Introduction
Arbuscular mycorrhizal fungi (AMF) perform a symbiotic association called mycorrhiza with roots of more than 80% of terrestrial plants; in general, AMF provides phosphorus (P), nitrogen (N), sulfur (S), potassium (K), calcium (Ca), copper (Cu), and zinc (Zn) in plant-soil (Smith, Facelli, Pope, and Smith, 2010; Tedersoo, Bahram, and Zobel, 2020). The AMF degrades organic soil matter, favoring mycelium growth and forming an extensive hyphal network that transports mineral nutrients in the water toward the plant interior (Jansa et al., 2019; Zhou et al., 2020).
Since AMF are obligate biotrophs, they require a host plant to complete their life cycle and substrate as propagation medium (Adinurani, Rahayu, Budi, Pambudi, and Soni, 2019). However, they are not host-plant specific, so under different conditions and crops, AMF species or consortia may be more efficient in promoting plant growth or productivity than others (Frew, 2019).
In particular, native AMF consortia are found better adapted to soil-climate conditions of a specific region than introduced ones, thus providing greater beneficial effects on plants (Mitra et al., 2019). Moreover, they have a different effect on plant growth than the use of inoculants with individual species (Vital-Vilchis, Quiñones, Hernández, and Rincón, 2020).
In the search for sustainable alternatives and environmental impact reduction, the use of organic waste, such as combined substrates, has turned out to be a viable alternative in plant production, aiming at reusing nutrients contained in these materials and reducing production costs (Mendonça et al., 2021).
Substrates play an important role in AMF reproduction and survival because they provide the necessary conditions for growth and root development in host plants, which allows greater efficiency of mycorrhizal symbiosis (Tzortzakis, Nicola, Savvas, and Voogt, 2020). The diverse substrates include leaf litter, rice straw, chickpea husk, dryland, and sand among others; in host plants, those that have been used are wheat, barley, sorghum, and maize, among others (Kadian, Yadav, and Aggarwal, 2018; Kadian, Yadav, Jangra, and Aggarwal, 2019; Sukmawati et al., 2021).
Bean is especially important for a developing world since it provides a source of proteins, calories, and trace elements to people that cannot allow themselves nutrition sources of high economic value (Myers and Kmiecik, 2017).
The objective of this study is to assess AMF consortia and three organic substrates to select those that produce greater agronomic quality and mineral content in common bean (Phaseolus vulgaris) plants.
Materials and Methods
Research was performed in a greenhouse at Universidad Autónoma Agraria Antonio Narro (UAAAN) in Saltillo, Coahuila, Mexico (25° 35’ 63” N and 101° 03’ 49” W, at 1581 m).
Arbuscular mycorrhizal fungi. The AMF used in this study were provided by the Microbiology Laboratory of the Horticulture Department at UAAAN, which were native of semi-arid zones of Coahuila endophytes and cataloged as: C1-ART (Glomus sp.1, sp. 2, and Claroideoglomus sp.1); C2-GEC (Glomus sp.1, sp. 2, sp. 3, Claroideoglomus sp. 1, Acaulospora sp. 1, sp. 2, and Gigaspora sp. 1.); C3-PAR (Acaulospora sp.1, sp. 2, sp. 3, sp. 4 and Glomus sp. 1.); C4-VIE (Acaulospora sp. 1. and sp. 2); C5-CUC (Glomus sp. 1, Acaulospora sp. 1, sp. 2, sp. 3, sp. 4, sp. 5., Funneliformes sp.1, and Acaulospora sp.1.); C6-SAC (Funneliformes sp.1, and Acaulospora sp.1.); C7-SAB (Acaulospora sp.1, sp. 2, sp.3, Claroideoglomus sp.1 and Glomus sp.1.); and C8-MUZ (Acaulospora sp.1, sp. 2, Glomus sp.1, and sp. 2).
Experimental design. The treatments were established under a complete randomized design, with a factorial arrangement including three substrates and eight consortia. Each treatment had four replicates, one plant per pot as an experimental unit.
