INTRODUCTION
Free-ranging sea turtles, due to their complex life cycle, long lifespan, and migratory behavior, are exposed to several threats and stressors of natural and anthropogenic origin that can affect their health, such as exposure to marine pollution, malnutrition, vessel strikes, entanglement, and infectious diseases (Work et al. 2015). Among these, fibropapillomatosis (FP), a neoplastic disease associated with chelonid herpesvirus 5 but mainly triggered by anthropogenic disturbances, has a circumtropical distribution (Rodenbusch et al. 2014, Duffy and Martindale 2019). Since its first reports in the 1930s in the Florida Keys, FP has reemerged in the past 3 decades as a conspicuous disease in green sea turtles (Chelonia mydas), reaching an epizootic status and potentially threatening the survival of this endangered species (Aguirre and Lutz 2004).
Fibroepithelial lesions of different size (up to 30 cm in diameter), morphology (from flat to verrucous), consistency (e.g., soft or firm), and color (e.g., pink, grey, black) can be present on the neck, flippers, periocular tissue, inguinal region, tail, cloaca, carapace, and plastron but can also occur internally as fibromas, myxofibromas, and fibrosarcomas (Jones et al. 2016, Page-Karjian 2019). The presence of these tumors, especially when numerous (up to 91 on a single juvenile green turtle in Brazil, Santos et al. 2010), is likely to interfere with regular activities such as swimming, diving, feeding, and predator avoidance, and it also might hamper vision and swallowing. Juvenile individuals are the most affected by FP, particularly after their recruitment in neritic waters, and some authors suggest that the few instances observed in adults can be due to the fact that afflicted immature turtles might be either dying off or, conversely, surviving, recovering, and developing greater immunity, which causes spontaneous regression of tumors (Van Houtan et al. 2010, Guimarães et al. 2013).
The etiology of FP is still being discussed, but some possible causes or risk factors associated with it are the presence of ectoparasites (Greenblatt et al. 2004); ingestion of arginine (immune response regulator) linked to feeding on invasive macroalgae in eutrophic habitats (Van Houtan et al. 2010); increase in warm water temperatures (Page-Karjian et al. 2014); pollution (Torezani et al. 2010); and increased ultraviolet exposure, which could induce genomic mutations, immunosuppression, or the conversion of chemicals into oncogenic compounds (Duffy and Martindale 2019). FP prevalence in different populations varies worldwide, both spatially and seasonally. For example, FP prevalence in Brazil from 2005 to 2014 ranged between 17.4% and 44.6% according to the geographic location (Rossi et al. 2019). In the coastal areas of Texas, prevalence increased from 5.0% in 2010 to 35.2% in 2018 (Shaver et al. 2019). In Hawaii the disease increased rapidly after an outbreak in the 1980s, peaked in the 1990s, and then declined from 1999 onward, without an apparent reason (Chaloupka et al. 2009). In Mexico, few reports of FP in green turtles have been documented to date. Recently, one case was described in Baja California Sur, where one juvenile out of 22 captured green turtles, exhibited lesions on the eyes and hind flippers (Reséndiz et al. 2016). Other cases were recorded in 2015 in Akumal, Quintana Roo, where 5 out of 22 captured individuals (22.7% prevalence) presented fibropapillomas (Labrada-Martagón et al. 2017).
The Veracruz Reef System National Park is a particularly important study area due to the activities that are being carried out within the current Port of Veracruz expansion, one of the major infrastructure projects in Mexico’s recent history. This marine protected area is also affected by organic pollution from rivers with wastewater discharge from human activities, by tourism infrastructure, and by commercial and recreational fishing (Jordán-Dahlgren and Rodríguez-Martínez 2003, Ortiz-Lozano 2012). In this region of the Gulf of Mexico, information is still lacking on the presence of turtles with FP and the possible effects of chemical contaminants and organic pollutants on its prevalence. Since previous research has found higher prevalence of FP in green turtles living close to regions where agriculture, industrial activity, and urban development are occurring (van Houtan et al. 2010), this study provides baseline information for long-term monitoring programs that can be implemented to evaluate the current health status of green turtle populations in this area of Mexico. Here we report the first findings on FP recorded in free-ranging green sea turtles (C. mydas) in the Veracruz Reef System National Park during a year.
