Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Atmósfera
versión impresa ISSN 0187-6236
Atmósfera vol.23 no.3 Ciudad de México ene. 2010
Chemical composition of rainwater in northeastern México
E. RAMÍREZ LARA, R. MIRANDA GUARDIOLA, Y. GRACIA VÁSQUEZ, I. BALDERAS RENTERÍA
Facultad de Ciencias Químicas, Universidad Autónoma de Nuevo León, Av. Pedro de Alba s/n Ciudad Universitaria, San Nicolás de los Garza NL, C.P. 66451, México. Corresponding author; E. Ramírez Lara; e–mail: evangelina.ramirezlr@uanl.edu.mx
H. BRAVO ÁLVAREZ, R. SOSA ECHEVERRÍA, P. SÁNCHEZ ÁLVAREZ, A. ALARCÓN JIMÉNEZ, M. C. TORRES
Centro de Ciencias de la Atmósfera, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, México, D. F. 04510 México
J. KAHL
Department of Mathematical Science, University of Wisconsin Milwaukee, EMS Building Rm W 435, P.O. Box 413, Milwuakee, WI 53201, USA
Received September 9, 2009; Accepted February 15, 2010
RESUMEN
En el presente trabajo se investigó la composición química del depósito atmosférico húmedo en Monterrey capital del Estado de Nuevo León la ciudad industrial más importante del noreste de México, y donde la calidad del aire presenta serios problemas debido a las partículas suspendidas. El período de muestreo fue de enero a diciembre de 2007. La estación se localiza en la azotea de la Facultad de Ciencias Químicas de la Universidad Autónoma de Nuevo León la cual está localizada al norte del Área Metropolitana de Monterrey. Treinta y dos muestras de depósito húmedo fueron recolectadas utilizando un muestreador automático analizando concentración de iones (SO4–2,NO3–,Cl–,Ca2+, Na+,K+,NH4+) y conductividad. Los resultados muestran que el valor de pH promedio ponderado fue de 6.58 debido a la neutralización. La química del agua de lluvia muestra una alta concentración de Ca+2 y Mg+2 como especies catiónicas y de SO4–2 y Cl– como especies aniónicas. La baja concentración de H+ encontrada en las muestras de agua de lluvia de Monterrey N.L. sugiere que una parte importante de H2SO4 y de HNO3 fue neutralizada por partículas alcalinas presentes en la atmósfera. Para analizar la posible asociación entre los iones en las muestras de lluvia y consecuentemente la correlación con fuentes de emisión se aplicó el programa estadístico SPSS v.12. Se encontró una baja correlación entre los iones H+, SO4–2 y NO3– posiblemente debido a la neutralización. Las pedreras ubicadas en la localidad y la composición propia de las montañas pueden estar contribuyendo a las altas concentraciones de Ca+2 y Mg+2. Los resultados de esta investigación serán utilizados para evaluar la composición de la depositación atmosférica, la calidad del aire y para desarrollar estrategias para implementar medidas de control de las emisiones atmosféricas en el Estado. El presente trabajo representa la primera contribución de la química del agua de lluvia en el noreste de México.
ABSTRACT
The present study reports the chemical composition of atmospheric wet deposition in Monterrey, capital of the state of Nuevo León and the most important industrial city in northeastern México, where air quality presents a serious problem due to dust particles emitted. The sampling period was from January to December 2007. The station was mounted on the roof of the College of Chemical Sciences at the University of Nuevo León which is located in the north of the Metropolitan Area. Thirty–two samples of rain were collected with an automatic sampler and analyzed for pH, ion concentrations (SO4–2, NO3–, Cl–, Ca2+, Na+, K+, NH4+) and conductivity. The results show that the average weighted pH value of the rainwater was 6.58 due to neutralization. Rainwater chemistry showed high contribution of Ca+2 and Mg+2 in cations and SO4–2 and Cl– in anionic species. Low concentration of H+ found in rainwater samples from Monterrey N.L. suggests that an important portion of H2SO4 and HNO3 have been neutralized by alkaline particles in the atmosphere. In order to find possible association between ions in precipitation, and consequently, the possible sources of pollutants correlation study was applied using the program SPSS v.12. Weak correlations were found between the H+ ions and SO4–2 or NO3–1 because of neutralization. The local dust cement factories and surrounding limestone environment might be causing high concentration of Ca+2 and Mg+2. The results of this research will be used to evaluate the composition of atmospheric deposition, to evaluate air quality and to develop strategies to implement preventive measures and control of the atmospheric emissions in Nuevo Leon. This work represents the first study of rainwater chemical composition in the northeast of México.
