Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de análisis de la conducta
versión impresa ISSN 0185-4534
Rev. mex. anál. conducta vol.36 no.2 México ene. 2010
Artículos de investigación empírica
Pre–exposure to sweeteners on glucose and sucralose consumption
Pre–exposición a endulzantes sobre el consumo de glucosa y sucralosa
Alma Gabriela Martínez Moreno, Felipe de Jesús Díaz Reséndiz, Claudia Patricia Beltrán–Miranda, José Guadalupe González Rentería, Laura Margarita Munguía Hernández y Diana Merced Venancio López
Centro de Investigaciones en Comportamiento Alimentario y Nutrición (CICAN) CUSur — Universidad de Guadalajara
Correspondence to:
Alma Gabriela Martínez Moreno,
Centro de Investigaciones en Comportamiento Alimentario y Nutrición,
CUSur–Universidad de Guadalajara. Av. Prolongación Colón s/n,
Cd. Guzmán, Mpio. de Zapotlán el Grande,
Jalisco. CP. 49000. Tel. (01 341) 575 22 22, ext. 6127.
E–mail: alma.martinez@cusur.udg.mx
Recibido: Abril 17, 2010
Revisado: Junio 9, 2010
Aceptado: Junio 28, 2010
Abstract
Previous research reported that rats demonstrated large consumptions of solutions made up with glucose and that they did not seem to show preference for solutions made up with sucralose. Those findings suggested that the animals showed large consumptions of the sweet solution, as long as it contains calories. Although, the sequence to which the animals are exposed could contribute on the observation of these responses: history of flavor consumption may alter the consumption of new foods. In the present study, a group of rats was exposed to two sweeteners: glucose and sucralose at different times, with the purpose of determining whether the history of consumption of a sweetener with calories may modify the consumption of another sweetener without calories, and vice versa. The results demonstrated that the subjects showed great consumptions of glucose, regardless of the previous exposure to sucralose, and that the consumption of sucralose was not affected by the previous consumption of the glucose. These findings suggest that the previous consumption of sugars seems not to have an effect on subsequent sweet solution consumption.
Key words: sweeteners, history of consumption, glucose, sucralose, rats.
Resumen
Investigaciones previas reportaron que las ratas muestran grandes consumos de soluciones compuestas por glucosa y que no parecen mostrar preferencias en el consumo de soluciones compuestas por sucralosa. Estos hallazgos sugieren que los animales muestran grandes consumos de agua endulzada mientras la solución disponible contenga calorías. Sin embargo, la secuencia en la que los animales son expuestos a los endulzantes podría contribuir para observar estas respuestas alimentarias: la historia previa de sabores puede alterar el consumo de nuevos alimentos. En el presente estudio se expuso a un grupo de ratas a dos endulzantes: glucosa y sucralosa en diferentes momentos, con la finalidad de determinar si la historia de consumo de un endulzante con calorías puede modificar el consumo de otro endulzante sin calorías y viceversa. Los resultados mostraron que los sujetos mostraron grandes consumos de glucosa independientemente de la exposición previa a la sucralosa y que el consumo de sucralosa no fue afectado por el consumo previo de la glucosa. Estos hallazgos sugieren que el consumo previo de azúcares parece no afectar la conducta de consumo posterior.
Palabras clave: endulzantes, historia de consumo, glucosa, sucralosa, ratas.
Introduction
Several studies have evaluated the nourishment pattern of the rat since the availability of natural and artificial sweeteners (Ackroff, Manza & Sclafani, 1993; Ackroff & Sclafani, 2004; Bello & Hajnal, 2005). Martínez and López–Espinoza (2007) presented three groups of rats with free access periods of food+water, alternating them with periods of food deprivation. After the subjects were deprived, they had food and a water+glucose solution available. Each group received a different solution: high, medium, or low. They reported that the subjects doubled, tripled, and quadrupled the consumption of water+glucose regarding the intake of water, demonstrating that the increase in beverage consumption could have resulted from the conditions generated by the deprivation periods and to the characteristics of the sweetener used: the flavor and energy content.