MATERIALS AND METHODS
The Veracruz Reef System National Park (19º00ʹ00ʺ- 19º16ʹ00ʺ N; 95º45ʹ00ʺ-96º12ʹ00ʺ W) has an area of 65,516 ha and harbors 45 coral reef structures. The Park includes the Cabezo Reef, a shallow water area extending 1,538.7 ha (Liaño-Carrera et al. 2019) (Fig. 1), where systematic boat based surveys (outboard motor, speed: 12-15 km·h-1) were carried out from February 2017 to February 2018 at least once per month, depending on weather conditions. Capture per unit effort was calculated as the number of individuals captured per year divided by the total effort units (68.3 navigation hours). Turtles were captured by hand by an experienced swimmer following the technique described by Limpus (1978) and brought onboard. On the boat we recorded curved carapace length (CCL), notch to tip, with a flexible measuring tape (Bolten 1999) and, considering external physical evaluation (sensuSantos et al. 2017), the number, size, anatomic region, gross morphology, consistency, and color of cutaneous tumors present on each animal. Tumor size was recorded with a digital caliper, measuring the longest axis (Rossi et al. 2016). Only one tissue sample from a verrucous tumor could be collected. It was fixed in a 10% buffered formaldehyde solution and sent to the laboratory (ESCA Laboratorio Veterinario, Xalapa, Veracruz) for histopathology analysis. All turtles were marked with metal tags (National Band & Tag Company, Newport, KY, USA) on the anterior flippers following the methodology of Balazs (1999), and they were released in situ after being handled according to the proper recommendations (Hargrove et al. 2016). Field work was carried out under permits SGPA/DGVS/07034/16 and SGPA/DGVS/000999/18 from the Mexican Secretariat of Environment and Natural Resources.
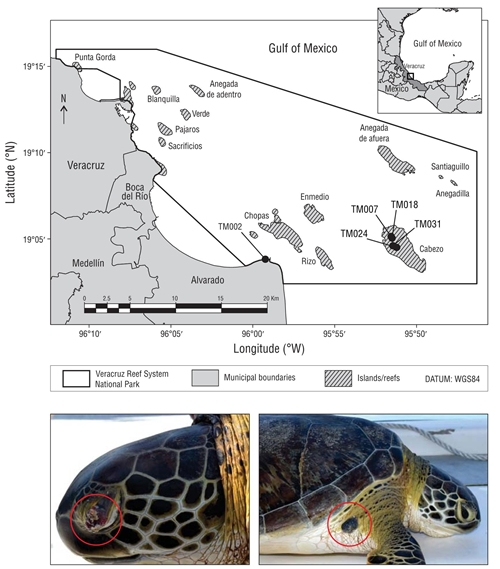
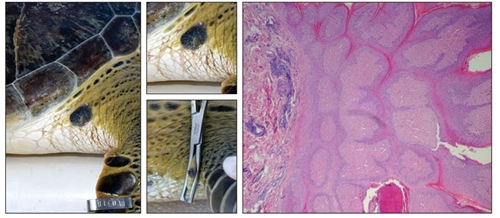
Figure 1 Map of the Veracruz Reef System National Park showing the location where the 5 green turtle individuals (Chelonia mydas) with fibropapillomas (filled circle with corresponding tag ID) were captured. Pictures at the bottom show 2 of the captured individuals with fibropapilloma tumors (red empty circles) (photos by M Lourdes Robledo-Catalina).
Turtles were categorized into 6 size classes according to CCL (class I: 21.0-34.0 cm; class II: 34.1-47.0 cm; class III: 47.1-60.0 cm; class IV: 60.1-73.0 cm; class V: 73.1-86.0 cm; class VI: 86.1-100.0 cm). Individuals with CCL > 86.1 cm were considered sexually mature adults (Labrada-Martagón et al. 2017). Cutaneous tumors were classified into 4 size categories (Work and Balazs 1999, Trigo-Tavera and Valero-Elizondo 2004): A (<1 cm), B (1-4 cm), C (>4-10 cm), and D (>10 cm). Also, a classification was assigned according to the anatomic region, adapted from Rossi et al. (2016): head and eyes (1); neck and shoulders (2); forelimbs and hind limbs (3); carapace and plastron (4); inguinal region, cloaca, and tail (5). A FP index (FPI) was calculated for each individual, based on the sum of tumors in each size category:
where N is the number of tumors of different sizes (A-D) (Rossi et al. 2016). The FPI value was then used to obtain a general score to classify tumor severity (mild: FPI < 40; moderate: 40 ≤ FPI < 120; severe: FPI ≥ 120) (Rossi et al. 2016). To analyze the relationship between turtle size (CCL) and average tumor size, a Spearman correlation was run in PAST v.3.25 (Hammer et al. 2001).
