Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.39 no.3 Texcoco Set. 2021 Epub 13-Dez-2021
https://doi.org/10.18781/r.mex.fit.2106-7
Scientific articles
Morphological characterization and biocontrol potential of Trichoderma species isolated from semi-arid soils
1 Departamento Académico de Agronomía, Universidad Autónoma de Baja California Sur. Carretera al sur km 5.5. Colonia El Mezquitito, CP 23080, La Paz, Baja California Sur, México;
2 Centro de Investigaciones Biológicas del Noroeste. Avenida Instituto Politécnico Nacional 195. Colonia Playa Palo de Santa Rita Sur, CP 23096, La Paz, Baja California Sur, México;
3 Ministerio de Educación Superior, Universidad de Granma. Carretera a Manzanillo km 17 Peralejo, CP 85149, Granma, Cuba;
4 Facultad de Ciencias Agrotecnológicas, Universidad Autónoma de Chihuahua. Escorza 900, Colonia Centro, CP 31000, Chihuahua, Chihuahua, México;
Species of Trichoderma spp. were isolated, identified and characterized associated with Pachycereus pringlei and Jatropha cinerea as biocontrol agents against phytopathogenic fungi. The antagonistic agents were isolated from six sites in Baja California Sur, Mexico. The identification was made based on its morphological characteristics and abundance, frequency of occurrence and mycelial growth of Trichoderma spp. and in vitro antagonism against F. oxysporum, F. solani, R. solani, C. gloeosporioides and A. alternata was determined. Eighteen Trichoderma isolates concentrated in seven species were obtained: T. asperellum, T. atroviride, T. harzianum, T. koningii, T. viride, T. longibrachiatum and Trichoderma spp. Duncan’s test (p<0.05) showed significant differences in the abundance of the species (CFU/g of soil) and the frequency of occurrence. The largest population was found in El Saltito, Los Encinos and Las Pocitas with CFU of 2.1, 1.8 and 0.7 × 103 g-1 of soil respectively. In the in vitro antagonism, T. koningii was the one that significantly inhibited the growth of phytopathogenic fungi. The antifungal activity of the various of Trichoderma spp. can be an alternative for the biocontrol of diseases caused by phytopathogenic fungi.
Key words: Fungus; soil; diversity; population; phytopathogens; biological control
Se aislaron, identificaron y caracterizaron especies de Trichoderma spp. asociadas a plantas de Pachycereus pringlei y Jatropha cinerea como agentes de biocontrol hacia hongos fitopatógenos. Los agentes antagónicos se aislaron de seis sitios en Baja California Sur, México. La identificación se realizó en base a sus características morfológicas y se determinó; abundancia, frecuencia de ocurrencia y antagonismo in vitro hacia F. oxysporum, F. solani, R. solani, C. gloeosporioides y A. alternata. Se obtuvieron 18 aislamientos de Trichoderma concentrados en siete especies: T. asperellum, T. atroviride, T. harzianum, T. koningii, T. viride, T. longibrachiatum y Trichoderma sp. La prueba de Duncan (p<0.05) mostró diferencias significativas en la abundancia de las especies (UFC/g de suelo) y la frecuencia de ocurrencia. La mayor población se encontró en El Saltito, Los Encinos y Las Pocitas con UFC de 2.1, 1.8 y 0.7 × 103 g-1 de suelo respectivamente. En el antagonismo in vitro, T. koningii inhibió significativamente el crecimiento de los hongos fitopatógenos comparado con el control comercial. La actividad antifúngica de las diversas especies de Trichoderma spp. pueden ser una alternativa para el biocontrol de enfermedades ocasionadas por hongos fitopatógenos de las especies analizadas.
Palabras clave: Hongo; suelo; diversidad; población; fitopatógenos; control biológico
Trichoderma (Ascomycota: Hypocreales) is a cosmopolitan fungus that includes over 100 species found in different climatic zones and colonizes a wide range of niches, including living and dead plants, soil, sediment, organic matter, animal tissue, and others (Wang and Zhuang, 2020; Nuangmek et al., 2021). Trichoderma spp. has a versatile metabolism that gives it the ability to control diverse phytopathogens, due mainly to the production of hydrolytic enzymes, competition for space and nutrients, resistance induction in the host, antibiosis, mycoparasitism, among others (Gamarra et al., 2017; Zhou et al., 2020). There has been significant progress on the regulating mechanisms used by different species to establish themselves in terrestrial or marine habitats (Gal-Hemed et al., 2011; Su et al., 2018). One of the differences is reportedly the more efficient production of secondary metabolites (6-pentyl-α-pyrone and trichodermaketones), along with a greater production of enzymes (chitinase and β-1,3-endoglucanase) (Kim et al., 2020). Some of the most important Trichoderma species acting as biocontrol agents against phytopathogens are T. reesei, T. koningii, T. asperellum, T. viride, T. harzianum, T. aureoviride, and others (Brito et al., 2020; Alfiky and Weisskopf, 2021).