Keywords: Wet deposition, acid rain, ionic species, conductivity, pH, chemical composition, precipitation chemistry.
1. Introduction
The issue of acid precipitation has received much attention in the international community for the last several decades because of its notable direct adverse effects on ecosystems and indirect effects on human health (Hu et al., 2003).
Recently innumerable efforts have been made to understand the physical and chemical processes responsible for the formation of acid species and their removal from the atmosphere. The chemistry of the rainwater has been subjected to numerous investigations during the last two decades due to the increase of environmental problems caused by acid rain. The composition of rainwater is important in understanding the role of transport of the soluble components of the atmosphere and the contribution of different sources of atmospheric pollutants.
The chemical composition of rainwater varies from site to site and from region to region, due to the influence of local sources. The processes controlling the composition of rain are complex and influenced by both natural and anthropogenic sources. If the source is influenced by increasing made man activities, the rainwater will become acid because the anthropogenic activities contribute acidic gases like SO2 and NOx and basic gases like NH3 (Kulshrestha et al., 2003). Acidity is mainly due to sulfur and nitrogen oxides emissions from fossil fuel combustion, which after being dispersed and being transported react chemically in the atmosphere before becoming wet or dry deposited as sulfuric and nitric acid or neutralized ammonium salts (Possanzini et al., 1988).
Several studies have reported potential ecological deterioration caused by acid rain, such as deterioration of forests, acidification of lakes and grounds, decay of marble, and degradation of buildings and ancient monuments (Samara et al., 1992; Galloway, 2001). The phenomenon known as acid rain, along with its effects on the deterioration of buildings and monuments, have reached the level of public awareness.
Special attention has been drawn to deterioration of buildings and ancient monuments (Dikaiakos et al., 1990; Samara et al., 1992; Cobourn et al., 1993; Bravo et al., 2006).
Considering the importance of acid rain and the relation that exists with the population growth of large cities, the aim of this paper is to gain an initial understanding of rainwater chemistry, including its composition and possible sources, at an urban locality in northeast México, where these kind of data have not previously been available.
2. Experimental
2.1 Demographic details of the study area
Monterrey, the third largest city in México, is located in Nuevo León State (Lat. 25°40'N; Long 100°18'W). Its average altitude is 537 m above sea level, and has an area of 580.5 km2 (Fig. 1).
The Monterrey Metropolitan Area is the third most populous city in México and is considered a high profile center of education, tourism and business, with a population of 4 000 000 inhabitants with 85% in urban areas.
The regional climate is classified as semi–arid, with tropical conditions interspersed with cold air incursions associated with North American frontal systems. Its weather, though reasonably pleasant in spring and autumn, is hot in the summer; the temperature average high reaches 35 °C (95 °F) in August, with an average low of 23 °C (74 °F). Winters are cool with an average January high of 19 °C (67 °F) and low of 8 °C (48 °F) The average annual precipitation is 615 mm, and is more prominent during May through September. Humidity in winter can be high, although snowfall is a very rare event.
Industrial activities and vehicular emissions are common potential pollution sources. More than 60% of the total emissions of NOx, CO, HC and Pb are due to vehicles, and 92% of SO2 emissions are to due industrial activities (INEGI, 2002).
2.2 Sample collection
Rainwater samples were collected continuously by event from January to December 2007. An automated wet–dry sampler was used (Anchor International, model TE–78–100), with high–density polyethylene buckets. The sampler was located on the roof of the Ciencias Químicas Building (12.3 m above ground level) of the Universidad Autónoma de Nuevo León, located in the urban municipality of San Nicolás de los Garza. Following rainfall events, the bucket was tightly sealed with a clean plastic lid to avoid contamination during transport to the laboratory (ASTM, 2004).
Upon arrival at the laboratory, samples were immediately measured weight, volume, pH and conductivity (Hanna HI model 255 pH/conductivity/temperatures).
