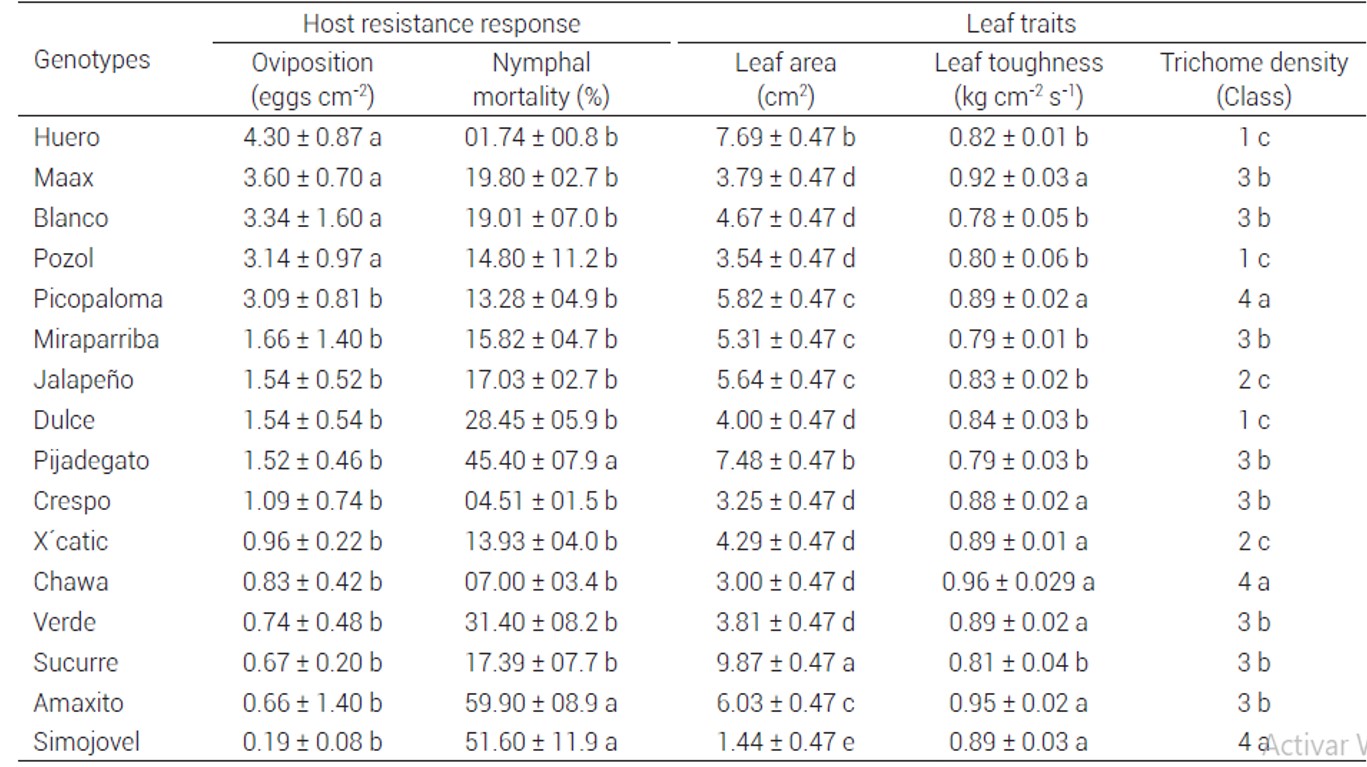
Mean values ±standard error with different letter within the same column are significantly different (Scott-Knott test, P ≤ 0.05). Values of trichome density are in categorical data, from a five-classes scale described by Matos et al. (2011).