In another study, Martínez, López–Espinoza, Díaz and Valdés (2009) exposed rats to sweetened solutions in free access periods. A group was exposed to food+water, followed by a period of access to food+water+glucose and a second group received food+water+sucralose. This cycle was repeated two more times. They pointed out that even though the glucose and the sucralose have the same flavor, only the first contains calories. The results demonstrated that the group that received the glucose showed great consumption of water+glucose, in relation to the registered consumption in the periods of water access, while the group that received the sucralose sweetened solution did not show variations between the consumption of water and water+sucralose. The differences of sweetened water consumption between the groups were considerable. The authors concluded that the energy content seems to be a predominant stimulus for animals to increase the consumption of sweetened water.
This evidence shows that the post–ingestive consequence of the energy content of glucose represents a more powerful stimulus than the flavor for the presence of large consumptions. To strengthen this hypothesis, the current experiment has as its purpose, to analyze water consumption, water+glucose, water+sucralose, food, and calories in free access conditions, when the animals receive in their diet a sweetener with calories and a sweetener without calories. In this way it would be possible to determine if the increase of the consumption of water+glucose may be affected by the previous consumption of a sweetener without calories, or if the previous consumption of a natural sweetener can produce an increase in the consumption of water+sucralose. In other words, we asked whether the history of consumption of a sweetener would condition the consumption of another sweetener.
Method
Subjects
A group of 16 naïve Wistar rats, eight females and eight males from the colony housed at University Center of Health Sciences of University of Guadalajara were used. Subjects were three months old at the beginning of the experiment. All procedures of this study were realized according to ethical guidelines for research with animals of The Official Mexican Norm (NOM–062–ZOO–1999).
Apparatus and materials
Sixteen individual home boxes, 13cm high, 27cm wide, and 38cm long, made up from metallic bars and a section for a feeder and trough. To record food consumption and body weight a precision electronic scale was used. The animals received food from the commercial brand Harlan 501, specific for laboratory animals, with its nutritional formula was as follows: 3% fats, 7% ashes, 1% calcium, 23% protein, 6% fiber, 49% NFE (nitrogen–free extract), 0.6% phosphorus, and 12% humidity. Three different solutions were used water was used and during the experimental manipulation, a solution of diluted 8% glucose and a solution composed of 2% sucralose. Two sweeteners were chosen for their calories and flavor. Each gram of sugar contributes 4 calories. In turn, the sucralose is a sweetener without calories that is produced from sugar; therefore its flavor is similar to table sugar. The sucralose molecule passes through the body without being metabolized and is eliminated after consumption. The liquid was provided in graduated troughs of 270 ml, unique for laboratory rats, they had anti–spill nozzles designed to prevent gas entry.
Procedure
The subjects were identified with a number, date of birth, and body weight at the beginning of the experiment and were placed in individual home boxes. Body weight, liquid consumption, food, and calories were registered daily at 8:00. The subjects were taken from its home box to the work table. They were placed on the scale to register its body weight and afterwards returned to its box. Then, food was weighed and the total consumption was calculated by subtracting the obtained amount from the daily given amount (50 g), then the food was replaced in the feeder. Finally, the solution consumption was registered by subtracting the marked amount on the trough to the daily amount of liquid offered (270 ml). Afterwards the solution was replaced and the troughs were sealed and placed in their designated space in the box. To calculate the calories consumed, the number of grams consumed was multiplied by 3.4, the amount of calories per gram in food. The number of calories per gram that the food provides is adequate, according to the standardized average in animal nutrition stated by the Subcommittee on Laboratory Animal Nutrition, Committee of Animal Nutrition, Board on Agriculture, National Council (1995). The solution made from sucralose did not provide calories, whereas each milliliter of water+glucose consumed provided 0.9 calories. This amount was obtained calculating the amount of glucose in water and the number of calories that a gram of the carbohydrate provided.