Crop establishment. 1-kg capacity polystyrene pots (N = 96) were prepared to contain one substrate each one (Table 1): sugarcane bagasse (SB), coffee pulp (CP), and bovine manure (BM) and sand (1:1 p/p) mixture previously sterilized (thrice) in an autoclave (Felisa FE-398) at 121 °C for 15 min. Common bean (Phaseolus vulgaris) var. Pinto Saltillo seeds were inoculated at the moment of sowing with 50 g of soil containing 20 ± 3 spores of each consortium of the original isolate. The experiment was maintained for 75 days, (average temperature of 26 °C and 42% RH) under greenhouse conditions and with every other day irrigation with nutrient Steiner (1984) whose content is; 11.92, 6.92, 3.95, 1.31, 5.42, and 5.03 meq L‑1 de NO3, K, Ca, H3PO4, Mg and SO4 respectively with P at 75% during plant growth. The Irrigation sheet was from 0.50 to 1.50 L plant-1∙day-1 according to plant growth, in seedlings 25 to 50%, during plant development to 75% until flowering starts.
Table 1: Chemical properties and substrate mineral content.
| Substrate | pH | EC | N | P | K | Ca | Mg | Fe | Zn | Mn | Cu | |
| 1:2 | dS m-1 | % | µg g-1 | - - - - g kg-1 - - - - | - - - - - - µg g-1 - - - - - - | |||||||
| SB | 7.27b | 1.14b | 0.49ª | 27.25a | 7.59a | 4.38a | 2.68b | 6.19b | 9.81a | 16.20a | 0.33a | |
| CP | 7.57ab | 1.44a | 0.59ª | 5.43c | 2.72b | 3.94a | 2.98ab | 12.37a | 8.14a | 8.96b | 0.45a | |
| BM | 7.65a | 1.06b | 0.66ª | 21.06b | 2.36b | 3.41a | 3.12a | 6.53b | 3.26b | 5.88b | 0.48a | |
Data (measurements, n = 3). SB = sugarcane bagasse; CP = coffee pulp; BM = bovine manure; EC = electrical conductivity.
Determined variables. All the plants were measured in height, stem diameter, leaf number, root length, and fresh biomass. Then, samples were dried in a stove for 48 h at 75 °C and weighed to obtain dry biomass. The number of spores was counted by the method of Gerdemann and Nicolson (1963). For this purpose, 100 g of substrate were weighed and placed in 1 L-1 capacity volumetric flasks adding distilled water until the gauging volume was completed; the content was shaken for several minutes until completely homogenized, and then passed through three sieves vertically set from greater to lesser pore diameter of 400, 125, and 38. The residues (125 and 38 µm) obtained in the sieves were recovered with 20 mL of water and placed in 50-mL centrifuge tubes, to which 30 mL of 70% sucrose were added for centrifuging at 3000 rpm for 5 min. To the residue obtained, 5 mL of distilled water were added in a Petri dish and observed in the stereoscope with 40X for spore counting.
Colonization percentage was performed in triplicate in bean roots (Phillips and Hayman, 1970), which were cut into 1 cm pieces and placed in test tubes. Then, 10 mL of KOH at 10% were added and placed in a water bath for 5 min at 75 °C, adding H2O2 at 3% (1 min) and acidifying with HCl at 2% (5 min). The roots were washed with distilled water between each reagent added and finally stained with Trypan Blue Solution (Thermo Fisher Scientific, Waltham, MA, USA) at 0.05% (10 min water bath or 24 h at room temperature). Subsequently, color was eliminated, and the roots were placed in a lactoglycerol solution. Once stained, they were placed on lamellae, using lactoglycerol, observing with an optical microscope at 40X, and recording mycorrhizal fungal structures (arbuscules, vesicles, or hyphae) in cortical cells and root segments. Three fields of each segment were observed applying McGonigle, Miller, Evans, Fairchild, and Swan (1990) formula.