Results showed significant differences in oviposition preference of B. tabaci among C. annuum genotypes (P= 0.0042). The density of whitefly eggs on the genotypes Jalapeño, Pijadegato, Crespo, X´catic, Chawa, Verde Sucurre, Amaxito and Simojovel (range of 0.19 to 1.7 eggs cm-2) was significantly lower than that of the genotypes Huero, Maax, Blanco, Pozol and Pico Paloma, with 4.3, 3.6, 3.3, 3.1 and 3.1 eggs cm-2 on their leaves, respectively (Table 1).
Nymphal mortality was significantly different among genotypes (P = 0.001). Nymphal mortality was higher on genotypes Pijadegato (45.4 %), Simojovel (51.6 %) and Amaxito (59.9 %) than on the other genotypes (Table 1).
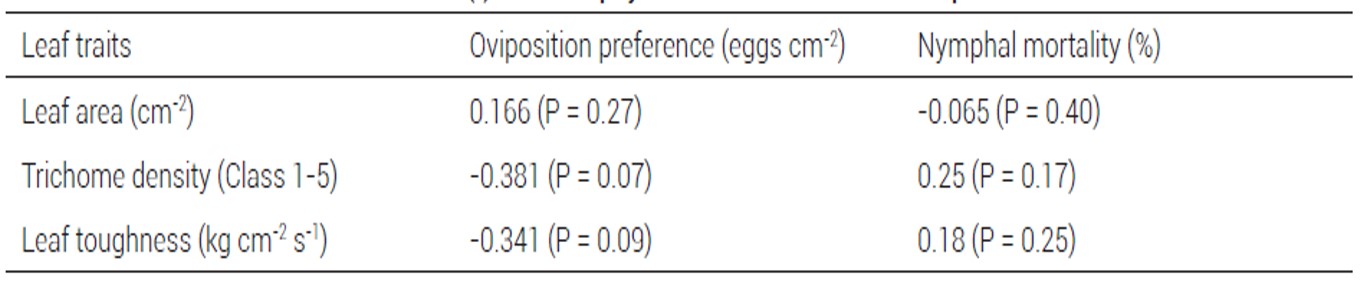
Table 2. Pearson correlation coefficients (r) between physical leaf traits and host response to Bemisia tabaci.
Physical leaf traits
Physical leaf traits (trichome density, leaf area and leaf toughness) varied among C. annuum genotypes. Leaf area showed significant differences among genotypes (P = 0.001). High density of trichomes (class 4) was observed in leaves of the genotypes Picopaloma, Chawa and Simojovel, whereas the lower density of trichome (class 1) was observed in leaves of Huero, Pozol and Dulce (Table 1). The highest leaf area was observed in the genotypes Sucurre (9.87 cm2), Huero (7.69 cm2) and Pijadegato (7.48 cm2), and the lowest leaf area was recorded in Simojovel (1.44 cm2) (Table 1). Leaf toughness was higher (P = 0.0001) in the genotypes Maax (0.92 kg cm-2 s-1), Picopaloma (0.89 kg cm-2 s-1), Crespo (0.88 kg cm-2 s-1), X´catic (0.89 kg cm-2 s-1), Chawa (0.96 kg cm-2 s-1), Verde (0.89 kg cm-2 s-1), Amaxito (0.95 kg cm-2 s-1) and Simojovel (0.89 kg cm-2 s-1) relative to that of the other genotypes (Table 1).
Slight negative correlation was observed between trichome density and B. tabaci egg density; likewise, between leaf toughness and egg density; however, these correlations were not significant (P > 0.05) (Table 2).
Chemical composition of leaves
The second assessment of nymphal mortality on four genotypes with differential response to B. tabaci showed that nymphal mortality on Simojovel (85.1 ± 3.9 %) and Amaxito (98.3 ± 2.3 %) was significantly higher than that on X´catik (11.8 ± 4.1 %) and Jalapeño (25.7 ± 6.5 %). Analysis of mineral elements in leaves of these genotypes showed that the content of N in Simojovel was significantly higher than that in Amaxito. In contrast, no differences were observed in content of P and K among genotypes (Table 3). Total phenolics and flavonoids in leaves were significantly different among genotypes. The genotype X´catic had the highest content of total phenolics, whereas the content of flavonoids was significantly higher in leaves of Jalapeño compared to that in Amaxito (Table 4).
