BACKGROUND
Schizophrenia (SZ), a cognitive disorder with an estimated global prevalence of .28% in 2016 (Charlson et al., 2018), ranks among the twenty-five leading causes of disability worldwide (Institute for Health Metrics and Evaluation, Human Development Network, & The World Bank, 2013).
Clinically, it is characterized by the presence of three groups of symptoms: positive (loss of contact with reality), negative (social isolation and communication problems), and cognitive (difficulty making decisions or inability to pay attention). Consequently, people diagnosed with SZ have a high probability of being unemployed and unable to care for themselves, as well as living in poverty and having problems relating to other people (Charlson et al., 2018).
The relatively early onset of symptoms (between adolescence and adulthood) impacts the economically productive sector of society (Owen, Sawa, & Mortensen, 2016). In 2016, it was reported that SZ accounted for 13.4 million years lived with disability worldwide (Charlson et al., 2018). In 2013, likewise, an estimated expenditure of between $94 and $102 million USD was reported in various countries, equivalent to .02% to 5.46% of the gross domestic product. These estimates include items such as direct medical expenses (hospitalization, medication, and non-hospital care), direct non-medical expenses (shelter for the poor, food, social care, and the cost of suicide), and indirect costs (productivity lost due to premature mortality, disability, and unemployment; Chong et al., 2016).
In the field of basic research, efforts have been made to define the etiology of SZ to design prevention, early diagnosis, and treatment strategies. From 1891 onwards, SZ was addressed from a neurodevelopmental perspective, which only changed with the emergence of the “neurodevelopmental hypothesis” in the 20th century. This hypothesis posits that disruption by genetic and epigenetic factors of brain development during the initial stages of life precedes the emergence of psychosis in adolescence (McGrath, Féron, Burne, Mackay-Sim, & Eyles, 2003). It should be noted that this approach is limited to the early influence of risk factors. However, the neurodevelopmental hypothesis has now evolved into the “Risk Factor Model of Psychosis” (RFMP; Murray, Bhavsar, Tripoli, & Howes, 2017). This proposal is an integrative, flexible model which, in addition to risk factors in the prenatal and perinatal stages, includes adversities that occur during subsequent stages such as adolescence and early adulthood.
The information available on the etiology of EZ has grown exponentially due to the growing use of new techniques and the implementation of a multifactorial model such as the MFRP. There is therefore a need to collect and organize the latest information obtained on the subject to update our knowledge of SZ. Accordingly, this article seeks to answer questions such as what the prodromes and biological markers detected in patients with SZ or UHR subjects are, and which of them can specifically be used in a schizophrenia diagnosis.
METHOD
This article is a narrative review of the minor physical abnormalities, functional, neuroanatomic, and synaptic connection abnormalities, and cell markers present in SZ patients and/or UHR subjects. Information was obtained through a search of relevant sources in the Medline-Pubmed database using Language filters (English and Spanish), Text availability (Free full text and full text), Publication date (Range: 2000-2020) and Article type (Clinical Trail, Randomized Controlled Trial, Review, Meta-Analysis and Systemic Review). The keywords used in the search engine were: “Schizophrenia” in combination with the Boolean operator “AND”, and each of the following terms: “MPA,” “Minor Physical Abnormalities,” “Dermatoglyphic abnormalities,” “Cognitive antecedents,” “Childhood cognitive problems,” “Olfactory Bulb abnormalities,” “fMRI structural brain anatomical abnormalities,” “Functional Magnetic Resonance Imaging structural brain anatomical abnormalities,” “*fmri structural brain abnormalities,” “*Dopamine system dysregulation model,” “*Neurotransmission dysregulation,” “*Oxidative stress in NMDA receptors,” “*Redox dysregulation,” “*Origin of NMDA receptor hypofunction,” and “microtubule alterations.” The “Associated Data” filter was used to reduce the number of articles in the searches that used terms marked with *. Articles resulting from the search and those marked under the term “Found citation matching” were selected. Fifteen articles were added by the authors because of their relevance to the topic, and their usefulness for contextualizing concepts and/or contrasting the information collected. All articles unrelated to the objectives of the present study or minor physical abnormalities, functional, neuroanatomical, and synaptic abnormalities or cell markers present in schizophrenic and/or UHR patients were discarded.
RESULTS
The neurodevelopmental hypothesis has suggested that early interruption of key events during brain development, such as cell migration, proliferation, differentiation, and death results in an increased risk of developing SZ. The result is not only evident in the development of symptoms on the schizophrenia spectrum, but also in discrete cell alterations that are imperceptible during medical evaluations (such as biological markers), as well as signs or symptoms that precede the onset of the disease and constitute high-risk clinical states (prodrome). Despite their differences, both have the capacity to be pre-morbid markers of brain dysfunction, possibly reflecting early manifestation of the development of the pathology. In this article, we seek to classify prodromes into minor physical abnormalities and functional abnormalities, and biological markers into neuroanatomical abnormalities, synaptic abnormalities, and cell markers.
