Background
Importance of language disorders in psychiatric diseases
One of the most relevant characteristics of the human species is the ability to communicate through language. This skill is acquired by imitation in the first years of life. Impairment in the learning process to communicate is observed during child development, around five years old. These processes are crucial for the optimal development of social relationships.
There is limited information about the prevalence of language disorders. Epidemiologic studies have estimated the rate of speech and language impairments among populations. According to data from the XII Censo General de Población y Vivienda en México 2000 (Instituto Nacional de Estadística, Geografía e Informática [INEGI] 2000), the prevalence of language problems fluctuates between 4.7% and 14.2% across the country (Instituto Nacional de Estadística, Geografía e Informática [INEGI], 2004). Since language impairment detection involves early stages of childhood, it has been estimated a prevalence of 2-3% for language disorders and 3-6% for speech disorders in school children without signs of neurological or genetic alterations. However, this prevalence reaches 15% in preschool age. Language problems are more frequent in boys than girls and in individuals who have a family history with language or speech delays and reading problems (Moreno-Flagge, 2013; Barragán & Lozano, 2011). Language and communication difficulties can be attributed to several causes, ranging from a physical disability to a learning difficulty, often due to deficits in cognitive functioning (Chan & Fugard, 2018).
A wide range of neuropsychiatric disorders has been reported in children from preschool to school ages, and in some cases continues into adolescence and adulthood (Chan & Fugard, 2018). In addition of symptoms in first episodes of mental disorders, other conditions frequently co-occur, such as anxiety, conduct, and learning disorders, but more importantly for this review, speech, language, and communication difficulties (SLCDs), which are among the most common (Chan & Fugard, 2018).
It has been reported that SLCD goes undetected in the clinical practice of psychiatric diseases. However, 70% of children referred to psychiatric services have shown a diagnosis of language impairment (Cohen, Davine, Horodezky, Lipsett, & Isaacson, 1993; Giddan, Milling, & Campbell, 1996). Two major and frequent mental disorders in humans, autism and schizophrenia, are characterized by language and communication problems in a different age of appearance. Patients with autism show a reduced interest and ability in communication, while in schizophrenia speech is disorganized leading to failure in communication (Wang, Jeffries, & Wang, 2016).
The attention-deficit hyperactivity disorder (ADHD) is a prevalent childhood neuropsychiatric condition that shows comorbidity with speech, language and communication difficulties. Bruce, Thernlund, and Nettelbladt (2006), reported that about half of children with ADHD were referred to speech and language therapists. Likewise, other authors found that 68% of children with ADHD had SLCDs (Trautman, Giddan, & Jurs, 1990).
Some clinical studies have reported the presentation of specific language impairment (SLI) in patients with bipolar disorder, suggesting that deficiencies in verbal functions are among the most outstanding. For example, interpretation, writing, and oral comprehension are presented as being below the expected considering the age and intellectual coefficient (Mattos, Rabelo, Gueiros, Soares, & Coutinho, 2009). Alterations in verbal fluency, both formal and semantic fluency, have also been reported in patients with bipolar disorder (Radanovic, Nunes, Gattaz, & Forlenza, 2008).
Brain organization of language
From the second half of the 19th century, the neural basis of human communication has been centered with the activity of three regions of the left hemisphere: Broca’s area in the region of the frontal gyrus (center of the representation of the movements), Wernicke’s area in the temporal gyrus (center of the images of the words), and the insula (center of speech) (Fisher & Marcus, 2006; Ardila, Bernal, & Rosselli, 2016). These areas represent the key-language specific substrates.
Neuroimaging techniques as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have greatly contributed to the advancement in the understanding of the cerebral organization of language (Ardila et al., 2016). Using these techniques, it has been possible to locate the areas of language processing in the brain: language-comprehensive area in the temporal lobe (BA22, BA21) and language production area in the frontal lobe (BA44, BA45) (Ardila et al., 2016). Likewise, different language disorders have been observed according to the specific location of the damage in these areas. Injuries involving the lexical/semantic system (Wernicke’s aphasia) and the grammatical system (Broca’s aphasia) allow the development of interpretative models of language evolution (Lieberman, 2015). The presence of these neural networks is considered a crucial evolutionary advantage for the unique faculty of language in humans (Friederici & Chomsky, 2017).
