Introduction
Mexico is the most important center of the world of cacti concentration. Cactologists recognize the existence of 913 taxa, making up 669 species, which are grouped into 63 genera (Guzmán et al., 2003). By geographic region, it can be found the greatest diversity in the Tehuacán - Cuicatlán valley (Puebla and Oaxaca), followed by the Barranca de Metztitlán and the Balsas depression (Guzmán et al., 2003). The “cañada del zopilote” is an area located within Balsas river depression and is recognized as a physiographic area with a high concentration of taxa, among them is S. zopilotensis (Arreola-Nava and Terrazas, 2004). In this region, cacti have great cultural, nutritional and ecological importance, being the sustenance and shelter of great diversity of other plants and animal species (Alanís and Velazco, 2008). The natural populations of many species have been affected by the pressures of human development, mainly due to the conversion of natural areas for agricultural and/or livestock uses, also the plants extraction from their habitat for sale as decoration plants in national and international markets affect their populations, so it is important to implement effective propagation technologies to minimize the impact on wild species (Jarvis, 1979; Sánchez-Mejorada, 1982; Fuller and Fitzgerald, 1987).
Most plant seeds are exposed to very extreme seasonal periods of time during which they can be damaged or die if they do not have some protection mechanism. Dormancy is the most widely used mechanism in many seeds from cacti species, and is commonly induced by unfavorable conditions (cold or extreme dry heat). This phenomenon can be defined as a state of suspended or slowed growth and metabolism. Lethargy is a survival mechanism against frost, drought, etc. and it is necessary for populations of many plant species; it must occur in due time (that is, before adverse conditions reach lethal intensity) and last long enough, it must be interrupted when conditions are ideal for growth and development to resume (Matilla, 2008). The main mechanisms that cause lethargy in the seed are: 1. Environmental factors such as light, temperatures and absence of water; 2. Internal factors: such as test characteristics, embryo immaturity, ethylene concentration, presence of inhibitors, absence of growth promoters, and 3. Timing mechanisms: post-maturation, inhibitors blocking and synthesis of growth promoters (Benítez-Rodríguez et al., 2004; Benítez, 2009
1; Flores et al., 2006; Sánchez-Soto, 20032).
The germination process consists in water absorption, metabolism reactivation and growth initiation; most seeds begin to germinate as soon as they are wet, as long as the temperature, light and cold pretreatment conditions are optimal (Baskin and Baskin, 1998; Gutterman, 1993). It is important to know the optimal germination conditions for the efficient establishment of production units such as seedlings and nurseries. Germination and establishment are the most critical stages in life cycle of plants, because they are more vulnerable to environmental stress, competition, predation and diseases (Angevine and Chabot, 1979; Fenner, 1985). Little information was found on the optimal germination conditions of S. zopilotensis; many cacti need specific conditions to carry many of these physiological processes. Ithas been reported that in some Stenocereus species, that the seeds increase their germination percentage with the storage age both in constant and fluctuating temperatures (Rojas-Aréchiga et al., 2001). The effect of previous soaking in seeds of different cacti has been reported as a treatment can increase the germination percentage (De Carvalho et al., 2008; Gonzalez-Cortés et al., 2018). Based on the above, the objective of this study was to evaluate treatments of immersion in water and hormonal solutions of S. zopilotensis seeds on the break of dormancy and germination.
Materials and Methods
Description collecting area. 150 fruits were collected from five S. zopilotensis plants near the community of Xalitla, municipality of Tepecoacuilco de Trujano, Guerrero. The research material collection area was located on slopes with slight slopes, due to hills located to the east, with regularly flat areas that have been used for agriculture; The area is located at an altitude of 740 meters above sea level. In the western part is the Zopilote River, which receives the Coloapan and Huacapan rivers currents, which descend from the south-eastern region and flow only in the rainy season (INEGI, 2017). Based on the Köppen classification, the climate is warm semi-humid with summer rainfall; the annual mean temperature was 29.7 °C and precipitation was 586.16 mm (CFE, 20173). In the study area, the vegetation type is low deciduous forest, in which predominate species are: Ceiba aesculifolia "ceiba", Cyrtocarpa procera "berraco", around 20 Burseras species, as B. morelensis, B. xochipalensis, B. lancifolia, B. longipes among others (Ávila et al., 2010); Zizyphus acuminata "corongoro", Lysiloma tergeminum "goat leg", Euphorbia schlechtendalii "milkweed", Neoevancia zopilotensis and Neobuxbaumia sp. "cactus mezcala ".