The experiment had five phases and the subjects were divided into two groups: Group A and Group B, each one formed with four females and four males. All the subjects received 50 g of food and 250 ml of the solution daily under free access conditions. In the first phase, Group A received food and water for ten consecutive days. In the second phase, food and a solution composed of water+glucose were provided for five days. They returned to baseline conditions during ten days in the third phase. In the fourth phase they received food and a solution composed of water+sucralose during five days. Finally, they received food and water in the last phase. Group B received the same manipulation, except that during the second phase they received a solution composed of water+sucralose and in the fourth phase a solution composed of water+glucose. The experiment was run for 40 days.
Results
Figures 1, 2, and 3 showed the mean and standard deviation of the consumption of liquid, food, and body weight respectively. The white circles represent phases 1, 3, and 5 (food+water). The black circles represent the phases of access to the sweetener with calories (food+water+glucose) and the grey circles the phases of access to the sweetener without calories (food+water+sucralose). The abscissa shows the number of days of the experiment, and the ordinate the consumption of food or liquid. The individual data from the females and males of groups A (top panel) and B (bottom panel) are presented in the left and right columns, respectively.
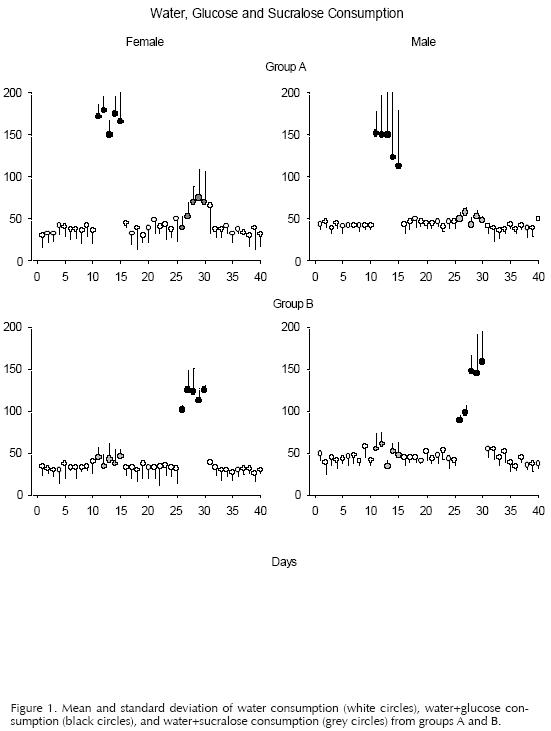
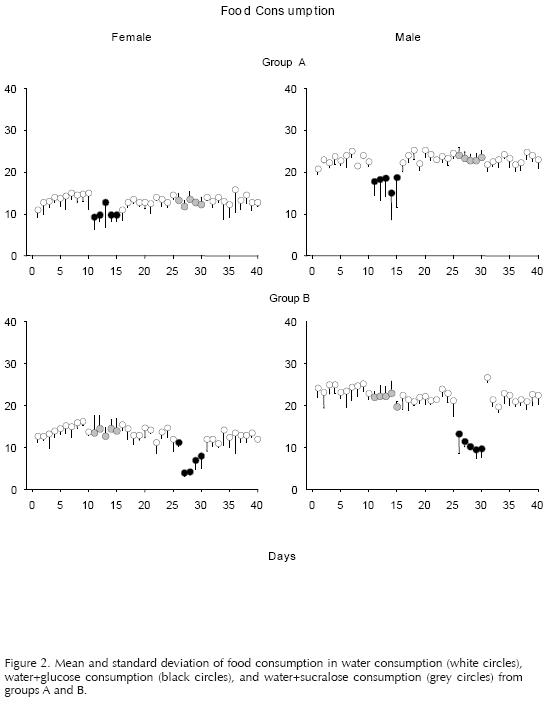
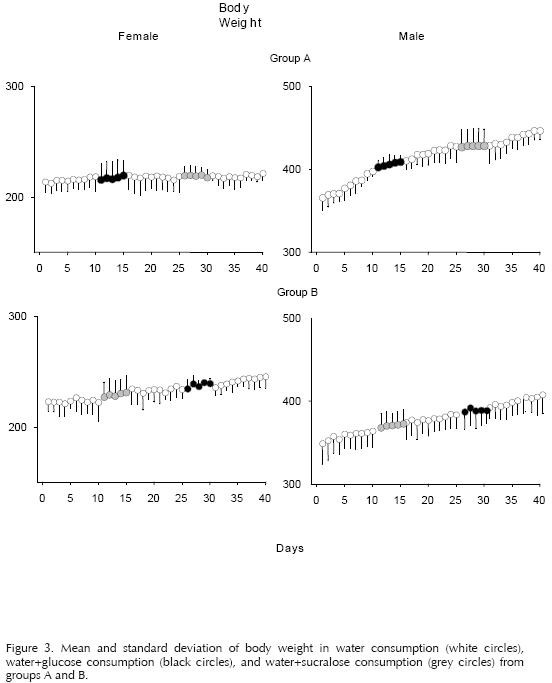
Figure 1 shows the large water+glucose consumption registered in Phase 2 in relation to the water consumption and water+sucralose recorded in the rest of the experiment. Additionally, differences are observed between the consumption of water, water+glucose, and water+sucralose in females and males. While females tripled their water+glucose consumption in relation to the other phases, males from Goup A tripled their consumption of water+glucose during the first three days of free access to the sweetener solution and then began to decrease their consumption. The males from Group B showed the opposite effect during their exposure to the sweetener with calories: they began drinking a mean of 100 ml the first two days of access and ended up tripling the consumption of water+glucose the last three days of this phase. It can also be observed that the mean consumption of water+glucose of the females is more systematic as seen in their standard deviation in relation to the mean of males, in which it is observed a greater variation in their standard deviations. On the other hand, the water consumption was maintained stable during the whole experiment: females consumed a mean of 40 ml and males a mean of 50 ml. The consumption of water+sucralose was almost the same except in females from Group A, they increased their water+sucralose consumption to a mean of 70 ml in relation to the consumption of water, starting on the third day of access to the sweetener. However, this tendency was made even with the first day of water access of the last phase and began decreasing to a mean of 40ml.