Each sample was later filtered through 0.25 mm, 0.22 µm pore diameter cellulose membranes (Millipore) in order to remove particulate matter. All the filtered samples were stored in precleaned polyethylene bottles and refrigerated to 4 °C while awaiting major inorganic ion analysis by ion chromatography.
2.3 Chemical analysis
After collection and weighing, an unfiltered aliquot of each sample was used for pH and conductivity determination. The pH meter was calibrated before every measurement using standard buffer solutions of pH 4.00 and 7.00 (SCFI, 2000a). For conductivity measurements (25 °C), a conductivity meter was used after calibration with KCl standard solutions (SCFI, 2000b). Concentrations of H+ were calculated from the pH values.
A second aliquot of each sample, retrieved from the refrigerated polyethylene bottle, was used for the determination of major ion concentrations. The cations Na+, K+, Ca+2 and NH4+ were determined by ion chromatography using a Hewlett Packard 1090 with Hamilton PRP –x100 (150 x 4.1 mm) separation column and an Alltech 550 conductivity detector and for the anions Cl–, NO3– and SO4–2. All solutions were prepared with deionized water supplied by a Milli–Q–Millipore system, and with standards solutions prepared by dissolution of 99% purity analytical grade salts. In order to check the instrumental errors a quality standard solution of the anions and cations, as well as blank samples, were prepared with different concentrations and given random numbers among real samples. The quality assurance procedures included the routine running of blanks and control samples as well as replicate samples. The column and the other operating conditions employed for analysis of both the ions are reported by Bravo et al. (2006).
3. Results and discussion
3.1 Rain quantity
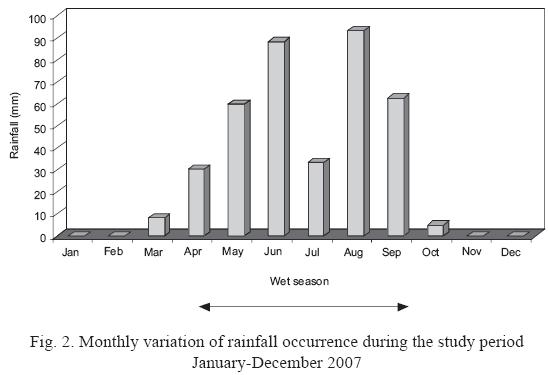
A total of 32 rainwater samples were collected. The largest number of monthly events occurred in August (7) followed by May (6), June (5) and July (5); no rain events were observed on January, November and December. Monthly rainfall during the study period is presented in Fig. 2. The highest rainfall was during August (93.5 mm), followed by June (88.5 mm) and September (62.8 mm). The months of January, February, March, October, November and December are considered the dry season.
3.2 Variation of pH
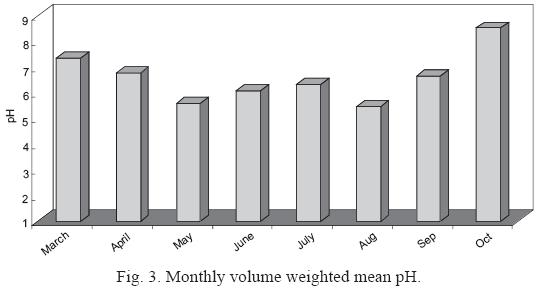
The pH of individual precipitation events ranged from 5.44 to 8.5, with both acidic and alkaline values. In this study the volume–weighted mean pH (VWM) value was 6.58, higher value than the widely accepted background rain pH of 5.6 (Charlson and Rhode, 1982). In comparison with other results (pH = 5.6) reported in México by Bravo et al. (2006), the results are quite similar. The pH of natural precipitation is controlled by dissolved CO2, due the interaction between water droplets and carbon dioxide. Precipitation pH is modified by the addition of both acidic and alkaline components (Topçu et al., 2002; Singh and Mondal, 2008).
Fig. 3 shows the temporal variation of volume weighted pH. The highest precipitation acidity (5.44) was associated with summer samples, especially in the period May to August 2007.