RESULTS
During the study period, 41 green turtles were captured, which corresponded to 0.6 individuals per unit effort. Turtles varied in size from 26 to 100 cm (CCL), with 2 individuals in class I, 7 in class II, 14 in class III, 8 in class IV, 5 in class V, and 5 in class VI. Five turtles presented cutaneous tumors (12.2% prevalence) and none of them were considered adults, ranging in size from 41.7 to 80.0 cm (Table 1, Fig. 1). A total of 22 tumors were recorded on the 5 individuals, and the majority (41%) was found on flippers (region 3), followed by the head and eyes (region 1, 27%) and the carapace and plastron (region 4, 23%). Tumor frequency was lowest on the neck and shoulders (5%) and the tail and cloaca (5%) (Table 1).
Table 1 Characteristics of fibropapillomas found in 5 green sea turtles (Chelonia mydas) tagged in the Veracruz Reef System National Park in 2017-2018.
Tag number |
Characteristics of fi bropapillomas |
|||||||||
ID |
Left |
Right |
CCL (cm) [size class]a |
Geographic coordinates |
Number |
Longest axis (cm) [size category]b |
Anatomical regionc |
Gross morphologyd |
FPIe |
Severity |
TM002 |
UV350 |
UV1349 |
41.7 [II] |
19º3ʹ49.356ʺ N |
1 |
2.80 [B] |
1 |
R, S, V |
47.1 |
Moderate |
95º59ʹ14.063ʺ W |
2 |
1.28 [B] |
1 |
SR, S, V |
||||||
3 |
1.15 [B] |
4 |
R, S, V |
|||||||
4 |
4.49 [C] |
3 |
R, P, M |
|||||||
5 |
1.85 [B] |
3 |
R, P, M |
|||||||
6 |
1.05 [B] |
3 |
SR, S, V |
|||||||
7 |
1.59 [B] |
3 |
R, P, V |
|||||||
8 |
7.16 [C] |
4 |
R, CM, M |
|||||||
9 |
1.11 [B] |
3 |
SR, S, V |
|||||||
10 |
0.86 [A] |
4 |
SR, S, SM |
|||||||
TM007 |
UV1326 |
UV1327 |
51.1 [III] |
19º5ʹ8.412ʺ N |
1 |
0.16 [A] |
1 |
SR, S, V |
42.4 |
Moderate |
95º51ʹ33.371ʺ W |
2 |
0.32 [A] |
3 |
SR, S, V |
||||||
3 |
2.36 [B] |
3 |
SR, S, V |
|||||||
4 |
1.62 [B] |
2 |
R, S, V |
|||||||
5 |
4.39 [C] |
3 |
R, P, M |
|||||||
6 |
5.38 [C] |
4 |
R, P, V |
|||||||
7 |
0.70 [A] |
4 |
SR, S, V |
|||||||
8 |
0.73 [A] |
5 |
SR, P, V |
|||||||
TM018 |
UV0322 |
UV0321 |
80.0 [V] |
19º5ʹ2.220ʺ N |
1 |
0.01 [A] |
1 |
F, S, SM |
0.1 |
Mild |
95º51ʹ29.628ʺ W |
||||||||||
TM024 |
UV0318 |
UV0319 |
50.0 [III] |
19º4ʹ30.396ʺ N |
1 |
1.31 [B] |
3 |
SR, S, V |
1.0 |
Mild |
95º51ʹ13.100ʺ W |
||||||||||
TM031 |
UV0658 |
UV0657 |
67.0 [IV] |
19º4ʹ36.264ʺ N |
1 |
0.48 [A] |
1 |
R, S, V, U |
0.2 |
Mild |
95º51ʹ26.676ʺ W |
2 |
0.32 [A] |
1 |
R, S, V |
||||||
aSize class of individuals, based on curved carapace length (CCL): I = 21.0-34.0 cm, II = 34.1-47.0 cm, III = 47.1-60.0 cm, IV = 60.1-73.0 cm, V = 73.1-86.0 cm, VI = 86.1-100.0 cm.
bTumor size categories: A (<1 cm), B (1-4 cm), C (>4-10 cm), and D (>10 cm).
cAnatomical regions of sea turtle where fibropapillomas were found: 1 = head and eyes; 2 = neck and shoulders; 3 = forelimbs and hind limbs; 4 = carapace and plastron; 5 = inguinal region, cloaca, and tail.
dGross morphology: R = raised, SR = slightly raised, F = flat, S = sessile, P = pedunculated, CM = coalescing masses, V = verrucous, SM = smooth, M = mixed (verrucous and smooth), U = ulcerated.
eFibropapillomatosis index (FPI) = (0.1 × NA) + (1 × NB) + (20 × NC) + (40 × ND), where N is number of fibropapillomas and subscript uppercase letters are the tumor size categories.