Figure 2 shows the results obtained from food consumption. A decrease in the consumption of food is observed during the glucose access phases in relation to the other phases. The females from Group A continued with this tendency on the following phases, however, the decrease in food consumption is of only three g. Regarding food intake during the sucralose phase, no changes were observed in relation to the registered intake in the water access phases. Figure 3 shows the mean and standard deviation of body weight throughout the experiment. There were no changes observed during the whole experiment in spite of the change in liquid diet of the experimental subjects.
Discussion
Results demonstrated that the animals increase the consumption of water+glucose regardless of the exposure history to other artificial sweeteners, as in this case the exposure was to sucralose. Additionally, the previous consumption of glucose did not affect the consumption of sucralose, in other words, the water+sucralose consumption was maintained stable and similar to water consumption, just as it has been reported in previous research (Bello & Hajnal, 2005; Martínez, López–Espinoza, Díaz & Valdés, 2009).These findings suggest that the history of exposure to sweeteners does not modify: 1) the preference and consumption for natural sweeteners over artificial sweeteners (Ackroff, Manza & Sclafani, 1993); 2) the large consumptions of glucose solutions (Martínez & López–Espinoza, 2007); 3) the increasing pattern of water+glucose consumption (Martínez, López–Espinoza, & Martínez, 2006); 4) the flavor perception of artificial sweeteners (Sims, Roberts, Daniel, & Renwick, 2000); and 5) the intake of sucralose observed under different experimental procedures (Bello & Hajnal, 2008; Martínez, López–Espinoza, Díaz, & Valdés, 2009).The evidence in scientific literature shows that rodents, specifically rats, perceive in a different way the sweet flavor of diverse sweeteners (Ackroff, Manza, & Sclafani, 1993; Myers & Sclafani, 2003; Pérez & Sclafani, 1990; Vigorito & Sclafani, 1988). Most of this evidence has been collected from studies made with sucrose, that is, common table sugar (Agmo & Marroquin, 1997; Sclafani, 1987). It has also been showed that sucrose is preferred over all other natural sweeteners such as fructose (Ackroff & Sclafani, 2004). Even though it is well known that animals develop a strong preference for the sweet flavor of sugar, it is hard to explain the ascending response in the observed intake pattern (Martínez & López–Espinoza, 2007; Martínez, López–Espinoza & Martínez, 2006; Martínez, López–Espinoza, Díaz & Valdés, 2009).One of the most convincing arguments is the possible addiction that animals may develop in the presence of constant exposure to this sweetener (Brito, 2004), it is even affirmed that the chronic intake of sugars is similar to the state that causes dependence to some opiates in laboratory rats (Colantuoni et al., 2002). These statements have reinforced the theory to addiction to carbohydrates presented by Wurtmand and Fernstrom in the 70's (Ottley, 2000).