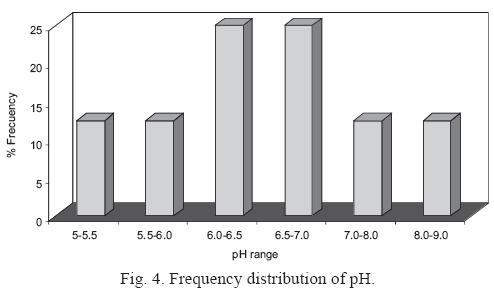
The frequency distribution of pH is shown in Fig. 4. Only 25% of the 32 rain samples showed a pH below 5.6, indicating that rainfall with above–background acidity is not common in Monterrey. Instead, the abundance of pH values above 5.6 indicate the presence of alkaline substance in the rainwater (Hu et al., 2006).
Alkaline precipitation has been reported in different parts of the world. In India, previous studies have recorded pH values ranging from 6.0 to 7.5 (Kemani et al.,1985; Saxena et al.,1996). Chandra Mouli et al. (2005) reported an alkaline VWM of 6.78. Another study, in the urban area of Ankara, Turkey, reported alkaline pH values due to high calcium (CaCO3) loading from alkaline soil (Topçu et al., 2002). Thus, the VWM pH of 6.58 for our Monterrey samples likely reflects a strong impact of alkaline soil dust on rainwater composition.
3.3 Chemical composition
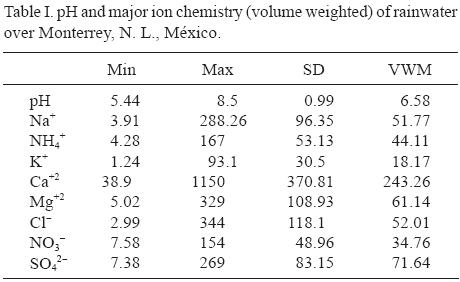
The variation of monthly mean concentration of major inorganic ions in rain water is illustrated in Table I. Volume weighted mean concentrations of cations can be ordered in descending order as Ca2+ > Mg2+ > Na+ > NH4+ > K+, with values ranging from 1150 to 93.1 µeq/L. The corresponding order of anions volume VWM concentration was Cl–, SO4–2, and NO3–, with values ranging from 344 to 154 µeq/L.
The variation of monthly mean concentration of major ions in rain water is illustrated in Fig 5.
Ca+2 and SO4–2concentrations are large because the Monterrey Metropolitan Area is surrounded by several mountains with numerous quarries and associated factories. The rock is composed basically of calcium carbonate. Aggregation operations are installed around those hills and represent a significant source of airborne particulate matter.
The high concentration of cations observed in March may be due to the small volume of rainfall. (Recall, from Fig. 2, March rainfall was only 8.2 mm compared with 93.5 mm in August.) Only one rain event was present in this month.
The smaller amount of rainwater is expected to produce higher concentrations of chemical species in rainwater due to reduced removal of suspended particles by wet deposition (Chandra et al., 2005).
During January, February, November and December no rain events were present in Monterrey's Metropolitan Area.
On the other hand, the increased anion concentrations during October may be due to the prevailing meteorological conditions in Monterrey that do not allow complete dispersion of NO2 and SO2 (INEGI, 2002).
3.4 Comparison with other selected sites
Table II shows volume–weighted mean concentrations in rainwater for this study and other results reported for different regions of México and selected sites worldwide. The pH of the present study (6.58) is higher than the other Mexican regions like El Tajín, Veracruz (4.58) (Bravo et al., 2006) and Estado de México (4.54) (García et al., 2006), but is similar to Tirupati, India (6.78) (Chandra et al., 2005). This may be due to the latter region having a high load of suspended alkaline atmospheric particles.
In this study Ca+2 is the most abundant cation. The relative high concentration of this ion suggests the effect of local anthropogenic emissions. The Monterrey Metropolitan Area is a major industrial city which has a serious atmospheric pollution problem due principally to the surrounding mountains, quarry hills and factories. These are composed basically of calcium carbonate and the excess calcium is believed to be due to particles originating from these sources (INEGI, 2002). India soils have also been considered to be a major contributor of particulate matter in the atmosphere and to alkalinity in the rainwater (Chandra et al., 2005)
The Ca+2 and Mg+2 concentrations in Monterrey are higher than concentrations reported by Bravo et al. (2006) for the El Tajín archeological zone in Veracruz, México and by García (2006) for Rancho Viejo, a rural zone close to México City, but lower than the concentrations reported by Nastos (2007) for Athens, Greece.