Minor physical abnormalities
Minor physical abnormalities (MPA) are nonspecific, morphogenetic variants resulting from possible genetic or epigenetic damage in fetal development during the first two trimesters of gestation (Franco, Valero, & Labad, 2010).
Since the development of the fetal nervous system, the craniofacial, dermal, and other structures are of ectodermal origin, this group of markers can reflect morphogenesis and brain functionality and function as prodromes in people susceptible to SZ. They therefore have a high prevalence in patients with SZ or UHR subjects (Hennessy, McLearie, Kinsella, & Waddington, 2005; Compton & Walker, 2009).
The Waldrop Scale (WS) or its modified version, the Gourion Scale (WS-M), is one way to assess MPA by observing the characteristics of six main anatomical points: head, eyes, ear, mouth, hands, and feet (Weinberg, Jenkins, Marazita, & Maher, 2007; Tenyi, 2011). Franco et al. (2010) mention that there is a higher frequency of MPA in subjects with SZ than in control subjects. In the WS-M evaluation (p < .05), a size greater than two was observed in the mouth (2.65), head (2.55), eyes (2.47), hands (2.14), and feet (2.15) compared to control subjects. No significant differences were observed between ethnic groups. In a clinical trial in first-degree relatives, sixty Mexican families with at least one member with SZ and sixty-one control families were compared using the WS-M. A mean of 5.72 ± 2.3 MPA was obtained counting the subject with SZ in experimental families (p < .001), 1.8 ± 4.46 MPA (p < .001) in control families and 6.14 ± in isolated subjects with SZ (p = .092; Ambrosio-Gallardo, Cruz-Fuentes, Heinze-Martin, Caraveo-Anduaga, & Cortés-Sotres, 2015).
Since the development of epidermal ridges is temporally related to neuronal migration, dermatoglyphic abnormalities are another type of MPA, characterized by alterations in the pattern of epidermal ridges in the hands, fingers, and soles of the feet (Golembo-Smith et al., 2012). There are various methods of measuring dermatoglyphic abnormalities, the most studied and conclusive ones being the total finger ridge count (TFRC) and the a-b ridge count (ABRC). Golembo-Smith et al. (2012) report a lower TFRC and ABRC in patients with SZ. These authors suggest that SZ is a disease that impacts various stages of embryonic development, since there is a relationship between the fetal stages affected and the dermatoglyphic abnormalities that occur. Abnormalities in TFRC are associated with alterations in the first months of prenatal development, whereas abnormalities in ABRC are associated with later stages. It should be noted that there is a higher degree of dermatoglyphic abnormalities in UHR than control subjects (Dondé, D’Amato, & Rey, 2018).
It is important to recall that MPAs are not specific to SZ and are also seen in other psychiatric disorders. However, they are more prevalent in schizophrenic patients than control subjects, or patients with other psychiatric disorders (Xu, Chan, & Compton, 2011; Lobato et al., 2001). The development of more standardized tests based on MPA is required for their clinical application, such as the WS-M, which makes it possible to detect families with members with SZ at a borderline value of seven (specificity: 94.8%, sensitivity: 23.2%; Ambrosio-Gallardo et al. 2015). Failing that, Kim et al. (2011) proposes using MPAs in human subjects to associate them with biological markers present in the pathophysiology of SZ, in the same way as genes associated with SZ development (such as Neuregulin-1) have been associated with MPA in animal models (Waddington, Katina, O’Tuathaigh, & Bowman, 2017).
Functional abnormalities
As a possible result of alterations in prenatal neurodevelopment, discrete prodromal cognitive deficits appear, visible from the perinatal period to adolescence, even before the first psychotic episode (FPE). Cognitive deficits remain and worsen with the development of SZ (Sheffield & Barch, 2016). Prospective studies show that the childhood of subjects who subsequently develop SZ is usually preceded by abnormalities in psychomotor development, socio-emotional-behavioral problems (SEB), problems adapting to the environment, cognitive abnormalities (IQ, working memory, and attention), linguistic problems and abnormalities in reading or visual perception compared with control subjects (Laurens et al., 2015; King, Laplante, & Joober, 2005; Parellada, Gomez-Vallejo, Burdeus, & Arango, 2017).