This genus is used to develop commercial bioproducts for the control of plant diseases (Singh and Jadon, 2019; Carrillo et al., 2020). However, there are cases of low efficiency in the control of phytopathogens, since the diverse species of Trichoderma that make up the commercial bioproducts are originally from different edaphoclimatic regions to the geographic area in which it is applied (Harman et al., 2010). It is therefore important to select native microorganisms adapted to the edaphoclimatic conditions of the place in which the biocontrol of plant diseases is intended to be carried out (Al-Mekhlafi et al., 2019; Tegene et al., 2021). Worldwide, agricultural activities are carried in deserts with limited yearly rainfall and extreme temperatures of over 40 °C. Soils in semiarid areas are commonly alkaline with poor organic matter (Yang et al., 2019; Elnashar et al., 2021), therefore, crops that grow under these conditions require bioproducts that help achieve a greater productivity and quality of the harvest, hence the study of Trichoderma spp. native to desert areas are an important resource for the sustainability of agricultural crops (Torres-De la Cruz et al., 2015; Michaud, 2018). In Mexico there are scarcely any studies related to obtaining native Trichoderma isolations in desert areas. Due to this, the aim of the present study was to isolate, identify and characterize Trichoderma species as biocontrollers of semiarid areas against phytopathogenic fungi.
Materials and methods
Sampling and study site. During the year 2019-2020, strains of Trichoderma spp. were isolated in Baja California Sur, Mexico. In the area of study, the predominant climate is semiarid with a maximum temperature of 44 °C and a minimum of 16 °C with rainfalls of 122.5 mm/year. The predominant vegetation is the xerophilous scrub. Samples were taken from six places (Figure 1): Las Pocitas (24° 24’ 30.13N-111° 5’ 53.55” W and 57 masl), El Cajete (24° 12’ 56.95”N-110° 35’ 17.21” W and 17 masl), El Saltito (24° 14’ 8.03” N-110° 12’ 10.36” W and 55 masl), Los Encinos (24° 0’ 9.02” N-110° 9’ 29.95” W and 530 masl), El Triunfo (23° 47’ 39.2” N-110° 7’ 6.56” W and 516 masl) and Los Barriles (23° 42’ 7.72” N-109° 44’ 39.69” W and 170 masl). In each site, 10 soil samples were collected, each weighing 2 kg, at a depth of 30 cm of the Pachycereus pringlei and Jatropha cinerea rhizosphere (representative species of the xerophilous scrub) (Siddiquee, 2017). The samples were stored in sterile bags at 20 °C.
Isolation and identification. The isolation of Trichoderma spp. was performed in the Phytopathology Laboratory of the Autonomous University of Baja California Sur, Mexico. The procedure was carried out using the method proposed by Karthikeyan et al. (2008). From each homogenized soil sample, a 50 g subsample was taken and placed in a beaker with 450 mL of sterile distilled water and stirred for 20 min. One milliliter of the mixture was taken to carry out a series of dilutions until 10-3, 10-4 and 10-5 were obtained. Out of each dilution, a 200 μL aliquot was uniformly striated in Petri dishes with Potato-Dextrose-Agar (PDA, Bioxon) and incubated at 28 °C for seven days. After five days of incubation, the culture forming units (CFU), which displayed a green color, were quantified. They were replanted in PDA until pure cultures were obtained. The Trichoderma spp. isolations were identified with the taxonomic keys proposed by Rifai (1969), Barnett and Hunter (1972) and Bissett et al. (2015). The macroscopic morphological characteristics observed were the color of mycelia, the texture of mycelia and the formation of concentric rings. The microscopic characteristics determined under a compound microscope (Labomed LX 400) were the shape of conidia, phialides and the presence of chlamydospores.