3.5 Source contribution
In order to find association between ions in precipitation, and consequently, the possible sources of pollutants and the gaseous reaction occurring in the atmosphere, the correlation between ions was calculated for all samples and presented in Table III. The acidic ions SO42– and NO3– present high correlation (r = 0.817) indicating their origin from similar sources, because of the similarity in their behavior in rain water and the co–emissions of their precursors SO2 and NOx (Chandra et al., 2005; Singh and Mondal, 2008). A significant correlation was observed between NO3– and Cl– (r = 0.648). On the other hand a strong correlation was observed between Ca2+ and Mg 2+ (r > 0.9), Ca2+ and NH4+ (r = 0.895) and Ca2+ and K+ (r = 0.984), suggesting the common origin of these ions from natural sources associated with crustal rather than anthropogenic or marine origin (Singh and Mondal, 2008). The high loading of Ca2+ and Mg2+ and the negative correlation between NH4+and NO3– (r = –0.439) and NH4+ and SO42– (r = –0.405) suggests a buffering factor for the acidity of the rainwater.
There is also a significant correlation between ion components and conductivity, except for the H+, NO3– and SO42– ions. Because of the significant neutralizing effect of calcium ions, H+ was not expected to have a good correlation with SO42– and NO3–.
Hydrogen ions are weakly correlated with SO42– and NO3–. Topçu et al. (2002) found similar results in Ankara, Turkey.
As can be seen in Table III, all ionic species except sulfate correlate negatively with rainfall amount (P), indicating an atmospheric washout process.
4. Conclusions
A study of the chemical composition of rainwater was carried out over a one–year period at Monterrey, Nuevo León, México the most important industrial state in northeastern México. This represents the first contribution to the literature of rainwater chemistry in the region.
The study reveals the following principal conclusions:
1. The rainwater is typically alkaline, with strong correlations between Ca+2 and other ionic species, revealing that the acidity is being neutralized.
2. The average pH of the rainwater was 6.58, perhaps due to neutralization. Only 25% of the rain samples had a pH below 5.6. This shows strong inputs of alkaline species to rainwater samples in this region. The average pH of samples higher than 5.6 is due to high loadings of calcium ions in the form of CaCO3. This may be due to a high load of alkaline dust particle by numerous quarry hills and factories. These are composed basically of calcium carbonate.
3. The rainwater chemistry is dominated by Ca2+, Mg2+ and SO42–. The principal cations and anions, in decreasing order, are Ca2+> Mg2+> Na+> NH4+> K+ and Cl–> S04–2> NO3–.
4. The cations Mg2+, NH4+, Na+ and K+ correlated strongly with Ca2+, suggesting a common natural source of crustal origin.
5. The low concentrations of H+ found in Monterrey rainwater samples suggest that an important portion of H2SO4 and HNO3 has been neutralized by alkaline particles in the atmosphere.
6. Due to neutralization by alkaline particles, the anions SO42– or NO3– are only weakly correlated with H+.
7. The monthly variation in ionic deposition is influenced by rainfall rate and ionic species concentration.
8. The correlation study and the comparison of major ion composition with other sites revealed that rainwater ion composition is strongly influenced by anthropogenic sources rather than terrestrial and marine sources.
Acknowledgements
The authors thank Universidad Autónoma de Nuevo León for financial support of this work.