The motor approach to language development
It has been widely reported that children with SLI also perform more poorly in fine and gross motor abilities than their normative peers (Iverson & Braddock, 2011). In recent years, a novel proposal for language learning and development has been proposed: the embodied cognition theory (Schoemers & Pulvermüller, 2016). Infants experience an accelerated neuromotor development with better movement’s capacities each month in crawling, walking, running, climbing, hugging, etc. According to this theory, these sensorial and motor experiences may represent one of the brain platforms for language learning when the children learn to identify and produce the words that describe these actions. The brain networks that support the execution of these actions also support the comprehension and production of this type of words (Kemmerer & Gonzalez-Castillo, 2010).
An important aspect in this field is the presence of abundant hand-arm and body gestures in children’s oral language communication. This duality (oral language/gestures) is considered part of a complex form of semantic communication. Accordingly, more than half of children with SLI also present clinical performance on gesture imitation tasks (Iverson & Braddock, 2011). Recently, Trevisan, Sedeño, Birba, Ibáñez, and García (2017) studied the effect of body actions training in the reading performance of narrative events (naturalistic texts) in children with dyslexia. They found that this type of body actions has a significant effect on reading performance. The same effect has also been found in normative studies in adults with hockey-type actions (Beilock, Lyons, Mattarella-Micke, Nusbaum, & Small., 2008). Recent reviews have confirmed the effect of motor experience on linguistic and cognitive comprehension (Schomers & Pulvermüller, 2016; Yang, 2014).
Most language expressions in infant and younger children describe body actions (walking, running, climbing, kissing), or specific actions (drinking, playing). All these actions are marked by a verb; verbs selection and use (grammar and semantics) are highly dependent on prefrontal cortex. Particularly, motor and actions verbs are highly dependent on premotor cortex-basal ganglia activation (Andres, Finocchiaro, Buiatti, & Piazza, 2015). During verb fluency testing, Broca’s area activation in children is not as specific and localized as in young adults; progressive parcelling is achieved in Brocas’s area as age increases (Holland et al., 2001). These findings indicate a prolonged period of language development. Weakness in verb-argument structures (grammatical simplicity) has been described in children with SLI (Thordardottir & Weismer, 2002).
Neuroimaging studies in children with language learning difficulties have found that subcortical structures like the basal ganglia show diminished volume compared to normative children, additional to the cortical compromise in development (Krishnan, Watkins, & Bishop, 2016). Studies of SLI children, including non-affected siblings, reported that the basal ganglia were also compromised (reduced white matter) compared to control children (Badcock, Bishop, Hardiman, Barry, & Watkins, 2012).
Frequent neuroimaging findings in children with ADHD are alterations in gray or white matter at premotor, motor cortex, and cortico-striate components, mainly basal ganglia (Sutcubasi Kaya et al., 2018). Alterations of the striatal connectivity contribute to deficits in motor and inhibitory control (Barber et al., 2019). The embodied cognition theory may explain some of the language difficulties that are also frequent in children with ADHD. In fact, the prevalence of language difficulties in pediatric ADHD is higher than in normative population (Sciberras et al., 2014). In addition, ADHD children present alterations in fine motor coordination (Fenollar-Cortés, Gallego-Martinez, & Fuentes, 2017). Very recently, Barber et al. (2019) examined striatal maturation in a cross-sectional sample of 926 participants of the general population, spanning 8 to 22 years of age. The authors hypothesized that developing striatal connections would be predictive of clinical symptom domains (ADHD severity, psychosis, depression, and general psychopathology) in the general population.
The sensorial and motor support of early language development, specifically the cortico-striatal circuits that support naturalistic actions and motor actions, may represent an important pathway to genomic studies on language learning and development.
Genetics of language disorders
The process of speech and language acquisition is extraordinarily complex because it is one of the most distinguished cognitive developments in human evolution. With adequate opportunity, the vast majority of children rapidly develop efficient spoken language skills during the first few years of life. Sometimes children fail to acquire normal language, and this could be due to a secondary result of physical or neurological problems such as mental retardation, deafness, or cleft-lip or cleft palate defects (Fisher & Scharff, 2009). However, significant speech and/or language difficulties present in a subset of children remain unexplained (Bishop, 2003) and are heritable disorders. These cases represent a useful way to identify genes involved in the biological bases of human spoken language.