Hodgins & Klein (2017) report that 20% to 40% of individuals with SZ present behavioral disorders before the age of fifteen. In fact, two subgroups of patients can be identified and classified: those with a history of antisocial-aggressive behavior before the FPE and those whose aggressive behavior began after the onset of the disease. Externalizing antisocial behavior from the age of five, social internalizing behavior at the age of eleven, and unusual speech (such echolalia) between the ages of four and seven appeared to be sensitive, specific predictors in people who subsequently developed SZ according to Liu, Keshavan, Tronick, and Seidman (2015).
In a cohort study of 118 patients with onset of SZ in childhood, it was found that each child had an average of 3.89 abnormalities and that 55.8% had academic problems, 72.03% SEB problems, 50.83% language problems, and 44.07 % motor abnormalities (Driver, Gogtay, & Rapoport, 2013). This suggests that the presence of a delay in the acquisition of motor and behavioral abilities (developmental milestones) in early childhood could be considered prodromal indicators of SZ in pediatric population of UHR subjects.
In a London cohort of 1,504 children within a general population aged 9 to 11, Laurens and Cullen (2016) found that 9.4% had a substantial risk of developing the full psychotic spectrum due to the presence of a symptomatological triad: SEB, psychotic-like experiences, and linguistic-motor abnormalities. It should be recalled that since functional abnormalities are prodromal symptoms reflecting the development of a broader pathology, their use is crucial for early intervention. Certain functional abnormalities statistically increase the relative risk (RR) of developing SZ. According to various prospective studies, Artigue and Tizón (2014) report the presence of a delay in early learning (smiling, holding one’s head up, sitting up, crawling, and walking) during the first year of life (RR = 3.3; CI 95%), a delay in acquiring the ability to walk alone (RR = 4.8; 95% CI), language acquisition problems (RR = 2.8; 95% CI), and refusing to eat (30.8% of those diagnosed with SZ have this antecedent; p < .005).
The combination of functional abnormalities may be reflected in both interpersonal relationship skills and school productivity and may continue to act as modifiers of RR, as in the case of aggressive behaviors (RR = 3.65; CI 95%), poor school results (RR = 4.4; CI 96%), reading and writing disorders (53.8%), graphism disorders (69.2%; p = .003), and a preference for solitary play in early childhood (RR = 2.1; 95% CI; Artigue & Tizón, 2014). Jarcho, Mayer, Jiang, Feier, and London (2012) mention that patients with SZ are hyposensitive to pain, which can lead to delayed diagnoses and increased mortality in the patient (for example, as a result of painless myocardial infarction).
In clinical settings, the finding and interpretation of functional abnormalities should be done with caution, despite their high prevalence in early life in subjects with EZ compared to control subjects or patients with other mental disorders. As mentioned by Parellada et al. (2017), the isolated presence of a functional abnormality has a low positive predictive value. This is partly because the similarities in the profile of cognitive abnormalities of patients with diverse types of disorders (bipolar, major depressive, schizoaffective) are greater than the differences, which suggests the same etiology or a similar final pathophysiological mechanism (Barch, 2009). Further studies are required to analyze functional abnormalities in psychotic disorders to develop a statistical limit applicable in diagnosis. Despite these limitations, finding functional abnormalities continues to provide windows of opportunity. Their use has been reported (for example, in cognitive and premorbid functioning) as a predictor of treatment response in FPE patients (Levine & Rabinowitz, 2010).
A combination of retrospective studies that report abnormalities in subjects with SZ in childhood and prospective studies that confirm the findings, such as the one proposed by King et al., (2005), is suggested to develop more accurate behavioral clinical markers.
Neuroanatomic abnormalities
Magnetic resonance imaging (MRI) has made it possible to study structural changes in the brains of SZ patients. Most of the affected brain areas are related to cognitive-linguistic, affective-emotional and olfactory functioning (Nickl-Jockschat et al., 2011).
The olfactory bulb is significantly diminished in patients with chronic SZ, associated with a decrease in olfactory capacity. Chronic SZ patients scored lower on the University of Pennsylvania Smell Identification Test. Conversely, subjects at an UHR of SZ had a volumetric decrease in the olfactory bulb, yet normal olfactory ability (Turetsky, Hahn, Borgmann-Winter, & Mober, 2009).