Both pH and electrical conductivity were measured with a potential-conductivity meter (Hanna HI 98129, Woonsocket, RI, USA) in an aqueous medium substrate (1:2 v/v for pH and 1:5 v/v for EC). Substrate and dry plant biomass N were determined by micro Kjeldahl (Kjeldahl, Novatech, Avante Tecnología, Jal, MX) (Bremner and Mulvaney, 1982); P in substrates, dry biomass with acid digestion in HNO3 and HClO4 in ultraviolet-visible (UV-vis) spectrophotometry (Biomate 5, Thermo Electron Scientific Madison, WI, USA) (Hanway and Heidel, 1952); and minerals, such as K, Ca, Mg, Zn, Cu and Fe in atomic absorption spectroscopy (Xplor AA dual, GBC Scientific Equipment (Hampshire IL USA) (AOAC, 1990).
Statistical analyses. A factorial analysis (substrates and consortia) and Tukey’s test (multiple comparisons of means, P ≤ 0.001) were performed with SAS 9.2 software for Windows (SAS Institute Inc. 2010). For mineral content in bean plant leaf and root, a principal component analysis (PCA) was performed in biplot charts with Infostat software version 2020.
Results and Discussion
Substrate characteristics. Substrate pH was slightly alkaline and statistical differences were observed among them (Table 1); pH may influence substrate nutrient availability toward the plant, which is why pH should be regulated when this type of substrate is used (Gayosso-Rodríguez, Borges, Villanueva, Estrada, and Garruña, 2018); electrical conductivity was higher in coffee pulp (CP) substrate but lower than 2 dS m-1 in all the other substrates, which is acceptable for Pinto bean crop (Quintana-Blanco, Pinzón, and Torres, 2016); N, Ca and Cu contents did not show significant differences; sugarcane bagasse (SB) substrate showed greater contents of P, K, Zn and Mn whereas CP was higher in Fe and SB in Mg. The use of substrates with greater nutrient quantity increases plant biomass content (Caballero-Salinas, Ovando, Núñez, and Aguilar, 2020).
Agronomic parameters in bean plants. Significant differences (P ≤ 0.001) were found among the substrates (Table 2). The plants that reached the greater height, root length, and total fresh biomass were those cultivated in the (CP) substrate; those that had greater stem diameter, leaf number, and total dry biomass were cultivated with (SB).
Table 2: Comparing agronomic variable averages of the different substrates and consortia in Pinto bean plants.
| Substrate | Plant height | Stem diameter | Leaf number | Root length | Fresh biomass | Dry biomass |
| cm | mm | cm | - - - - - g - - - - - | |||
| SB | 39.94b | 5.00a | 25.88a | 21.00b | 28.44b | 6.23ª |
| BM | 27.66c | 3.87b | 15.88c | 16.59c | 12.59c | 2.14c |
| CP | 41.72a | 3.70b | 22.84a | 23.81a | 33.94a | 5.60b |
| Consortia | ||||||
| C1-ART | 28.42d | 4.28ab | 18.50c | 19.58bc | 20.58cd | 2.51e |
| C2-GEC | 33.58c | 4.26ab | 15.50d | 15.42d | 20.17d | 3.56d |
| C3-PAR | 37.83b | 4.66a | 22.83b | 18.58c | 28.75a | 4.16d |
| C4-VIE | 36.25bc | 4.08b | 24.67ab | 22.17a | 24.08b | 5.26c |
| C5-CUC | 43.08a | 3.58c | 18.25c | 22.25a | 23.00bc | 4.97c |
| C6-SAC | 32.67c | 4.43ab | 23.33b | 23.17a | 28.58a | 5.97b |
| C7-SAB | 37.92a | 4.14b | 25.58a | 22.25a | 24.33b | 7.09a |
| C8-MUZ | 41.75a | 4.09b | 23.58ab | 20.33b | 30.42a | 3.71d |
| Substrate*Consortia | ** | ** | ** | ** | ** | ** |
| C.V. (%) | 7.80 | 7.61 | 7.99 | 6.70 | 7.75 | 10.88 |
Data (means, n = 4) SB = sugarcane bagasse; CP = coffee pulp; BM = bovine manure; C.V. = Coefficient of variation; ** = highly significant. Averages with the same letter within each column do not differ statistically (Tukey’s test, P < 0.001).