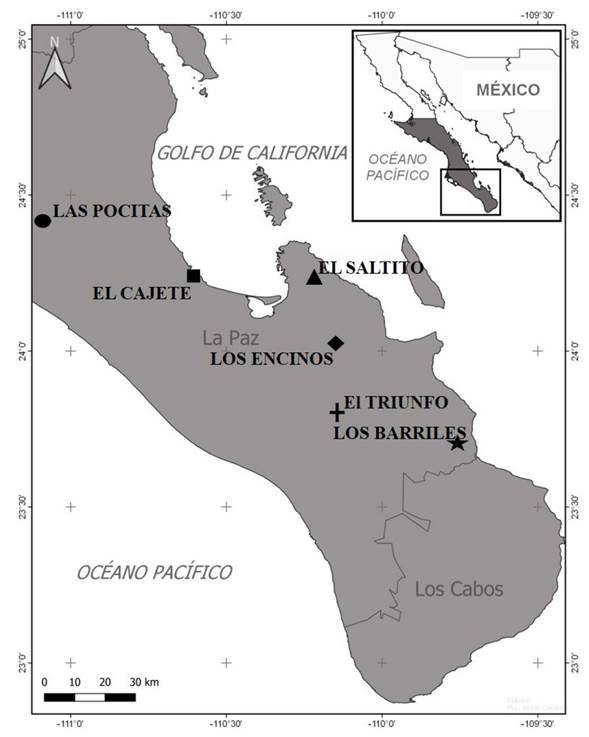
Figure 1 Study sites for soil sampling and isolation of Trichoderma species in Baja California Sur, Mexico.
Trichoderma abundance and frequency of occurrence. The abundance and frequency of Trichoderma spp. were determined with the quantification of the cultures of each area of study using the formula proposed by Muniappan and Muthukumar (2014): Abundance = Number of CFUs from a fungus in the sample /total number of CFUs from all the fungi in each sample × 100 and it was expressed in CFU g-1. The frequency of occurrence (F) was calculated using the formula F (%) = # agroecosystems with a species of fungus /# agroecosystems examined × 100. These experiments were carried out with five repetitions and evaluated twice.
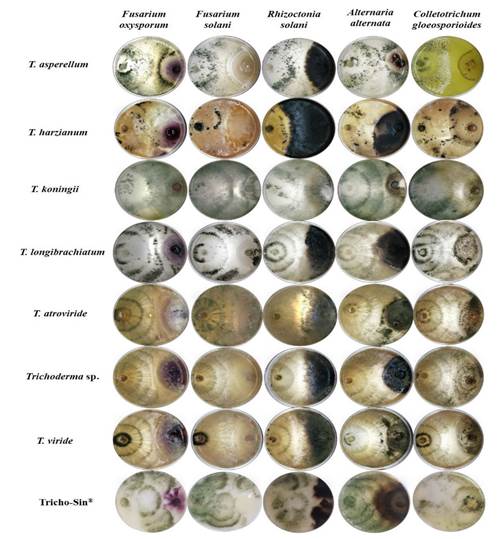
Antagonism of Trichoderma spp. vs. phytopathogenic fungi. The antagonistic activity was evaluated in vitro using the dual culture method of the Trichoderma isolations that presented the greatest speed of mycelial growth towards five phytopathogenic fungi (Fusarium oxysporum, Fusarium solani, Rhizoctonia solani, Colletotrichum gloeosporioides and Alternaria alternata) obtained from the ceparium of the Phytopathology Laboratory. Its pathogenicity was evaluated in earlier studies (Camacho-Aguiñiga, 2016; Núñez-Madera et al., 2016; Rodríguez-Macías, 2016). The microorganisms were cultivated in Petri dishes with PDA for seven days at 28 °C. Later, a disk, 5 mm in diameter, was taken from each Trichoderma and phytopathogen and they were both placed on the edges of the dish, 6 cm away from each other. The Petri dishes were incubated at 28 °C for five days and the mycelial growth of the pathogen was measured in cm, in relation to the Trichoderma. A group of Petri dishes were planted with a Trichoderma from the commercial product Tricho-Sin® based on T. harzianum (commonly used in the organic agriculture of the region) along with the phytopathogens. As a control, Petri dishes were planted on one edge, only with each fungus. The percentage of inhibition (PI) was determined using the formula by Otadoh et al. (2011): PI (%) = R1-R2/R1 × 100, where R1 = mycelial growth of the fungus in the control dishes, and R2 = mycelial growth of the fungus in the presence of the antagonist. Ten repetitions were carried out per treatment and the experiment was performed twice.
Statistical analyses. The data were analyzed using a one-way analysis of variance (ANOVA) using the software STATISTICA 10.0 (StatSoft software package, Tulsa, OK) and Duncan’s test (p≤0.05) was used for the separation of means. Before the analysis of variance, the percentages were converted to arcsine-square root.