The first direct evidence of a specific gene that influences speech and language acquisition comes from an unusual autosomal dominant form of communication disorder in a large three-generation family, the KE family, a British family named in this way to maintain their anonymity (Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001). Approximately half the members of this family were affected by a severe speech and language disorder characterized by problems in the mount movements needed for speech (childhood apraxia of speech, also known as developmental verbal dyspraxia), together with impairment in receptive and expressive language (Vargha-Khadem, Watkins, Alcock, Fletcher, & Passingham, 1995; Watkins et al., 2002; Becker, Devanna, Fisher, & Vernes, 2018). Linkage studies in the KE family and mapping the translocation breakpoints in an unrelated child with similar language problems (Lai et al., 2000), guided the isolation of the Forkhead box protein P2 gene (FOXP2). All affected KE members were heterozygous for a point mutation in this gene, resulting in an amino-acid substitution in the encoded protein (Lai et al., 2001). As indicated in the next section, FOXP2 is a regulatory gene affecting the expression of a high number of downstream target genes.
Since these findings, other genes have been implicated in disorders of speech and language abilities (Table 1) and most of them are targets or interact with FOXP2 as indicated later. Pathogenic mutations in CHD3, SETD1A, WDR5, SETBP1, KAT6A, TNRC6B, and ZFHX4 genes have recently been identified by analyzing whole-genome sequences from nineteen unrelated individuals diagnosed with childhood apraxia of speech (Eising et al., 2019). Interestingly, these genes were highly co-expressed in the developing human brain. The axon guidance receptor gene ROBO1 was described as a risk candidate gene for dyslexia by mapping translocation breakpoints in a dyslexic patient and analyzing its expression in a large pedigree in which dyslexia was linked to the candidate chromosomal region (Hannula-Jouppi et al., 2005). Association studies in linkage regions determined KIAA0319 and DCDC2 as potential risk genes for dyslexia (Cope et al., 2005; Meng et al., 2005) and CMIP and ATP2C2 for SLI (Newbury et al., 2009) GNPTAB, GNPTG, and NAGPA show variants that have been associated with non-syndromic stuttering (Raza et al., 2016). Moreover, several polymorphisms in CNTNAP2 were associated with variations in language-related phenotypes that span different disorders, such as autism, and the general population (Anney, 2013; Newbury et al., 2011; Whitehouse, Bishop, Ang, Pennell, & Fisher, 2011).
Table 1 Phenotypes in relation to language and its genetic association
| Phenotype | Clinical observations | Gene | Chromosome | Protein name |
|---|---|---|---|---|
| Childhood apraxia of speech |
A disorder of speech motor programming that af- fects the production, sequencing, timing, and stress of sounds, syllables, and words. |
FOXP2 CHD3 SETD1A WDR5 SETBP1 KAT6A TNRC6B ZFHX4 |
7q31.1 7p13.1 16p11.2 9q34.2 18q12.3 8p11.21 22q13.1 8q21.13 |
Forkhead box protein P2 Chromo domain helicase DNA binding protein 3 SET domain-containing protein 1A WD repeat domain 5 SET binding protein 1 Lysine Acetyltransferase 6A Trinucleotide Repeat Containing 6B Zinc Finger Homeobox 4 |
| Developmental dyslexia | Difficulty in learning to read despite conventional instruction, adequate intelligence, and sociocultural opportunity: Prevalence from 5 to 12% of school- age children even though difficulties persist into adulthood. |
ROBO1 KIAA0319 DCDC2 DNAAF4 |
3p12.3 6p22.3 6p22.3 15q21.3 |
Roundabout homolog 1 Dyslexia-associated protein KIAA0319 Doublecortin domain-containing protein 2 Dynein axonemal assembly factor 4 |
| Specific language impairment |
It is characterized by difficulty in language acquisi- tion despite otherwise normal development and in the absence of any obvious explanatory factors. It affects 5 to 8% of preschool children and is highly heritable but multifactorial. |
CNTNAP2 CMIP ATP2C2 |
7q35 16q23.2 16q24.1 |
Contactin-associated protein-like 2 C-Maf-inducing protein Calcium-transporting ATPase type 2C member 2 |
| Stuttering | Disorder of the speech flow characterized by in- voluntary repetitions or prolongations of sounds or syllables, and by interruptions of speech known as blocks. It typically arises in young children, affecting at least 15% in an age range from 4 to 6 years. The disor- der usually resolves spontaneously before adoles- cence, leading to a population prevalence of 1 to 2% among adults. |
GNPTAB AP4E1 GNPTG NAGPA |
12q23.2 15q21.2 16p13.3 16p13.3 | N-Acetylglucosamine-1-phosphotransferase subunits α/β Adaptor related protein complex 4 subunit ε 1 N-Acetylglucosamine-1-phosphotransferase subunit γ N-Acetylglucosamine-1-phosphodiester Alpha-N-Ace-tyl- glucosaminidase |
Reviewed in National Center for Biotechnology Information (s.f.)