Early-onset SZ patients present rapid loss of cortical gray matter in the superior frontal and temporal lobes (3%-4% annual loss), white matter growth retardation, ventricle enlargement, cerebellar volume loss, with patients with greater cortical loss presenting the most severe symptoms (Toga, Thompson, & Sowell, 2006; Rapoport & Gogtay, 2011; Ordóñez, Luscher, & Gogtay, 2016). Areas that showed small to moderate structural changes in SZ and UHR subjects include cerebellar gray matter (associated with damage to emotional modulation, motor and postural control), subcortical structures (including the thalamus, hippocampus, globus pallidum [single with increased volume], and amygdala), the anterior cingulate cortex, frontotemporal cortex (involved in conceptual thinking, verbal memory, working memory, and presence of hallucinations), and the parietal cortex (related to attention, memory, and motor control). At the cortex level, abnormalities were found in the formation of cerebral sulci (gyrification), suggesting problems during prenatal neurodevelopment (Chan, Di, McAlonan, & Gong, 2011; Haukvik, Hartberg, & Agartz, 2013; Fornito, Yücel, Dean, Wood, & Pantelis, 2009; Nickl-Jockschat et al., 2011; Jackowski et al., 2012).
Longitudinal studies of patients at prodromal or initial stages of SZ observed progressive cognitive deterioration following the FPE, a continuous volumetric reduction of gray matter, and alterations of white matter and lateral ventricles. The volumetric decrease of gray matter in the left superior temporal gyrus (p = .022) is significantly and marginally related on the right side (p = .62) to the onset and severity of verbal auditory hallucinations (Haukvik et al., 2013; Modinos et al., 2013). van Erp et al. (2018) found that schizophrenic subjects had an overall decrease in cortical thickness and brain surface area after comparing 4,474 patients with SZ with 5,098 healthy controls, where the largest effect size was located in the frontal and temporal lobes. A significant negative correlation was observed between age and bitemporal cortical thickness in SZ subjects.
In UHR subjects, FPE patients, and chronic SZ patients, Chan et al. (2011) reported a progressive decrease in gray matter, associated with the course and severity of the disease. The decline initially occurred in the cingulate region, the amygdala, the insula, and the temporal gyrus. In the final stages of SZ, it extended to the parahippocampal gyrus and/or the stria terminalis. It is important to note that a decline in the volume of the prefrontal cortex is already observed in the prodromal stages of the disease (Chan et al., 2011). It has also been shown that there is a decrease in white matter growth and an acceleration in the reduction of frontotemporal cortical volume and limbic structures in patients with early-onset SZ and/or UHR subjects who developed the spectrum, which suggests abnormal maturation of the brain during adolescence (Brent, Thermenos, Keshvan, & Saidman, 2013; Miguel-Hidalgo, 2013; Mubarik & Tohid, 2016).
The dorsolateral prefrontal cortex is part of the “Positive Work Network,” defined as the set of neurons whose metabolic activity increases during the performance of tasks requiring focused concentration. Its regulatory counterpart, the “Default Network,” is defined as the set of neurons with increased metabolic activity during resting in a normal subject and are associated with reflective, introspective processes. The two circuits are expected to complement each other, synchronize, and compete for the subject’s attention to the external or internal environment, for good subject-environment interaction. The impact on prefrontal gray matter therefore suggests that this is the origin of attention problems in schizophrenic patients. Indeed, a positive correlation has been reported between the loss of prefrontal cortical thickness and the severity of negative symptoms (Walton et al., 2018). Atrophy in the prefrontal cortex and its fascicles may cause asynchrony between the “Positive Work Network” and the “Default Network.” Although associations have been reported between gray matter loss and hypoactivation of the “Default Network” in subjects with SZ, the heterogeneity of each study varies. It remains to be determined whether this is due to hyperconnectivity versus hypoconnectivity, or hypoactivation versus hyperactivation (Gao et al., 2018). Sheffield and Barch (2016) have stated that abnormalities in “Default Network” activity during rest have been associated with problems with memory (episodic and working), attention and verbal learning. It has also been suggested that there is an impairment in bilateral frontotemporal and frontolimbic connectivity in UHR subjects developing SZ, and FPE subjects. Structural changes are more evident and generalized as the pathology progresses until asymmetry develops in brain connectivity (Vijayakumar et al., 2016; Wheeler & Voineskos, 2014; Ribolsi, Daskalakis, Siracusano, & Koch, 2014; Kuswanto, Teh, Lee, & Sim, 2012; Granger, 1996).
Damage to the hippocampal formation (HF) present in SZ can explain part of the pathophysiology of this disease, since this structure participates in memory, verbal learning, and emotional regulation. In patients at prodromal stages or FPE subjects, there is a volumetric decrease in the hippocampus associated with an increase in the metabolic activity of the CA1 of the HF, suggesting possible unregulated hyperglutaminergic activity (Nakahara, Matsumoto, & van Erp, 2018).