Table 3 Mean content (± standard error) of N, P and K in leaves of Capsicum annuum genotypes.

.Mean values with different letter within the same column are significantly different (Tukey, P ≤ 0.05).
Table 4. Mean content (± standard error) of total phenolics and flavonoids in leaves of Capsicum annuum genotypes.
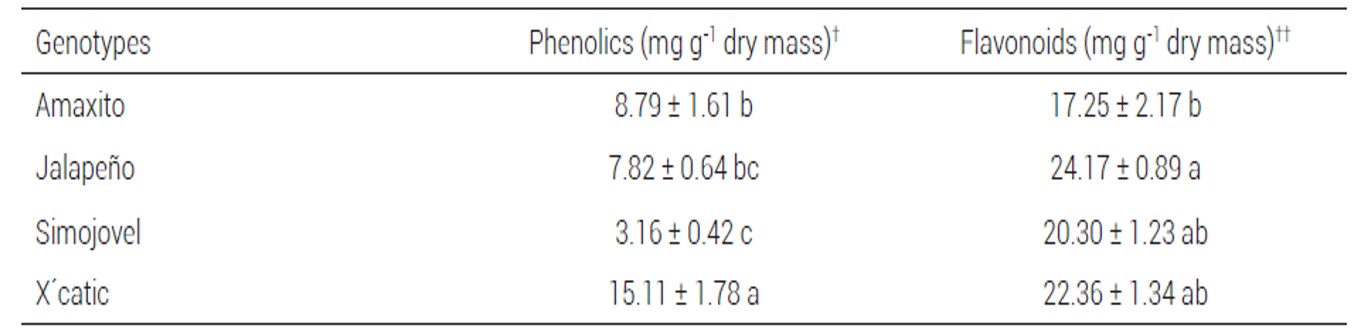
†For the calculation of total phenolics, the contents are presented as mg equivalent of gallic acid per g of leaf dry mass.
††For the calculation of total flavonoids, the contents are presented as mg equivalent of quercetin per g of leaf dry mass. Mean values with different letter within the same column are significantly different (Tukey, P ≤ 0.05).
DISCUSSION
Genetic diversity of pepper genotypes in Southern Mexico offers an excellent opportunity to study the response of landrace genotypes to B. tabaci, as well as to determine the possible influences of leaf physical and chemical traits on host plant response.
In the present study oviposition preference and nymphal mortality of B. tabaci varied among pepper genotypes. Genotypes that had low oviposition preference or caused high nymphal mortality on B. tabaci have strategies to counteract the settling and colonization by this pest. In general, low oviposition preference by B. tabaci has been related to antixenosis, whereas nymphal mortality has been related to antibiosis (Horas et al., 2018). The present study suggests that antixenosis was present in some genotypes as Simojovel, Amaxito and Sucurre, which showed low oviposition by B. tabaci. Also it is likely that antibiosis was present in some genotypes as Simojovel, Amaxito and Pijadegato, as high nymphal mortality was observed in these genotypes; however, this is difficult to confirm and more experimental evidence is needed to show that nymphs feed on these genotypes and nymphal mortality is the result of such feeding.
In other studies differential response of B. tabaci to C. annuum genotypes has been found, but the contribution of plant leaf traits was not assessed (Ballina-Gomez et al., 2013). In this study, in an attempt to determine possible associations between leaf traits and resistance to B. tabaci, the physical characteristics and the chemical composition of leaves were evaluated, with large variation only in physical leaf traits (leaf area, leaf toughness and trichome density) among genotypes. In agreement with this study, Matos et al. (2011) and Firdaus et al. (2011) documented high variation in morphological characteristics of leaves in C. annuum. Some of these characteristics, as trichome density and leaf toughness have been strongly related to host plant defense to phytophagous insects (Belete, 2018). In the present study there was only slight negative correlation between trichome density and oviposition preference, and between leaf toughness and oviposition preference, but no association was found between the morphological leaf traits and nymphal mortality.