Seed drying and storage. Physiological maturity fruits of S. zopilotensis "tuna pelona" were collected, were taken to the plant physiology laboratory of the Academic Unit of Agricultural and Livestock and Environmental Sciences in the University of Guerrero in Iguala, Guerrero, Mexico; where the seeds were extracted (5000 for all the study). The seeds were washed under running water and dried in the shade under room temperature conditions. The seeds were stored in cool conditions (17-19 °C) to try to keep their viability.
Latency break treatments. The collected seeds (same collection) were divided into two groups based on the storage time, the first by 70 dac (days after collection) and the second by 273 dac based on other studies (Alvarez-Espino et al., 2014). Each group seeds were stirred and randomly separated into sets of 100. The seeds were deposited in plastic containers, in each one a set of 100 seeds was placed (repeat) where the different treatments were applied. The established treatments were: T1: no soaking; T2: 6 hours soaking in 500 mL of water; T3: 12 hours soaking in 500 mL of water; T4: 24 hours soaking in 500 mL of water, and T5: 6 hours soaking in 500 mL plant-based hormonal stimulant Biozyme TF( (gibberellins - AIA - Zeatin, 32.3 - 32.2 - 83.2 mg L-1 respectively), Seed Anatomy and Water Uptake in Relation to Seed Dormancy in Opuntia tomentosa (Cactaceae, Opuntioideae) (Alvarez-Espino et al., 2014). Five repetitions per treatment were used, giving a total of 5000 seeds, 2500 for each group (70 and 273 dac). Once the latency break treatment was finished, the seeding was carried out, for this, plastic Petri dishes 8.5 cm in diameter and 1.4 cm high were used, to which circles of paper towels (sanitas() were placed, before placing the seeds on the paper, were moistened with running water and maintained at 20±2 °C.
Seed germination. From the seed placement, germination was recorded in all treatments every 24 h, this due to the responses observed in previous studies. for the statistical analysis, the germinated seeds were quantified and at 5, 10 and 14 days after treatments application (based on the germination period commonly found in cacti) in both groups (70 and 273 dac); during this time, moisture of Petri dishes was kept by adding running water. The experiment was replicated for each type of seed.
Experimental design and statistical analysis. The experimental design was a completely random factorial design, product of five treatments and two types of seeds (70 and 273 dac), using five repeats (100 seeds group) per treatment, giving 50 experimental units total. The experimental units where the treatments were applied were randomized using the “design.ab” procedure in the “R” statistical program, the above to ensure the independence of the observations. With the data without transforming of germinated seeds, an error normality distribution analysis was performed using the Shapiro-Wilk test and homogeneity of variances analysis with the Bartlett test; An analysis of variances test and comparison of means was performed using the Tukey test (P ≤ 0.05) with the statistical software SAS V.9.4 (SAS, 2013).
Results and Discussion
The germinated seeds records per treatment (five repetitions sum) in both groups are shown in Table 1. During the three evaluation dates, a constant behavior was registered in the treatments with six, 12 hours soaking in water and six hours soaking in Biozyme using seeds with 273 days of storage; Although germination in the first days was minimal, it increased significantly on the second evaluation date and an equally high peak was presented on the third (Table 1).
Table 1: Register of accumulate germinated seeds of S. zopilotensis using dormancy breaking treatments.
Control |
6 h in water |
12 h in water |
24 h in water |
6 h in Biozyme‡ |
||||||
dat† |
70 dac |
273 dac |
70 dac |
273 dac |
70 dac |
273 dac |
70 dac |
273 dac |
70 dac |
273 dac |
5 days |
0 |
0 |
8 |
8 |
6 |
0 |
0 |
0 |
0 |
2 |
10 days |
7 |
4 |
18 |
198 |
12 |
87 |
17 |
2 |
3 |
177 |
14 days |
12 |
10 |
30 |
243 |
15 |
124 |
25 |
14 |
11 |
222 |
† Days after treatments; dac = days after collection. ‡ Mix of Gibberellin - AIA - Zeatina, 32.3 - 32.2 - 83.2 mg L-1 respectively.
Consistently, the dormancy breaking treatments obtained significant results regarding the germinated seeds; Furthermore, it was evident that using of seed with 273 days of storage yielded the best results (Figure 1).