The above evidence suggests that UHR subjects, borderline psychosis and FPE patients are characterized by volumetric abnormalities in the prefrontal, temporal, and hippocampal cortex, which worsen and spread to other brain circuits until they become more evident during the course of the disease in both biological markers and cognitive and social deterioration (Fujiwara, Yassin, & Murai, 2015). An example of this is the relationship between cognitive damage, loss of gray matter, and altered brain metabolism (Wojtalik, Eack, Pollock, & Keshavan, 2012). It is important to note that the analysis of these structural changes by MRI, as well as other imaging techniques, are clinically valuable as predictors of the course of SZ. Further research is required due to the inherent complexity of SZ, the lack of a Gold Standard, the dearth of clinical trials conducted with this type of biomarker, its decreased sensitivity in early-stage patients, and the presence of similar abnormalities in other affective disorders (Smucny, Wylie, & Tregellas, 2014; Fu & Costafreda, 2013; Wise et al., 2017; Drevets, Price, & Furey, 2008).
Abnormalities in synaptic connections
Despite the questions about the pathophysiology of SZ, it is now possible to explain alterations at the neuronal level responsible for cognitive deficits (attention, processing speed, language and/or learning) and abnormalities in brain structure. This section describes the dopaminergic pathway that is altered in SZ and therefore essential to understanding the dopamine hypothesis of the etiology of SZ. The structures involved in dopaminergic modulation will therefore be described.
Dopamine is a neurotransmitter concentrated in large quantities within the ventral tegmental area (VTA), whose exocytosis is initially tonic (when it is continuously released to the extracellular medium in a slow, irregular manner). It subsequently changes to its phasic form (when it is released from the neurotransmitter at a higher frequency). It is essential for the tonic phase to be present to enable it to move to the phasic form (Grace, 2016; 2017).
Dopaminergic regulation is determined by various structures in a hierarchical manner, as shown in Figure 1. The pontine tegmental peduncle nucleus stimulates the change to the phasic form of the tonic neurons of the VTA when a threat/reward type stimulus is received, whereas the ventral pallidum reduces the number of active tonic neurons through GABAergic neurons. The exocytosis produced in the neurons located in the ventral pallidum depends on that produced in the infralimbic cortex. This stimulates the basolateral amygdala, increasing glutamate release, which in turn stimulates GABA secretion. CA1 and the subiculum of the hippocampus (ventral hippocampus) inhibit neuronal activity in the ventral pallidum through glutamatergic stimulation of the nucleus accumbens (ventral striae; Donegan & Lodge, 2017; Grace, 2017). In turn, the subiculum has inhibitory parvalbuminergic interneurons (IPI) that produce GABA-A, which decreases the stimulation of the nucleus accumbens. The ventromedial prefrontal cortex stimulates the nucleus accumbens through glutamate release, while the amygdala can stimulate the nucleus accumbens in response to stress. One possible pathway of altered dopaminergic modulation may be through the stimulation of D2 and D1 receptors in the mesolimbic and mesocortical pathways (Grace, 2016; Donegan & Lodge, 2017). Godsil, Kiss, Spedding, and Jay (2013) mention another altered communication pathway that can occur between the hippocampal formation and the prefrontal cortex, which is sensitive to stress.
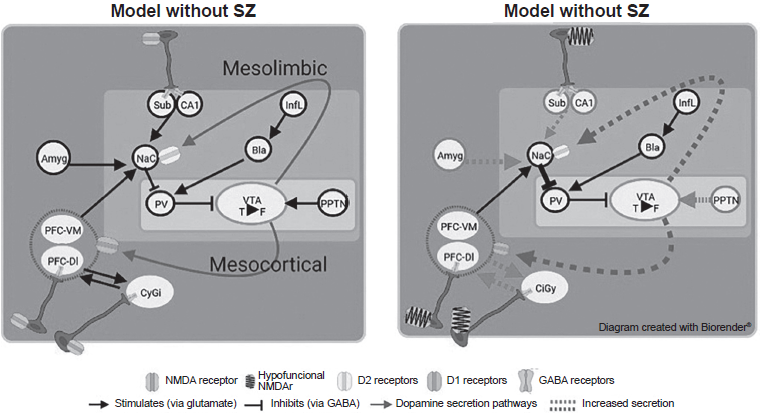
Figure 1 Representative diagram of dopaminergic circuitry in subjects with and without schizophrenia.Notes: Amyg: Amygdala; NaC: Nucleus accumbens; Sub: Subiculum; CA1: Cornu ammonis 1; PFC-VM: Prefrontal cortex ventromedial; PFC-DL: Prefrontal cortex dorsolateral; CiGy: Cingulate gyrus; VP: Ventral Pallidum; VTA: Ventral Tegmental Area; PPTN: Pedunculopontine tegmental nucleus; Infl: Infralimbic cortex; Bla: Basolateral amygdala'; T: Tonic form of secretion; F: Fascial form of secretion.