As for other human traits, most cases of developmental speech and language disorders have complex multifactorial inheritance, resulting from multiple risk factors of modest effect size (Graham & Fisher, 2015). Therefore, the identification of novel genes associated with these disorders is still a challenge. Advances in next-generation sequencing technologies as well as gene expression studies of developing brain could show new neurogenetic pathways implicated in language development.
FOXP2 and language impairment in psychiatric diseases
FOXP2 function
The family of transcription factors Forkhead box (Fox) was named after characterizing in the fork head gene (fkh) of Drosophila melanogaster mutations causing defects in head fold involution during embryogenesis resulting in spiked head in adult flies (Weigel, Jürgens, Küttner, Seifert, & Jäckle, 1989). Hundreds of Fox genes have been identified and classified into 19 subfamilies based on the evolutionary divergence of their forkhead DNA binding domain (FHD) (Hannenhalli & Kaestner, 2009; Morris, Stoychev, Naicker, Dirr, & Fanucchi, 2018). One of them is the FOXP subfamily, characterized by being comprised of four members (FOXP1-4) highly homologous (exhibit 55-65% sequence identity) and being multidomain proteins, giving them a peculiar molecular structure, containing a conserved DNA-binding forkhead box and functional domains: leucine zipper and zinc finger located N-terminal to the FHD. FOXP1, 2 and 4 contain a polyglutamine tract (Bowers & Konopka, 2012; Song, Tang, & Wang, 2016; Morris et al., 2018) (Figure 1). These proteins showed overlapping expression in the developing brain, as well as in other organs. FOXP3 disruption causes an immunological disorder: IPEX syndrome (immunodysregulation polyendocrinopathy enteropathy X-linked,). Mutations in/at FOXP1, FOXP2, and FOXP4 have each been linked to distinct neurodevelopmental disorders, which include global developmental delay an ID (intellectual disability), sometimes accompanied by features of autism and impaired speech and language abilities (McKusick, 1997).
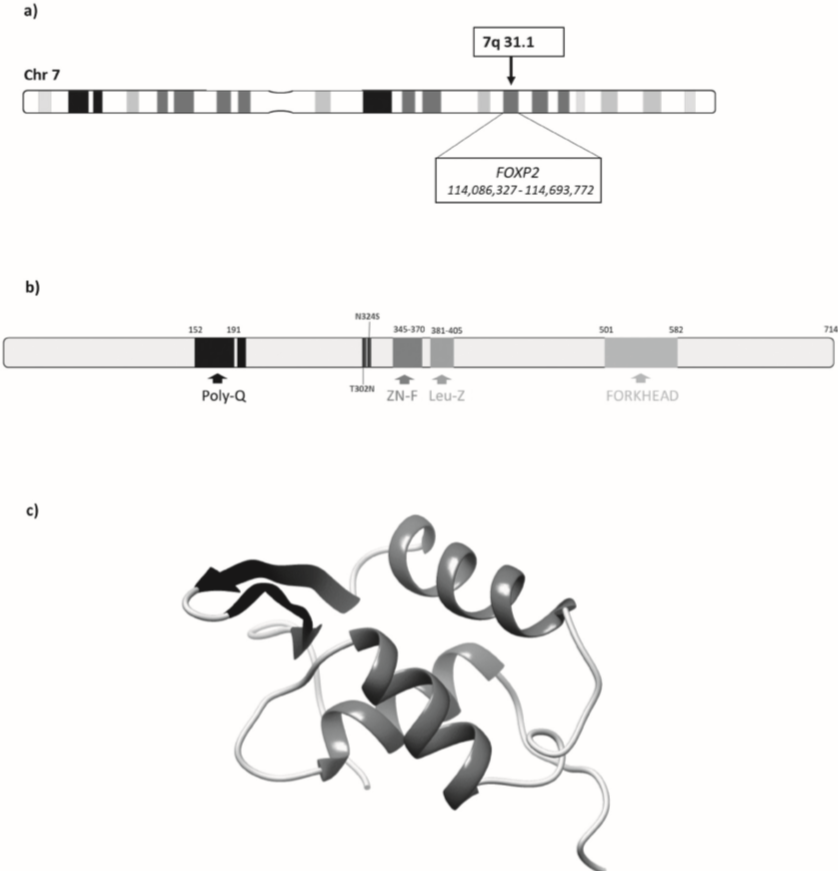
Figure 1 Human FOXP2. a) Chromosome 7 rerpesentation. Black arrow marks 7q31.1 band and box indicate the position of human FOXP2 gene (NCBI gene ID: 93986). b) Representation of structural and functional domains of human FOXP2 protein (GenBank: AAI43868.1). Poly-Q sequence, Zn finger, Leucine zipper and Forkhead domain (also known as a “winged helix”) are represented from black to light grey respectively. Evolutionary changes in two specific aminoacids between human and chimpanzee (T302N and N324S) are represented. This prediction has been performwed using SMART (Simple Modular Architecture Research Tool) software from EBI (European Bioinformatics Institute). c) 3D structure prediction of the FOXP2 protein using Phyre 2 (Kelley et al., 2015) and Chimera 1.12 software (Pettersen et al., 2004). It presents three α-helixes and two β-sheets.