Trichome density is considered one of the principal defense mechanisms against B. tabaci in solanaceaus crops (Leckie et al., 2016). Experiments on wild tomatoes and wild peppers showed that oviposition preference of B. tabaci and density of glandular trichomes had a negative strong correlation. High density of glandular trichomes resulted in high deterrence of landing and settling of B. tabaci and consequently in low oviposition rates (Firdaus et al., 2012; Rakha et al., 2017; Rodríguez-López et al., 2011). While glandular trichomes affect oviposition due to a wide array of volatiles and secondary metabolites that are emitted by these structures, regular trichomes affect oviposition as a physical barrier (Dalin et al., 2008; Rakha et al., 2017).
Leaf toughness is another well characterized mechanism involved in host plant resistance to phytophagous insects. In general, leaf toughness has been implicated in the decrease of feeding in phytophagous insects (Bellota et al., 2013; Dalin et al., 2008; Westbrook et al., 2011); however, in the present study, leaf toughness showed a slight negative correlation with B. tabaci oviposition preference, but not with nymphal mortality, suggesting that in pepper tough leaves could prevent oviposition by B. tabaci, but once oviposition has taken place, survival of nymphs is not affected.
The content of minerals in leaves may alter the preference and survivorship of a wide variety of phytophagous insects (Athey and Connor, 1989; Casotti and Bradley, 1991). Host plant resistance to phytophagous insects depends also on their nutritional components, such as the content of nitrogen, carbon and phenolic compounds (Lowman and Box, 1983; Ohmart et al., 1987). In the association B. tabaci-C. annuum, no studies have undertaken the influence of chemical composition of leaves on host plant response. In the present study, the content of N, P, K, total phenolics and flavonoids was measured in leaves of four genotypes with differential response to B. tabaci where two genotypes caused low nymphal mortality (Jalapeño and X´catic) and two genotypes caused high mortality (Amaxito and Simojovel).
Slight differences in foliar content of N were found among pepper genotypes, but no significant trend was observed between the content of N in leaves and the nymphal mortality. No clear trend were observed between the content of total phenolics/flavonoids and nymphal mortality either, even though the presence of these compounds in leaves has been strongly associated to the plant defense against leaf chewer and sap sucker insects (War et al., 2012). Overall then, no influence of chemical composition of leaves of C. annuum was found on nymphal mortality of B. tabaci.
In short, landrace genotypes of C. annuum have a differential response to oviposition preference and mortality of B. tabaci nymphs. Some genotypes, as Amaxito and Simojovel caused high mortality to B. tabaci nymphs. Physical traits of C. annuum leaves, as leaf area, leaf toughness and thrichome density were not associated to the oviposition preference or nymphal mortality of B. tabaci. Likewise, chemical traits of leaves, as the content of total phenolics, flavonoids and mineral content (N, P and K) were not associated with nymphal mortality of B. tabaci. Other host plant traits (chemical and physical) might be involved in mediating the interaction between B. tabaci and C. annuum.
CONCLUSIONS
Landrace genotypes of C. annuum showed differences in leaf traits (leaf area, leaf toughness and trichome density) and differential response to oviposition and nymphal mortality of B. tabaci. The genotypes Amaxito and Simojovel deter B. tabaci oviposition and caused high mortality on nymphs. Oviposition preference of B. tabaci had slight negative correlation with trichome density and leaf toughness, whereas nymphal mortality was not associated to any of these physical leaf traits. Genotypes with differential response to nymphal mortality showed no association between this trait and the leaf content of mineral elements (N, P and K), total phenolics or total flavonoids.











 nueva página del texto (beta)
nueva página del texto (beta)