Another possibility that might explain the ascending pattern of the consumption of sweetened solutions is that the consumption of the sweetener is forced: animals do not have another option of a liquid to drink in most experimental procedures (Davis, 1973; Ramírez, 1995). However, an argument that precludes this explanation is the temporal pattern in which the sweetener is made available, for they mostly refer to short term exposures: sessions lasting minutes or hours (Ackroff & Sclafani, 1997; Agmo & Marroquin, 1997). This is because some investigations have reported that long term intake of natural sweeteners (like sucrose and fructose), as well as their exaggerated intake, can provoke other effects like lack of mobility in the animal, a change of perception to other flavors, and the development of diabetes, which can affect the interpretation of the results regarding addictive behavior.
On the other hand, the intake of water+sucralose suggests that the history of chronic exposure to sweeteners results ineffective in producing large intakes of this sweetener. Also, consuming a sucralose solution previous to the exposure of a glucose solution does not seem to have an important effect in the feeding behavior of rats. Agmo and Marroguín (1997) stated that there are at least three reasons for which a sweetener without calories seems ineffective to condition certain responses in animals: 1) it is not possible to establish an association between the environment and a positive affective state on the subject; 2) it seems that taste stimulation does not induce an affective state capable of inducing consumption; and, 3) artificial sweeteners, such as saccharin or sucralose are weaker than glucose at producing long term responses. The authors suggested that the impact of artificial sweeteners is reduced gradually by the absence of post–ingestive effects. They added that even if they have a tasty flavor, this is not enough to establish specific responses.
Regarding food intake, it was observed that the subjects consumed practically the same amount of food during all the experimental phases, although there was a small decrease when glucose was available. Studies in humans and animals have shown that the intake of sweeteners have an effect on the intake pattern of other foods and also on body weight (Kanders, 1988; Rogers, Carlyle, Hill & Blundell, 1998). However, in the present study changes on body weight of the subjects were not observed, which could be due to two reasons: a) that exposure time to the sweeteners was not enough to observe important changes on the eating behavior and to be reflected on body weight, or b) that, the animals regulated their calorie intake adequately by substituting calorie intake from food, for the calories in the sweetened solution.
It would seem that the post–ingestive effects of the sweeteners, combined with the taste stimulation that produces the sweet flavor, leads to a positive state that determines a consumption response. According to the findings of our experiment, the flavor alone does not produce such an effect. The flavor can probably facilitate learning of certain behaviors and increase the motivation in animals to do a specific task, but in our particular case, the post–ingestive effect was what determined the consumption pattern of the sweetened solutions.
Finally, it is important to highlight that the effect of chronic intake of carbohydrates also causes organic alterations vastly described in medical scientific literature. Therefore, the knowledge of new findings concerning to psychology and behavioral sciences result add to medical and nutrition research data. It is possible that soon medical and nutritional recommendations on sugar consumption must be added with suggestions about the behavioral modifications regarding control and prevention of addictive responses generated by carbohydrates.