Its participation in the differentiation of the lung epithelium has been demonstrated through its function of transcriptional repressor (National Institutes of Health, The European Molecular Biology Laboratory, & State Secretariat for Education, Research and Innovation SERI, 2002). It has been shown that this gene orchestrates the expression of other genes by interactions with proteins that act upstream of FOXP2 to control its expression (Nudel & Newbury, 2013). Foxp2 contains a winged helix DNA binding domain and two subdomains of transcriptional repression in the N-terminal region, which contain various protein-protein interaction motifs, suggesting an interaction with other transcription factors with activator or repressor functions (Nudel & Newbury, 2013). It has also been reported that FOXP2 interacts synergistically with the co-repressor CtBP-1 to inhibit transcription (Li, Weidenfeld, & Morrisey, 2004).
Although the role in the activation/repression of genes involved in the neurogenesis or neuronal function remains unknown, it has been reported that FOXP2 is able to repress certain tissue-specific genes, for example, Li and colleagues classified the foxp subfamily as potent transcriptional repressors by inhibiting gene expression through their interaction with repressive chromatin remodeling complexes such as NuRD (Li et al., 2016). For its part, Shu and coworkers reported the repressive activity of Foxp2 through its binding to homologous N-terminal repression domain with promoters of lung-enriched genes (Shu, Yang, Zhang, Lu, & Morrisey, 2001).
The FOXP2 protein is also active from spin cord to organs such as spleen, intestine, skeletal muscle, lungs, and kidney (Shu et al., 2001; Becker et al., 2018; Song et al., 2016), including the fetal and adult brain (National Center for Biotechnology Information, U.S. National Library of Medicine. s.f.; OMIM, s.f.). In humans, high expression has been reported in the process of neuronal specification and brain configuration has been detected from as early as the 44th day of gestation (Lai, Gerrelli, Monaco, Fisher, & Copp, 2003). The expression of FOXP2 begins to decay postnatally until it becomes undetectable in the adult stage brain (Teramitsu, Kudo, London, Geschwind, & White, 2004). Unlike its expression in the brains of other vertebrates such as rodent and zebra finch, is found in constant and high expression from the embryonic to the adult stage (Song et al., 2016; Teramitsu et al., 2004; Ferland, Cherry, Preware, Morrisey, & Walsh, 2003). FOXP2 expression is first detected in the midline of the hindbrain, and subsequently becomes more complex as the embryonic development progresses. The expression of the FOXP2 gene in the human brain is limited to areas such as striatum, thalamic nuclei, hippocampus, cerebellum, and cortex, all of them belonging to the cortico-striatal region with great potential for psychiatric disorders and essential for the normal development of speech and language (U.S. Department of Health & Human Services, s.f.; Song et al., 2016). Some of these brain regions, such as the caudate nucleus, the putamen, and the cerebellum were previously implicated in the neuroimaging studies as regions in which the affected KE family members differed from the unaffected members or controls in terms of structural abnormalities (Bruce & Margolis, 2002; Watkins et al., 2002).