The pyramidal neurons of the dorsolateral prefrontal cortex are part of the “Positive Work Network” responsible for regulating the focus of attention in cognitive demand situations marked by the anterior cingulate cortex. Their control depends on IPIs in the prefrontal cortex, which make it possible to synchronize and modulate the activity of neuronal populations in the area. The activity of both types of IPI is usually reflected in the generation of the gamma oscillation frequency (30-80Hz) of the electroencephalogram (White & Siegel, 2016).
A decrease in the number of synapses and synchronizing/modulating IPIs plays a key role in the development of EZ symptomatology (Granger, 1996). The loss of these cells and synapses creates a state of dopaminergic hyperactivity and/or hyperreactivity in the VTA, susceptible to stimuli that activate the amygdala or NPPT (stress, threat, and reward situations; Grace, 2017). In addition to the Howes and Murray (2014) proposal, social adversities generate a biased cognitive scheme in patients with SZ, as a result of which they tend to interpret incoming stimuli as threatening.
The mesocortical pathway to the prefrontal cortex is thereby deregulated, meaning that the subject is unable to rank or interpret afferent stimuli appropriately (cognitive and negative symptoms; Howes & Murray, 2014). This could also explain why gamma activity in the prefrontal cortex is altered in SZ and is associated with impaired working memory (González-Burgos & Lewis, 2008). The increase in dopaminergic tone in the mesolimbic pathway due to dopaminergic regulatory input has been associated with the onset of positive symptoms (Donegan & Lodge, 2017).
The dopamine hypothesis is complemented by the “hypofunctional NMDA receptor” theory, which posits that when there is a defect in the NMDA receptors (NMDAR) that activate IPI, a deficient inhibition is produced, since there is no GABA release into the presynapsis of glutamatergic neurons in the prefrontal cortex, CA1, and subiculum (Howes, McCutcheon, Owen, & Murray, 2017; Coyle, Basu, Benneyworth, Balu, & Konopaske, 2012; Bast, Pezze, & mcGarrity, 2017; Rubio, Drummond, & Meador-Woodruff, 2012). This would explain the increase in blood flow and the loss of gray matter volume described by Nakahara et al. (2018). Likewise, the presence of hypofunctional NMDARs is associated with alterations in gamma oscillations correlated with cognitive damage (Gonzalez-Burgos & Lewis, 2012; Hasam-Henderson et al., 2018).
For this reason, glutamate increases in this area could serve as a SZ biomarker (Lieberman et al., 2018). Indeed, according to the model designed by Lesh, Niendman, Minzenberg, and Carter (2011), the decrease in the number of IPI in the prefrontal cortex produces dysfunction in the recruitment of networks for the performance of tasks, which is reflected in disorganization and the elaboration of erroneous behaviors.
Although each hypothesis and theory presented here to understand distinct aspects of SZ, this does not prevent them from participating in the study of other psychiatric disorders (such as loss of IPI in autism spectrum disorders and hypofunctional NMDAR in affective disorders; Filice, Janickova, Henzi, Bilella, & Schwaller, 2020; Lakhan, Caro, & Hadzimichalis, 2013). This phenomenon can complicate the development of specific biomarkers for SZ, but if they are actually developed, this would make it possible to diagnose a group of pathologies with a common origin.
Cell indicators
In addition to the dopaminergic and glutamatergic circuitry defects described in SZ, alterations in synapses have also been identified at the subcellular and molecular level.
The structural damage of IPIs occurs at two periods of neurodevelopment. Genetic abnormalities, such as the presence of hypofunctional NMDARs, mitochondrial changes, and aberrations in neuronal migration, originate during the pre-natal period. During the post-natal period, there is an increase in susceptibility to oxidative stress produced by environmental factors such as stress, hypoxic delivery and at the cellular level, hyperactivity with high demand and metabolic deficiency in processing oxidative stress (Hasam-Henderson et al., 2018; Barrón, Hafizi, Andreazza, & Mizrahi, 2017; Jiang, Cowell, & Nakazawa, 2013; Wang, Pinto-Duarte, Sejnowski, & Behrens, 2013). Figure 2 shows the risk factors present in the two periods of susceptibility during the life cycle and the consequences that lead to the onset of SZ in adolescence or early adulthood.