Results and discussion
Isolation and identification of Trichoderma isolations. Eighteen isolations were obtained and grouped in seven species: T. harzianum, T. viride, T. atroviride, T. asperellum, T. longibrachiatum, T. koningii and Trichoderma sp. (Table 1). Globular to subglobular conidia were found in T. atroviride, T. viride, T. longibrachiatum and Trichoderma sp. However, ellipsoidal conidia were found in T. asperellum, T. harzianum and T. koningii. Five species (T. atroviride, T. harzianum, T. longibrachiatum, T. viride and Trichoderma sp.) displayed two to three concentric areas of conidiation, whereas two species showed scattered conidiation in one ring (T. koningii and T. asperellum). Phialides displayed a globular shape in the center, except in T. viride, T. koningii and T. asperellum, which displayed a thin morphology. In most species, phialides tended to group into 2-3 whorls, except for T. harzianum and T. longibrachiatum, which presented a solitary arrangement. These characteristics coincide with those indicated in the taxonomic keys pointed out earlier. Although Trichoderma sp. displayed a colonial morphology, which is typical to the genus, and microscopic similarities, some characteristics varied, such as the shape of the conidia, their arrangement and the sporocarp.
The presence of these species may represent possible stress-resistant biotypes. Osorio-Concepción et al. (2013) mention that the variability of isolations in one place may be stimulated by stress factors such as light, a lack of nutrients or changes in pH. Al-Ani (2018) and Bononi et al. (2020) mention that the rapid growth of Trichoderma spp. and its ability to grow in different substrates has allowed its isolation in diverse soils worldwide. However, although this genus has been studied in diverse barren areas (Sharma et al., 2019; Ma et al., 2020), this is the first report of its isolation from a semiarid area in Northwestern Mexico. The identification of Trichoderma species by their morphology continues to be an efficient method to identify this fungus (Wu et al., 2017; Asis et al., 2021). The species of T. harzianum, T. atroviride, T. asperellum, T. koningii, T. longibrachiatum and T. viride have already been reported as biocontrol agents for phytopathogens in diverse crops (Miguel-Ferrer et al., 2021; Hewedy et al., 2020; Naeimi et al., 2020; Shamurailatpam and Kumar, 2020; Ayele et al., 2021).
Table 1 Morphological characteristics of different species of Trichoderma isolated from semiarid zone of the Northwest Mexico.
| Especie | Colonia | Micelio | No. anillos | Conidias | Fiálides |
|---|---|---|---|---|---|
| T. atroviride | Verde oscuro | Plano | 2 | Globosa | Agrupadas en 2-3 verticilos |
| T. asperellum | Verde oscuro | Plano | 1 | Elipsoidal | Agrupadas en 2-3 verticilos |
| T. harzianum | Verde oscuro | Algodonoso | 2 | Elipsoidal | Solitarias |
| T. longibrachiatum | Verde ligero | Algodonoso | 2 | Globosa | Solitarias |
| T. viride | Verde oscuro | Algodonoso | 3 | Globosa | Agrupadas en 2-3 verticilos |
| T. koningii | Verde ligero a azul verdozo | Algodonoso | 1 | Elipsoidal | Agrupadas en 2-3 verticilos |
| Trichoderma sp. | Verde ligero | Algodonoso | 2 | Sub-globosa | Agrupadas en 2-3 verticilos |
Trichoderma spp. abundance and frequency of occurrence. The abundance of Trichoderma spp. was significantly different (Duncan, p≤0.05) between study sites (Figure 2). Las Pocitas presented the highest abundance of Trichoderma spp., whereas the lowest fungal population was quantified in El Cajete. Regarding the frequency of occurrence of the Trichoderma spp. isolations, differences were found between sites under study (Figure 3). In El Saltito, a higher number of Trichoderma spp. species (6) were found: T. harzianum, T. atroviride, T. asperellum, T. longibrachiatum, T. viride and Trichoderma sp., and in Los Barriles, the lowest figure for occurrence was presented, with only three species: T. koningii, T. atroviride and T. asperellum.