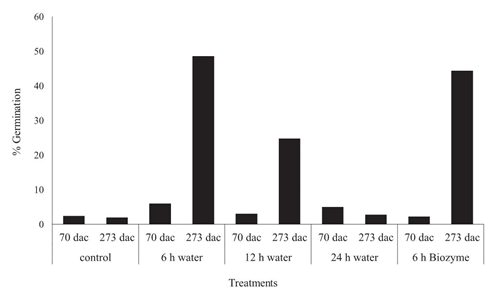
Figure 1: Seeds germination of S. zopilotensis through the interaction of dormancy breaking treatments and storage at 14 days after treatments application.
Analysis of variances test of third data record showed that, with respect to “storage time” factor, the highest levels of germinated seeds were obtained with the material of 273 days of storage (Table 2). Other authors have reported different germination patterns of cactaceous seeds with respect to storage time: seeds that lose viability at year, seeds that remain viable and germinate similarly for up to two years, and seeds where germination increases with breaking dormancy at 1 to 2 years old (Flores et al., 2005, 2008; Flores and Jurado, 2011). Similar to that reported in the present work, Flores et al. (2005) observed in some cacti greater germination levels in seeds of one-year-old or more, than in newly obtained seeds; this was also correlated with seed size; Flores et al. (2005) concluded that higher germination levels were determined in small 4-year-old seeds of Turbinicarpus sp. compared to larger young seeds; this could be related to the capacity of water absorption or as a survival strategy; thus they may become almost completely dehydrated during times of drought, shutting down all metabolic processes until water becomes available again (Ogburn and Edwards, 2010). It is documented that the hydrating capacity of water generates changes in the turgor of plant cells; This turgor is directly related to the enzymatic and metabolic activity; Usually this activity presents a Gaussian behavior with respect to the turgor and the water potential of the tissue, in this case of the seed; the above could explain the behavior of S. zopilotensis seeds in this study, where imbibition times greater than 6 hours caused a drop in germination percentage (Bradford, 1995; Kierzkowski et al., 2012). Embryonic immaturity is a factor that can cause innate dormancy, so seeds need a later maturation period to germinate, and this varies for each species (Rojas-Aréchiga and Vázquez-Yanes, 2000). This has been reported in various cacti species such as Eriocereus bonplandii and Mammillaria zeilmanniana, whose seed germination rate increased with age (Zimmer, 1967, 1969); likewise, young seeds of Ferocactus latispinus var. spiralis germinated less than 50%, while 45 month old seeds germinated more than 80% (Zimmer, 1980). This has also been demonstrated in Opuntia rastrera seeds (Mandujano, 19954, Mandujano et al., 1997) and Sclerocactus polyancistrus where the seeds must be "aged" before germination can occur (May, 1994). For several cactus species, a period of maturation or aging after harvest is necessary to occur germination or to be high (Rojas-Aréchiga and Vázquez-Yanes, 2000); Also, these authors mentioned that the Information concerning the dormancy and germination behaviour of cactus seeds comes from the pioneering studies of Alcorn and Kurtz (1959) and McDonough (1964), which demonstrated that light has a stimulating effect on the germination of Carnegiea gigantea and Stenocereus thurberi.
Table 2: Means multiple comparison of germination percentage of S. Zopilotensis seeds of 70 and 273 dac in the first trial.
dac = days after collection. * Means with different letters in the same column differ statistically according to the Tukey test (P < 0.05).
The statistical analysis determined that, with respect to the factor "latency breaking treatment", the best option to induce the germination starting process was T2: 6 hours soaking in water and T5: 6 hours soaking in hormonal stimulant Biozyme TF(, regardless of seeds storage time (Table 3). This indicates that in the presence of water, hydration sensitizes the seed tissues, and they respond by activating metabolic activity which starts the germination process, coinciding with what was proposed and previously mentioned by Ogburn and Edwards (2010); beyond 6 hours of soaking in water, the metabolic activity decreases, modifying this process. It is important to mention that, although the soaking treatment in running water for 6 hours was the most successful, a good part of the seeds did not germinate, so it is necessary to look for more alternatives that significantly increase the germination percentage (Matilla, 2008). A seed with 5-10% water content has a very negative water potential, so it tends to imbibrate very quickly, this rapid phase of water absorption causes temporary alterations in the seed membranes differential permeability, and consequently, a solutes loss to the surrounding environment; also lossing of different low molecular weight metabolites such as sugars, organic acids, ions, amino acids, germination inhibiting peptides (such as phenols) and ABA (Matilla, 2008). After the initial imbibition phase, the membranes regain their more stable configuration and the loss of solutes is reduced, a few moments after the imbibition of the viable seed begins, their metabolic activity resumes (Matilla, 2008).