This gene is required for the proper development of speech and language regions of the brain during embryogenesis, and may be involved in a variety of biological pathways and cascades that may ultimately influence language development. Mutations in this gene cause speech-language disorder 1 (SPCH1), also known as autosomal dominant speech and language disorder with orofacial dyspraxia. Multiple alternative transcripts encoding different isoforms have been identified in this gene (GeneCards®: The Human Gene Database, 1997). RT-qPCR and Western blotting indicated differential regulation of 13 new additional target genes in response to overexpression of human FOXP2 (Oswald et al., 2017).
FOXP2 evolution
The FOXP2 gene was the first to appear in the human capacity scene, named language. This gene has a sequence highly conserved notwithstanding the distance between vertebrate species. Despite this conservation, two aminoacids substitutions occurred on the human branch after splitting from the chimpanzee and absent in all the others primates, indicating a selective sweep that probably occurred within the past 200,000 years. These two nonsynonymous substitutions seem to have been subject to positive selection in recent human history that genetically mediated language (Fisher & Marcus, 2006). FOXP2 played a key role in the development of modern language, unique to Homo sapiens. Presumably, these aminoacid changes in exon 7 of the FOXP2 gene were selected because they allow to achieve a precise control of the orofacial movements and facilitates the refinement of the articulation of which human language makes use (Enard et al., 2002).
Despite the above, recent findings have shown controversy in the evidence of positive selection of FOXP2 in humans. Atkinson et al. (2018) reported not finding evidence that the two aminoacid substitutions from the FOXP2 gene have been associated with recent positive selection in humans < 200 kya (thousand years ago). Conversely, they have suggested an ancient selective sweep that could explain the presence of the two derived substitutions in humans (Atkinson et al., 2018).
FOXP2 disruption in language impairment
Up to now, data indicated that the disruption of one copy of FOXP2 is sufficient to derail speech development, causing a rare form of speech and language impairment. No human has yet been identified with the homozygous loss of FOXP2 function. Mice completely lack with functional Foxp2 (the murine orthologue) display severe motor impairment, reduced growth, and delayed cerebellar development, dying few weeks after birth. Disruption of one copy of Foxp2 provokes important alterations in ultrasonic sounds that juveniles produce in response to separation from their mothers (Shu et al., 2005).
Table 2 summarizes the genetic alterations found in FOXP2-related speech and language disorders. Chromosomal rearrangements and deletions disturbing one allele result in decreased FOXP2 protein level. Missense mutations produce abnormal proteins in which DNA binding activity, dimerization (transcriptional regulation of downstream target genes requires protein dimerization), or nuclear translocation are altered. These mutations result either in haploinsufficiency of FOXP2 or have a dominant-negative effect (Vernes et al., 2006; Estruch, Graham, Chinnappa, Deriziotis, & Fisher, 2016). The pathogenic variant in the KE family (p.R553H) changes a conserved residue in the DNA Forkhead binding domain, impairing the ability of FOXP2 to bind target sites (Stroud et al., 2006). In addition, the R553H mutation affects the nuclear localization signal at C-terminus and consequently hampers the nuclear localization of the protein (Mizutani et al., 2007). Maternal uniparental disomy of chromosome 7 has also been reported in several cases, suggesting a differential allelic expression of FOXP2, with a restricted expression of the paternal copy (Feuk et al., 2006). However, there are contradictory results concerning the imprinting status of the FOXP2 locus (Thomas et al., 2012).
Table 2 Genetic alterations in FOXP2 that cause speech and language disorders
| Mutation | Affected individuals with FOXP2-related language disorder (%)1 | Mode of inheritance and recurrence risk 1 |
|---|---|---|
| Gene deletion | ≈52 | About 80% of large contiguous gene deletion that includes FOXP2 are de novo; the remainder are inherited in an autosomal dominant pattern. |
| Chromosomal rearrangement (trans- location, inversion) | ≈8 | Sibs with a parent having a structural variant affecting chromosome 7 show increased risk, which depend on the specific structural variant. |
| Maternal uniparental disomy of chro- mosome 7 | ≈11 | Risk of recurrence is not augmented over that of the general population. |
| Sequence variant (missense, non- sense, and splice-site variants and small intragenic deletions/insertions) | ≈29 | Approximately 70% are de novo; the remainder are inherited in an autosomal dominant manner. |
1Reviewed in Morgan et al., 2017.