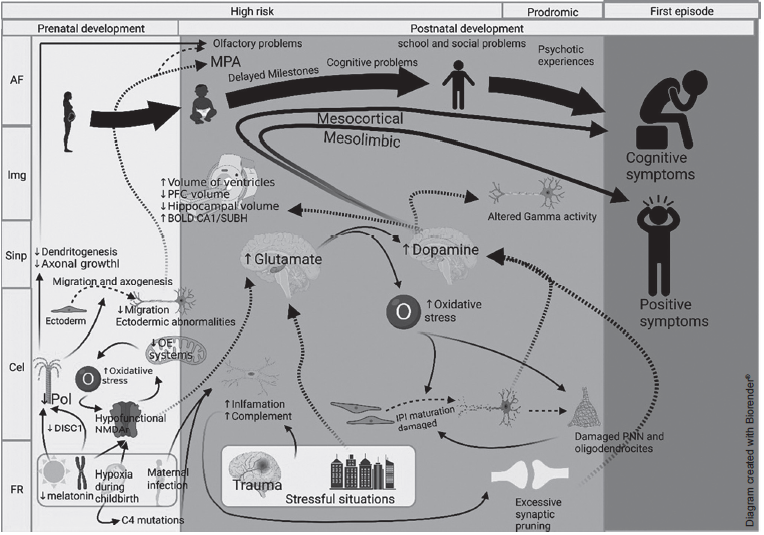
Figure 2 Diagram of correlation between risk factors, cell indicators, abnormalities in synaptic connections, neuroanatomical, minor physical and functional abnormalities developed in the prenatal and postnatal periods in subjects with schizophrenia.Notes: AF: Functional and minor physical abnormalities; Img: Neuroanatomic abnormalities revealed by imaging studies; Sinp: Abnormal synaptic connections; Cel: Cell indicators; RF: Risk Factors; Pol: Polymerization processes; NMDAR: NMDA receptor; PNN: Perineural networks; ↑: Increase, ↓: Decrease.
Moustafa, Hewdi, Eissa, Frydecka, and Misiak (2014) note that oxidative stress and mitochondrial dysfunction are associated with elevated levels of homocysteine, though the mechanism is unknown, and this marker is present in other affective disorders. Although attempts have been made to develop oxidative stress markers, further trials are required to obtain conclusive results (Barrón et al., 2017).
Post-natal neurodevelopment is characterized by IPI maturation processes, synaptic pruning, perineural network formation, and neurogenesis. During the course of these events, a critical period of increased neuronal plasticity and vulnerability to oxidative stress is produced (Do, Cuenod, & Hensch, 2015). IPI maturation requires glutamate and redox changes, as a result of which it is highly susceptible to hyperglutamatergic activity (accompanied by oxidative stress; Hardingham & Do, 2016). As mentioned earlier, the redox state modulates the gene expression of NMDAR. However, it has been suggested that oxidative stress induces the hypofunctionality of NMDARs. The latter downregulate antioxidant systems formed by glutathione peroxidase and thioredoxin-peroxidase, which leads to positive feedback with increased oxidative stress (Hardingham & Do, 2016; Carmeli, Knyazeva, Cuénod, & Do, 2012). Likewise, the alteration of other regulatory components of mitochondrial metabolism, such as the “transcriptional peroxisome proliferator-activated receptor-gamma coactivator” (PGC-1a) is associated with abnormal NMDAR functioning (Jiang et al., 2013).
In addition to the neuronal damage caused by oxidative stress, perineural networks are also susceptible to free radicals. Perineural networks are extracellular matrix structures responsible for stabilizing synapses after synaptic pruning and protecting them from oxidative stress. In particular, they protect the IPI synapse (Do et al., 2015). However, these structures, as well as oligodendrocytes, are vulnerable to redox dysregulation, which subsequently leads to white matter damage (Do et al., 2015; Steullet et al., 2016).
Patients who eventually develop SZ experience genetic and epigenetic events such as microglia-mediated inflammation, prenatal exposure to lead, malnutrition, maternal infection (cross-reaction with anti-NMDAR antibodies), and hypoxia during childbirth, which have been associated with damage in NMDARs (Nakazawa & Sapkota, 2020; Nakazawa et al., 2012; Guilarte, Opler, & Pletnikov, 2012).
Synaptic pruning is a natural phenomenon that allows the elimination of defective and unnecessary synapses (Cardozo et al., 2019). It has been hypothesized that excessive synaptic pruning could be part of the etiology of SZ. This increase may be multifactorial. Pro-inflammatory events such as alterations in the complement system, intrauterine infections (complement activation and inflammation), brain trauma, and sleep deprivation (microglia activation) have been associated with increased SZ-related synaptic pruning (Keshavan, Lizano, & Prasad, 2020).