Table 3: Means multiple comparison of the germination percentage of S. Zopilotensis seeds under latency breaking treatments.
Treatments |
Mean |
T 6 h |
27.60 a* |
T Bio |
23.30 ab |
T 12 h |
13.90 bc |
T 24 h |
3.70 dc |
T 0 h |
2.20 d |
* Means with different letters in the same column differ statistically according to the Tukey test (P < 0.05).
Similar results to ours were reported by Sánchez-Salas et al. (2006), who found that in Astrophytum myriostigma the best treatments for germination were distilled water and cooling, while the treatment with the lowest percentage of germination was mechanical scarification. Similar results were reported by Navarro and Deméneghi, (2007); who applied dormancy break treatments in M. pectinifera, they found that the seeds germination percentages varied between the different treatments, the highest percentage (95%) was with the Control (water) treatment, the gibberellin and sulfuric acid treatments registered similar values 85 and 80% respectively, while the lowest value (50%) was obtained when seeds immersed in Tween(. Similarly, Villanueva et al. (2016) reported that when applying dormancy breaking treatments in E. platyacanthus and M. pectinifera seeds, the highest average germination rates and germination speed were observed in water immersion treatments, this was previously reported by Mihalte et al., 2011. this suggests hydration sensitizes the seeds so that they can respond quickly to other environmental factors that trigger germination processes, such as enzymatic activation, synthesis of ABA inhibitors, as well as during the phase of seeds imbibition with PDND (physiological dormancy not deep) the several genes induction that respond to GAs (giberellins), phytohormones that lead to the embryo development and the subsequent emergence of the radicle through the testa takes place (Matilla, 2008). In addition to the above, the factorial design allowed analyzing the combinations between storage time and latency breaking treatments, ANOVA confirmed that the best combination to induce germination in "tuna pelona" was using seeds of 273 days of storage and apply treatments: soaking in water (6 hours) or soaking in Biozyme TF( hormonal stimulant (6 hours) and the second best option was soaking in water for 12 hours, these results could indicate the imbibition time as a determining factor, however, it is advisable to extend the study by adding combinations of hormonal solutions and soaking times to determine the effects of these variables. (Table 4).
Table 4: Means multiple comparison of germination percentage of S. Zopilotensis seeds of 70 and 273 dac under latency breaking treatments.
Treatments |
Group (dac) |
Mean |
T 6 h |
273 |
48.60 a* |
T Bio |
273 |
44.40 a |
T 12 h |
273 |
24.80 b |
T 6 h |
70 |
6.60 c |
T 24 h |
70 |
4.60 c |
T 12 h |
70 |
3.00 c |
T 24 h |
273 |
2.80 c |
T 0 h |
70 |
2.40 c |
T Bio |
70 |
2.20 c |
T 0 h |
273 |
2.00 c |
* Means with different letters in the same column differ statistically according to the Tukey test (P < 0.05).
Conclusions
During the three evaluation dates, a constant behavior was registered in the treatments with six, 12 hours of soaking in water and six hours of soaking in Biozyme using seeds with 273 days of storage, the best combination to induce germination in "tuna pelona" was using seeds with 273 days of storage and apply the treatments soaking in water for 6 hours or soaking in the Biozyme TF( hormonal stimulant for 6 hours. It is necessary to extend the study with other mixtures of hormonal solutions and imbibition times in order to confirm what is proposed here; It is also suggested continuing the study in the growth phase to know the effect of the treatments on the development of the seedling.
Data Availability
The data sets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Funds
There were no public or private financing funds for the execution of this study and all expenses were made by the authors.
Authors’ Contribution
Conceptualization: B.P.O., A.M.O., and T.R.R. Methodology and fund acquisition: A.M.O. and B.P.O. Formal analysis, writing, revision and edition: B.P.O., Y.D.T., and P.G.E. Supervision and project management: A.M.O., and T.R.R. All authors read and approved the final manuscript.











 nueva página del texto (beta)
nueva página del texto (beta)