The application of native consortia showed highly significant differences (P ≤ 0.001) in all the agronomic variables assessed (Table 2), of which the C7-SAB consortium was the one that showed differences in four out of six agronomic variables assessed (plant height, leaf number, root length, and dry stem biomass), while C3-PAR consortium reached greater results in stem diameter and fresh biomass. These results can be explained since a greater AMF diversity in consortia is considered beneficial for plant growth because they perform a variety of ecological functions, which supplement functionally by using resources more efficiently through differential spatial abilities (Crossay et al., 2020).
The interaction of both substrate*consortium factors (Figure 1) showed significant differences, obtaining the best results in plant height combined with SB substrate and C5-CUC consortium, as well as CP substrate and C3-PAR consortia; stem diameter was greater in the combination SB with C4-VIE and C6-SAC consortia; root length had a better result with CP substrate and C4-VIE consortium; leaf number was greater with CP substrate and C3-PAR consortium, as well as SB substrate and C6-SAC, C7-SAB and C8‑MÚZ consortia; fresh biomass was greater with CP and C3‑PAR consortium; dry biomass with SB substrate and C7-SAB consortium.

Figure 1: Interaction substrate*consortium effect (a) plant height; (b) stem diameter; (c) leaf number; (d) root length; (e) fresh biomass; and (f) dry biomass. C1-ART; C2-GEC; C3-PAR; C4‑VIE; C5-CUC; C6-SAC; C7-SAB; C8-MUZ. Data (averages ± standard deviation, n = 4) with different letters above the bars indicate significant different among interactions (Tukey’s test, P < 0.001).
Organic substrates provide nutrients that increase aerial and radicle biomass, which make host plants less limited in mineral resources to greater plant growth (Ma et al., 2021). The inoculated AMF consortia developed a symbiotic relationship with plant roots releasing a variety of organic acids modifying rhizosphere pH medium, improving growth and increasing nutrient and water absorption (Wahid et al., 2019).
The high nutrient content in the substrate and the biological efficiency of the AMF consortia increased the metabolic functions related to growth and development because of nutrient absorption and translocation by the roots to the plant (Tamayo-Aguilar, Riera, Alfonso, Juárez, and Rodríguez, 2019).
Other studies agree with the results obtained applying different AMF genera, using SB and CP substrates in maize (Quiñones-Aguilar, López, Hernández, Ferrera, and Rincón, 2014). Weisany, Zehtab, Raei, Sohrabi, and Ghassemi (2016) obtained greater plant height and underground and aerial biomass in chickpea and beans. However, the results differ from Wahid et al. (2019), who reported greater shoot and root length and biomass while assessing bovine manure soil inoculating AMF in mung bean (Vigna radiata).
The substrate with the adequate structure and porosity with native AMF causes greater radicle development and hypha exploration in the medium and with it water and nutrient supply besides biomass and leaf area in wheat and maize plants (Coccina et al., 2019; Al‑Maliki, Al-Amery, Sallal, Radhi, and Al-Taey, 2021).
Root colonization and spore number. A greater result was observed with the (CP) substrate and C2-GEC and C4-VIE with 46.01% and 45.61% of colonized roots, respectively (Figure 2a). The greatest spore number was obtained with the CP substrate and C2-GEC, C3-PAR, and C4-VIE consortia with 201, 188, and 198 spores in 100 g of soil, respectively (Figure 2b). The absence of radicle colonization and AMF spores in treatments where no consortium application existed showed the efficiency of the substrate sterilization procedure.
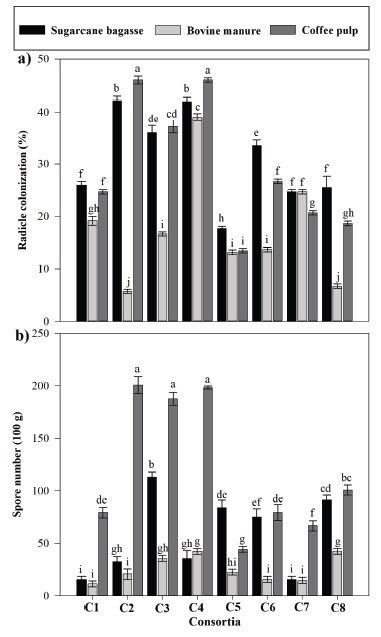
Figure 2: (a) Radicle colonization and (b) spore number in 100 g of Pinto bean plant sample. C1-ART; C2-GEC; C3-PAR; C4‑VIE; C5-CUC; C6-SAC; C7-SAB; C8-MUZ. Data (averages ± standard deviation, n = 4) with different letters above the bars indicate significant different among treatments (Tukey’s test, P < 0.001).