References
ASTM, 2004a. Standard guide for choosing locations and sampling methods to monitor atmospheric deposition at non urban locations. American Society for Testing and Materials. D 5111–95. USA, 1–9. [ Links ]
ASTM, 2004b. Standard guide of materials used for preparation of materials used for the collection and preservation of atmospheric wet deposition. American Society for Testing and Materials. D 5012–89, USA, 1–5. [ Links ]
Bravo Álvarez H., R. Sosa Echeverría, P. Sánchez Álvarez, R. Soto Ayala and A. Alarcón Jiménez, 2005. Precipitación ácida en la costa del Golfo de México. In: Contaminación e impacto ambiental: diagnóstico y tendencias (A. V. Botello, J. Rendón–von Osten, G. Gold–Bouchot and C. Agraz–Hernández, Eds.) Universidad Autónoma de Campeche, Universidad Nacional Autónoma de México, Instituto Nacional de Ecología, México, 535–552 pp. [ Links ]
Bravo H., R. Soto, R. Sosa, P. Sánchez, A. Alarcón and J. Kahl, 2006. Effect of acid rain on building material of the El Tajín archeological zone in Veracruz, México. Env. Poll. 144, 655–660. [ Links ]
Chandra Mouli P., S. Venkata Mohan, S. Jayarama Reddy, 2005. Rainwater chemistry at a regional representative urban site: influence of a terrestrial source on ionic composition. Atmos. Environ. 39, 999–1008. [ Links ]
Charlson R. J. and H. Rodhe, 1982. Factors controlling the acidity of natural rain water. Nature 295, 683–685. [ Links ]
Cobourn W. C., K. Lal Gauri, S. Tambe, S. Li and E. Saltik, 1993. Laboratory measurements of sulfur dioxide deposition velocity on marble and dolomite stone surfaces. Atmos. Environ. 27, 193–201. [ Links ]
Dikaiakos J. G., C. G. Tsitouris, P. A. Siskos, D. A. Melissos, P. Nastos, 1990. Rainwater composition in Athens, Greece. Atmos. Environ. 24, 171–176. [ Links ]
Galloway J. N., 2001. Acidification of the world: natural and anthropogenic, Water Air Soil Poll. 130, 17–24. [ Links ]
García R., M. C. Torres, H. Padilla, H. Belmont, E. Azpra, F. Arcega–Cabrera, A. Báez, 2006. Measurement of chemical elements in rain from Rancho Viejo, a rural wooded area in the State of México, México. Atmos. Environ. 40, 6088–6100. [ Links ]
Hu G.P., R. Balasubramanian, C.D. Wu, 2003. Chemical characterization of rainwater at Singapore. Chemosphere 51, 747–755. [ Links ]
INEGI, 2001. Estadísticas de medio ambiente de la Zona Metropolitana de Monterrey. 2002. Instituto Nacional de Estadística, Geografía e Informática, México. [ Links ]
Khemani L. T., G. A. Momin, M. S. Naik, R. Prakasa, R. Kumar and M. Ramana, 1985. Impact of alkaline particulates on pH of rainwater in India. Water Air Soil Poll. 24, 365–376. [ Links ]
Kulshrestha U. C., M. J. Kulshrestha, R. Sekar, M. Vairamani, A. K. Sarkar and D. C. Parashar, 2001. Investigation of alkaline nature of rain water in India. Water Air Soil Poll. 130, 1685–1690. [ Links ]
Nastos P. T., D. Alexakis, H. A. Kanellopoulou and A. E. Kelepertsis, 2007. Chemical composition of wet deposition in a Mediterranean site Athens, Greece related to the origin of air mass. J.Atmos. Chem. 58,167–179. [ Links ]
SCFI, 2000a. Análisis de Agua–Determinación de pH–Método de Prueba. Secretaría de Comercio y Fomento Industrial, México. [ Links ]
SCFI, 2000b. Análisis de Agua–Determinación de la Conductividad Electrolítica–Método de Prueba. Secretaría de Comercio y Fomento Industrial, México. [ Links ]
Possanzini M., P. Buttini and V. Di Palo, 1988. Characterization of a rural area in terms of dry and wet deposition. Sci. Tot. Environ. 74, 111–120. [ Links ]
Samara C., R. Tsitouridou, Ch. Balafoutis, 1992. Chemical composition of rain in Thessaloniki, Greece, in relation to meteorological conditions. Atmos. Environ. 26, 359–367. [ Links ]
Saxena A., U. C. Kulshrestha, N. Kumar, K. M. Kumari and S. S. Srivastava, 1996. Characterization of precipitation at Agra. Atmos. Environ. 30, 3405–3412. [ Links ]
Singh A. S. and G. C. Mondal, 2008. Chemical characterization of wet precipitation events and deposition of pollutants in coal mining region, India. J. Atmos. Chem. 59, 1–23. [ Links ]
Topçu S., S. Incecik and A.T. Atimtay, 2002. Chemical composition of rainwater at EMEP station in Ankara, Turkey. Atmos. Res. 65, 77–92. [ Links ]