Regardless of the mutation in FOXP2, childhood apraxia of speech constitutes a central phenotype of the resulting speech and language disorder. Additional findings include oral motor deficits, global developmental delay, and autism spectrum disorders that are more likely caused by large copy number variants or structural variants affecting FOXP2 (Morgan, Fisher, Scheffer, & Hildebrand, 2017). Among all cases of childhood apraxia of speech, which have a highly heterogeneous genetic basis, what fraction is caused by mutation in FOXP2 is not yet well established.
FOXP2 targets
The identification of novel genes associated with language disorders is a difficult process because their effect in this complex condition is low. Nowadays linkage studies have contributed to identify new genes, and GWAS and big cohorts of patients are the main and most useful tools to stablish genetic associations. As indicated before, FOXP2 has been the most studied and important gene related with human language, mainly by its biological relevance. Thus, the identification of FOXP2 targets related with language constitutes a new way to approach the role of genetics in human language development (Figure 2).
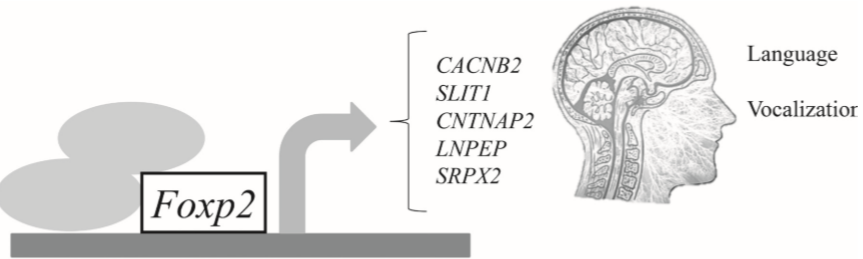
Figure 2 FOXP2 might control vocalization and language development in cooperation with other proteins (grey circles in the figure) such as FOXP1 (Xu et al., 2018), by regulating the expression of target genes (grey arrow) that participates in the formation of language related circuits in the brain.
As described in Konopka et al. (2009), CACNB2 (Calcium Voltage-Gated Channel Auxiliary Subunit Beta 2) gene expression is regulated by FOXP2. This gene encodes for a protein that contributes to the function of the calcium channel by increasing peak calcium current and shifting the voltage dependencies of activation and inactivation. Mutations in CACNB2 have been associated with Brugada syndrome (Brugada et al., 2005), but also common single nucleotide polymorphisms (SNPs) of CACNB2 have been shown to contribute to a range of neurodevelopmental disorders, including language disorders (Kim & State, 2014). Chip-microarray techniques have allowed to identify the SLIT1 (Slit Guidance Ligand 1) gene as another FOXP2 target (Spiteri et al., 2007). Along with the SLIT2 protein, SLIT1 is involved in neural axon guidance during neural development, acting as a molecular cue in cellular migration mediated by interaction with ROBO receptors (Plump et al., 2002). This interaction is the foundation of the SLIT-ROBO pathway, which has been related with speech sound language disorders and the evolution of vocal learning (Wang et al., 2015).
As mentioned above, polymorphisms of CNTNAP2 are related with a variety of language phenotypes in different disorders. This gene is down-regulated by FOXP2 (Vernes et al., 2008) and encodes a member of neurexin family required for the radial and longitudinal organization of myelinated axons by its mediation function between neurons and glia during nervous system development (Gao et al., 2019). According to some authors, CNTNAP2 is also linked to language development by its interaction with oxytocin (OXT) during critical developmental windows (Peñagarikano et al., 2015), as OXT would affect brain components closely interwoven to different facets of language (Theofanopoulou, 2016). Gregory et al. (2009) have reported that in patients with autism spectrum disorder, who present language deficits as a central feature of the phenotype, a mechanism of epigenetic misregulation of the OXTR (oxytocin receptor gene) through genetic silencing with DNA methylation associated with the activity and functional coupling of neurons impacting this on language performance (Puglia, Lillard, Morris, & Connelly, 2015). These studies also associate the LNPEP (Leucyl and Cystinyl Aminopeptidase) gene to language through its function as a peptidase that metabolizes oxytocin. Moreover, LNPEP controls synaptic transmission and formation, and was identified as a FOXP2 target, which regulates LNPEP by repressing its expression (Vernes et al., 2007). In addition, two variants of SRPX2 (Sushi Repeat Containing Protein X-Linked 2) gene (Asn327Ser and Tyr72Ser) have been described as pathogenic, causing rolandic epilepsy and intellectual disability and speech difficulties (Roll et al., 2006). SRPX2 encodes for a protein involved in the development of speech and language centers in the brain, acting as a ligand for the urokinase plasminogen activator surface receptor (uPAR) (Royer-Zemmour et al., 2008). Besides, Roll et al. (2010) showed that there is a transcriptional regulatory network between FOXP2 protein and the SRPX2/uPAR complex, so that FOXP2 regulates the expression of both.