The chemical composition and nutritional content in the substrate used were different, which caused diverse results in radicle colonization since organic and inorganic medium compounds act as an energy source for the microorganisms, causing an increase or decrease in microbial activity (Zancanari, Silva, Maltoni, and Cassiolato, 2020).
The P content in substrates where plants developed was lower in CP, which influenced being the substrate with the greatest colonization and spore number since an increase in this element availability affected AMF abundance and efficiency (Ceulemans et al., 2019; Ma et al., 2021). With this respect, Sharma, Sharma, and Aggarwal (2015) reported AMF increase in population and colonization in different substrates because the medium structure was modified when a different organic substrate was used. On the other hand, Zancanari et al. (2020) obtained low sporulation from 11-29 spores / 100 g of dry soil while using soil-sugarcane ash as substrate, the same as in this study.
Mineral content in bean leaf and root. The principal component analysis (PCA) revealed substrate and consortium grouping assessed in bean leaf mineral content (Figure 3a), of which (CP) substrate and consortia stood out with the increase in Mg, K, Mn, P, and N. In contrast, (SB) substrate and consortia were greater in Ca, Cu, and Zn whereas bovine manure (BM) substrate and consortia only increased Fe content. The PCA with the mineral content of bean root (Figure 3b) showed marked groupings of substrates and consortia, of which SB was superior in P, Mn, Cu and Fe content. In contrast, BM substrate increased mineral content of Ca, K, Mg, and Zn while CP and consortia increased N content in the root.

Figure 3: Principal component analysis substrate*consortium (a) mineral content in bean leaves and (b) mineral content in bean root.
The use of AMF genera of different substrates, showed favorable results in nutrient and mineral medium absorption, because these reduce the distance between cations and roots, and promote plant growth (Raklami et al., 2019).
Greater radicle biomass growth constitutes an important mechanism to improve nutrient availability (Wen et al., 2019). AMF application modifies Fe and Zn acquisition in the function of the chemical soil-substrate characteristics and efficiency of the radicle system; when levels are low and soil is alkaline, radicle colonization increases absorption of these micro elements in the leguminous plants (Ingraffia, Amato, Frenda, and Giambalvo, 2019).
In contrast to this research study, Martín-Alonso, Llerena, and Acosta (2018) reported that combining bovine manure and AMF increased P absorption because of an improvement in nutrient retention and exchange capacity. AMF inoculation increased N, P, Ca and Mg, contents in root shoot aerial biomass (Iqbal, Ahmed, Isik, Sultana, and Ortaş, 2021); in basil plants, with Glomus species and plant growth-promoting rhizobacteria (PGPR) P content increased (Khalediyan, Weisany, and Schenk, 2021); P and K increased in roots compared with sprouts in plants inoculated with AMF (Bi et al., 2021).
Conclusions
The results of this study conclude that the use of organic substrates and inoculation of native consortia increased plant development of bean crop positively, as well as mineral content in leaves and roots. Furthermore, colonization and number of spores increased where sugarcane bagasse (SB), coffee pulp (CP), and C2-GEC and C3-PAR were the best substrates and consortia, respectively, that produced better effects in bean, as an opportunity to being used as biofertilizer and applied in agricultural crops.
Availability of Supporting Data
The sets of data used or analyzed during this study are available through the corresponding author upon reasonable request.
Authors’ Contributions
In charge of the assembly of the experiment, sampling and field and laboratory work, in addition to the statistical analysis: J.R.P.J. Carried out the formulation of the project and participated in the data collection, revision and final edition of the manuscript: R.M.V. Contributed to the review and contribution of suggestions: R.G.C.C. and J.A.G.F. Collaborated in the interpretation of the results generated: V.R.T. Contributed to the review of the structure and conclusive wording of the paper: L.G.H.M.











 nueva página del texto (beta)
nueva página del texto (beta)