During adolescence, the use of marijuana variants with high THC concentrations before the age of 17 increases the risk of developing SZ. A possible explanation is the alteration of neuronal maturation due to the interaction between THC and type 1 cannabinoid receptors (CRB1) present in prefrontal GABAergic neurons (Renard, Rushlow, & Laviolette, 2018). This is consistent with the association between cannabis use, brain volume loss in CRB1-rich regions, and the development of SZ in UHR individuals (Rapp, Bugra, Riecher-Rössler, Tamagni, & Borgwardt, 2012).
Abnormal DISC1 (Disruption in Schizophrenia 1) expression has been observed in UHR subjects developing SZ and in animal models (Dahoun, Trossbach, Brandon, Korth, & Howes, 2017). Together with FEZ1 (fasciculation and elongation protein Zeta-1), they have been described as promoters of neurogenesis, as a result of which their disruption results in proliferation defects, axonal growth, and neuronal migration (Miyoshi et al., 2003; Wu et al., 2017). The alteration of neuronal migration leads to the onset of minor physical abnormalities, fragmented neuronal development, and structural changes at the subcellular level in the olfactory neuroepithelium, since they are all derived from the same embryonic tissue (ectoderm; Forni & Wray, 2012).
A decrease in tubulin B III has been reported in neuronal precursors obtained from patients with SZ, suggesting a deficit in axonal formation in a post-natal neurogenesis zone and therefore an abnormality in neuronal migration. Part of the inability of axonal polymerization would explain hyposmia in SZ, since L-type Ca+ channels (necessary for the transmission of the olfactory impulse in the olfactory neuroepithelium) depend on microtubular integrity to function properly (Benítez-King, Ramírez- Rodríguez, Ortiz, & Meza, 2004).
Microtubular integrity in patients with SZ has also been associated with melatonin levels. The decrease in this indolamine during the prenatal period acts as a risk factor, since, under normal conditions, it stimulates axogenesis and synaptogenesis and protects cells from reactive oxygen species. Therefore, minimal exposure to sunlight during pregnancy (for example in women from Nordic countries), leads to an alteration of the light-dark cycle and a subsequent decrease in the concentration of melatonin circulating (Benítez-King, 2006).
The inability to identify the subcellular mechanisms of SZ makes it impossible to distinguish structural-genetic changes from other psychiatric disorders and hinders the development of viable biomarkers (such as Tubulin B-III or DISC1 abnormalities in mood disorders; Marchisella, Coffey, & Hollos, 2016). A clear understanding of its pathophysiology would make it possible to identify specific structural abnormalities and increase the prevention of risk factors that lead to cell damage.
The use of the olfactory neuroepithelium as an object of study to characterize biomarkers in SZ would be a key topic for future research. It is a non-invasive model in sample collection that allows greater control of variables and enables one to study neuronal processes in vivo in humans, such as postnatal neurogenesis (Lim & Alvarez-Buylla, 2016; Egbujo, Sinclair, & Hahn, 2016). In addition, the olfactory neuroepithelium reflects the metabolism and biochemical processes of neurons in the central nervous system, making it possible to study neurodevelopment in this tissue or in vitro in neuronal precursors derived from it.
DISCUSSION AND CONCLUSION
From a holistic perspective, it is not possible to study the etiology of SZ based on a unicausal model or without temporal flexibility, since, as the neurodevelopmental hypothesis states, “schizophrenia is the behavioral manifestation of a disruption in the neurodevelopmental trajectory during the early stages of life…” (Bitanihirwe & Woo, 2014). This disruption predisposes a person to develop the full set of psychotic symptoms. For this reason, in studying SZ, it is helpful to use a graduated chronological model such as the one presented in this article based on the model by Lesh et al. (2011) and Howes and Murray (2014), where the behavioral, functional, biological, and morphological phenomena present in subjects with SZ or at UHR are the final result of an underlying anomalous cellular process, caused by multifactorial irruptions in pre- and post-natal neurodevelopment. Risk factors such as inflammation, oxidative stress and prenatal hypoxia produce irregularities in cells and synaptic formation. This is reflected in imaging studies and the cognitive, behavioral, and motor skills relevant to clinical diagnosis. However, the use of these biomarkers and prodromes within clinical practice remains limited because of their pathognomonic and etiologic similarity to other psychiatric disorders (low specificity) and their inability to detect subtle abnormalities in UHR subjects (low sensitivity). The use of study models closer to in-vivo human biology dynamics (such as olfactory neuroepithelium) and a full understanding of SZ etiology are required to identify and discriminate specific SZ biomarkers, or alternatively guide the initial diagnosis to a group of disorders with a common etiology.











 nueva página del texto (beta)
nueva página del texto (beta)