There are more than 100 genes considered as FOXP2 targets (Konopka et al., 2009) but their relationship with language remains unclear. Further investigations are needed to understand the genetics of human language.
FOXP2 and psychiatric diseases
Shared genetic vulnerability has been identified in different neuropsychiatric disorders (Ivorra et al., 2014). FOXP2 is one of the 23 clinically relevant genes shared by autism spectrum disorder (ASD), schizophrenia, and bipolar disorder (Khanzada, Butler, & Manzardo, 2017). This gene was considered as a potential susceptibility locus in neuropsychiatric disorders in which language impairment may occur, such as autism and schizophrenia. In the first condition, deficits in social communication are a key phenotype appearing in early childhood. In schizophrenia, language problems are characterized by disorganized speech and debut in young adulthood. Both conditions show complex etiology, with a genetic component as a central factor associated with environmental triggers that together may affect brain development.
Large chromosomal deletions involving the FOXP2 locus have been described in patients suffering both ASD and developmental verbal dyspraxia (Feuk et al., 2006). It has been proposed that genes regulated by FOXP2, such as the neurexin gene CNTNAP2, are involved in both language impairment and autism. Neuroimaging and genetic data support that CNTNAP2 is a risk factor for ASD and related neurodevelopmental disorders (Peñagarikano & Geschwind, 2012). Very recently, Zhou et al. (2019) reported rare functional variants of this gene when resequencing 358 ASD candidate genes in a case–control cohort from Chinese population. CNTNAP2 is proposed to play a role in controlling the assembly of functional network between important integrative connectivity hubs of the mammalian brain, thereby disruption of the gene function might predispose to autism and other neurodevelopmental disorders by specific alterations of connectivity in prefrontal areas (Liska et al., 2017).
FOXP1, a paralog of FOXP2, is considered a high-confidence causal ASD gene. Several studies have reported different mutations in FOXP1 related to the presence of ASD traits, intellectual disability, and psychiatric and language features (Meerschaut et al., 2017). Dimerization of FOXP proteins (homo- and hetero-dimerization) occurs for transcriptional regulation of target genes (Li et al., 2004). Interestingly, Foxp1 and Foxp2 of zebra finch, alone or in combination, can differentially control the CNTNAP2 promoter (Mendoza & Scharff, 2017). These results show how versatile the regulation of these proteins can be in respect to the transcriptional activity of the target genes and add complexity in their role in the development of neurodevelopmental disorders.
FOXP2 was first related with schizophrenia in a case-control study in which a FOXP2 polymorphism, rs2396753, was associated with thought disorders and auditory hallucinations in patients with schizophrenia (Sanjuán et al., 2006). This polymorphic variant was later correlated with grey matter reduction in patients with this disease (Španiel et al., 2011). Interestingly, rs2396753 was also linked to activity in brain areas related to language in healthy adults (Ocklenburg et al., 2013). Collectively, these data suggested that FOXP2 polymorphisms might play a neurogenic role in language impairment in schizophrenia. However, it remains controversial whether common FOXP2 polymorphisms could affect brain structures and play a major role in language and speech impairment in schizophrenia (Hoogman et al., 2014; McCarthy, Clark, Jablensky, & Badcock, 2019). In addition, the analysis of multiple classes of genetic variation in large genomic datasets concluded that CNTNAP2 may not be a robust risk gene for psychiatric phenotypes (Toma et al., 2018). The effect of the polymorphisms of FOXP2, and its target genes like CNTNAP2, in language dysfunction of neuropsychiatric disorders remains to be clarified.
Discussion and conclusion
Advances in the molecular study of psychiatric diseases have brought along findings that have allowed to unravel the clinical complexity that comprises them. However, in the same way they have been the gateway of a network interrelated mechanisms between the clinic they present.
The equation FOXP2-language impairment-psychiatric illness is still an interesting route to study the large percentage of patients with psychiatric diseases who continue to present some alteration of language within the clinical core.











 nueva página del texto (beta)
nueva página del texto (beta)