References
Ackroff, K., Manza, L. y Sclafani, A. (1993). The rat´s preference for sucrose, polycose and their mixtures. Appetite, 21, 69–80. [ Links ]
Ackroff, K. y Sclafani, A. (1997). Diabetic rats prefer glucose–paired flavors over fructose–paired flavors. Appetite, 28, 73–83. [ Links ]
Ackroff, K. y Sclafani, A. (2004). Fructose–conditioned flavor preferences in male and female rats: Effects of sweet taste and sugar concentration. Appetite, 42, 287–297. [ Links ]
Agmo, A. y Marroquin, E. (1997). Role of gustatory and postingestive actions of sweeteners in the generation of positive affect as evaluated by place preference conditioning. Appetite, 29, 269–289. [ Links ]
Bello, N. T. y Hajnal, A. (2005). Male rats show an indifference–avoidance response for increasing concentrations of the artificial sweetener sucralose. Nutrition Research, 25, 693–699. [ Links ]
Brito, J. X. (2004). Los azúcares y sus repercusiones en el metabolismo de la glucosa y los lípidos. Nutrición Clínica, 7, 185–190. [ Links ]
Colantuoni, C., Rada, P., McCarthy, J., Patten, C., Avena, N., Chadeayne, A. y Hoebel, B. (2002). Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obesity Research, 10, 478–488. [ Links ]
Davis, J. D. (1973). The effectiveness of some sugars in stimulating licking behavior in the rat. Physiology and Behavior, 11, 39–45. [ Links ]
Kanders, L. (1988). Sweet taste and food intake. Appetite, 11, 73–84. [ Links ]
Martínez, A. G. y López–Espinoza, A. (2007). Efectos post–privación con dos alternativas energéticas en ratas. Revista Mexicana de Análisis de la Conducta, 33, 43–60. [ Links ]
Martínez, A. G., López–Espinoza, A., Díaz, F. y Valdés, E. (2009). Consumo de soluciones endulzadas en ratas albinas: Sabor vs calorías. Psicothema, 21, 196–203. [ Links ]
Martínez, A. G., López–Espinoza, A. y Martínez, H. (2006). Efectos de modificar el contenido energético del agua sobre el peso corporal, consumo de agua, alimento y calorías en ratas. Universitas Psichologica, 5, 361–370. [ Links ]
Myers, K. P. y Sclafani, A. (2003). Conditioned acceptance and preference but no altered taste reactivity responses to bitter and sour flavors paired withintragastric glucose infusion. Physiology and Behavior, 78, 173–183. [ Links ]
Ottley, C. (2000). Food and mood. Nursing Standard, 15, 46–52. [ Links ]
Pérez, C. y Sclafani, A. (1990). Developmental changes in sugar and starch taste preferences in rats. Physiology and Behavior, 48, 7–12. [ Links ]
Ramírez, I. (1995). Is fructose sweeter tan glucose for rats?. Physiology and Behavior, 60, 1299–1306. [ Links ]
Rogers, P. J., Carlyle, J. A., Hill, A. J. y Blundell, J. E. (1988). Uncoupling sweet taste and calories: Comparison of the effects of glucose and three intense sweeteners on hunger and food intake. Physiology and Behavior, 43, 547–552. [ Links ]
Sclafani, A. (1987). Carbohydrate taste, appetite, and obesity: An overview. Neuroscience and Biobehavioral Reviews, 11, 131–153. [ Links ]
Sims, J., Roberts, A., Daniel, J. W. y Renwick, A. G. (2000). The metabolic fateof sucralose in rats. Food and chemical Toxicology, 38, 115–121. [ Links ]
Subcommittee on Laboratory Animal Nutrition, Committee of Animal Nutrition, Board on Agriculture, National Research Council. (1995). Nutrient Requirements of Laboratory. Washington, D. C.: National Academies Press. [ Links ]
Vigorito, M. y Sclafani, A. (1988). Ontogeny of polycose and sucrose appetite in neonatal rats. Developmental Psychobiology, 21, 457–465. [ Links ]
Note
Supported by CONACYT CB–2008/101314. Contribution of the authors: Martínez ABCDEF, Díaz ACDEF, Beltrán–Miranda EF, González BE, Munguía BE, Venancio BE. A = Experimental design, B = Data recording, C = Data interpretation, D = Manuscrpt writing, E = Bibliografy search, F = Traduction.