Regarding gross morphology, 95% of the tumors were raised or slightly raised, whereas only one (5%) was flat. They were mainly sessile (68%), and in fewer cases (27%) pedunculated. Few were smooth (9%), and the majority were verrucous (73%) or mixed (smooth and verrucous, 18%). In one case, a tumor on an individual’s eye was ulcerated. All tumors had a firm consistency, and their color was overall variable, from gray/black to red/pink, regardless of the anatomical region or tumor size (Table 1). The morphology of the tissue sampled was representative of the majority of the observed tumors and its histopathology analysis revealed moderate hyperkeratosis and epidermal hyperplasia with papillary projections. In the dermis, moderate fibroblastic proliferation and angiogenesis could be detected, as well as observing plasma cells, heterophiles, some melanomacrophages, and varying quantities of lymphocytes (Fig. S1).
Despite having a sample size of only 5 individuals, a trend toward a negative relationship between turtle size (CCL) and average tumor size could be inferred (Spearman correlation, r s = -0.9, n = 5, P = 0.01). Overall, FP was not severe, being moderate in 2 individuals (FPI = 42.4 and 47.1) and mild in the other 3 (FPI ≤ 1) (Table 1).
DISCUSSION
The proliferative changes observed in the analyzed tissue indicated that the tumors found in this study were benign neoplasms consistent with previous descriptions of fibropapillomas. Our results revealed 12.2% FP prevalence in green turtles captured in the Veracruz Reef System National Park during a year-long monitoring. This value is similar to those reported for other regions, such as the Turks and Caicos Islands during 2008-2010 (13%) (Stringell et al. 2015) and Puerto Manglar, Puerto Rico, during 1997- 2010 (12%) (Patrício et al. 2011). However, more recent reports across different populations, including those in the Caribbean off Mexico (Labrada-Martagón et al. 2017), high- light a generally higher prevalence (e.g., 43.1% in southeast Brazil [Tagliolatto et al. 2016]). Thus, despite the fact that the Veracruz Reef System is increasingly being impacted by anthropogenic activities, the observed tumors were not severe, and the recorded prevalence was not high compared to recent data reported at other sites, for example Espírito Santo and São Paulo, Brazil, and the coast of Texas, USA (Rossi et al. 2019, Shaver et al. 2019).
During our year-long survey we did not encounter any dead or stranded turtle showing signs of FP. Severely affected green turtles show extensive tumor growth, are more vulnerable to infection than mildly affected turtles and can develop anemia, look emaciated, and be susceptible to stranding (Stacy et al. 2019). It is important to consider that depending on the location of the tumor, the severity can be underestimated. For example, tumors on the eyes or mouth, even if small in size, can impair vital functions such as vision, breathing, and feeding (Stacy et al. 2019). Of the 5 individuals reported in this study, 4 had tumors on their eyes and 3 had the highest number of tumors on the flippers, which may cause greater exposure to predators and may represent a potential impediment to foraging and adequate food intake for juvenile turtles (Jones et al. 2016, Reséndiz et al. 2019). In particular, survival might have been compromised in one of the captured individuals (TM002), since 9 of its 10 tumors were both verrucous and mixed, with 2 located on the eyes and 5 on the flippers. Verrucous and pedunculated tumors are less likely to show spontaneous regression, and euthanasia is suggested for cases similar to that of this turtle (i.e., with bilateral ocular tumors) (Page-Karjian et al. 2014).
All the individuals affected by FP-like lesions were juveniles, and the fact that average tumor size was inversely proportional to turtle size confirms that this age class can be more affected and potentially vulnerable to FP. Research on turtle immature life stages is limited (Wildermann et al. 2018). However, juveniles are key to maintaining stable green turtle populations (Hirth and Schaffer 1974), and monitoring their health and recording the occurrence of FP in the coming years is thus recommended.
In the Veracruz Reef System National Park more studies are needed on the direct effects of environmental pollution on marine vertebrates. Recent research in this area has evidenced the presence of bacterial infections affecting coral reefs, which are at the bottom of the food chain (García- Fuentes et al. 2014); the occurrence of harmful algal blooms, related to increased ocean temperature, and nitrogen and phosphorus enrichment due to fertilizers (Aké-Castillo et al. 2014); and metal enrichment in the sediments supplied by rivers to coastal areas near the city of Veracruz (Celis-Hernández et al. 2013). The Park represents a dynamic system subject to environmental and anthropogenic pressures, which must be evaluated as possible stressors for marine turtles. A better understanding of FP has been listed within the 5 global research priorities for sea turtle conservation (Hamann et al. 2010). Thus, here we provide valuable information documenting for the first time the prevalence of FP in this area of the Gulf of Mexico that can serve as a baseline for future studies.











 nueva página del texto (beta)
nueva página del texto (beta)