In some geographical sites with greater rainfalls, there is availability of organic matter and host plants, and the abundance of Trichoderma is higher (Harman et al., 2004; Garnica-Vergara et al., 2016). In the semiarid areas in which high temperatures prevail almost all year round, a limiting factor is humidity, which affects plants negatively and indirectly reduces the diversity and abundance of microorganisms in the soil (Silva et al., 2020), such as bacteria, fungi, actinomycetes, related to plants of agronomic or forestry interest, among others (Long et al., 2021; Yang et al., 2021). Although Trichoderma spp. is one of the most abundant fungi in the soil, its occurrence is generally low in desert soils and is related to the scarce presence of plant species and to extreme edaphological conditions. In this regard, Gherbawy et al. (2004) identified only two species in soils of the Nile Valley: T. harzianum and the anamorphic Hypocrea orientalis, a member of the T. longibrachiatum genus. Montoya-González et al. (2016) point out that the dry environment and the lack of organic matter reduce the presence and diversity of soil microorganisms. These characteristics may be related to the low population found in the site of El Cajete due to the scarce vegetation and the sandy texture of the soil, lacking in organic matter. However, it is worth mentioning that the number of species found was higher than in Las Pocitas. These species may have a greater ability to produce compounds to adapt to extreme environments.
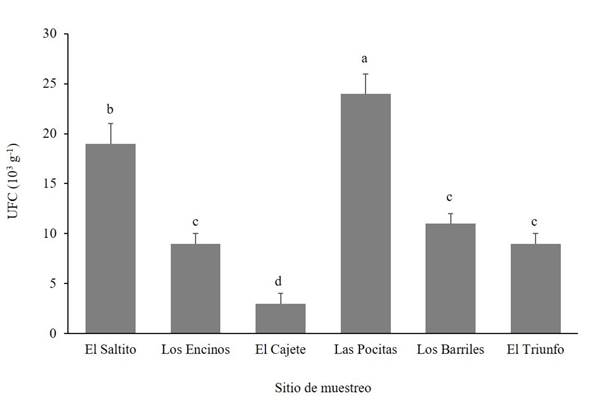
Figure 2 Abundance of Trichoderma species in different semiarid sites in the Northwest of Mexico. CFU = Colony Forming Units.
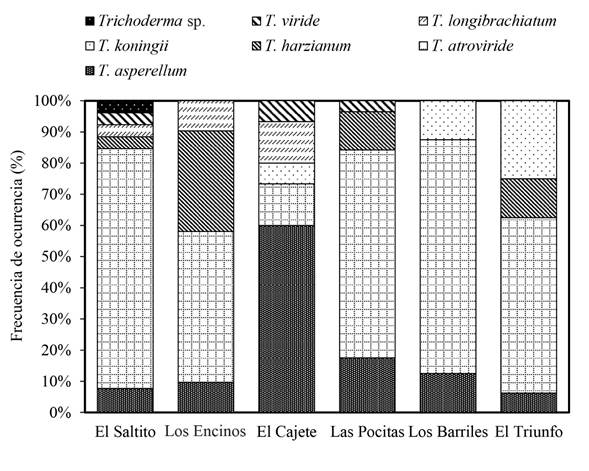
Figure 3 Frequency of occurrence of Trichoderma species in different semiarid sites belonging to the municipality of La Paz, in the Northwest of Mexico.
Antagonism in vitro. The percentage of inhibition (PI) of diverse phytopathogenic fungi was significantly different (Duncan, p≤0.05) between species of Trichoderma (Table 2). This response may be due to the different ability of each species in the production of compounds, as well as the speed of reaction when faced with different factors. Regarding this, Hewedy et al. (2020) point out that the difference in the inhibition of pathogens between Trichoderma spp. isolations is mostly due to its ability to adapt and grow under different substrates and to its antagonistic ability, mediated by its versatility to exert diverse antagonistic mechanisms. Due to this, its plasticity helps it survive in soils with extreme climates, such as in Northern Mexico. T. koningii inhibited all the phytopathogenic fungi (F. oxysporum, F. solani, R. solani, A. alternata and C. gloeosporioides), surpassing the antagonism exerted by the rest of the microorganisms, including the commercial product Tricho-Sin®. T. harzianum caused the least inhibition in phytopathogens (Figure 4). These results coincide with a report by Elshahawy et al. (2017), who proved the effectiveness of T. koningii in comparison with T. album, T. harzianum, T. virideand T. virens in the control of Sclerotium cepivorum. Meanwhile, Katyayani et al. (2020) evaluated the inhibiting effect of T. harzianum, T. viride and T. koningii on Fusarium oxysporum f. sp. ciceri and determined T. koningii to be the most efficient in inhibiting the mycelial growth of the pathogen. This effectiveness of T. koningii may be due to higher efficient mechanisms, not only for the control of pathogens, but also in the tolerance to different types of stress. Regarding this, Nykiel-Szymańska et al. (2018) reported that T. koningii specifically produces dechlorinated and hydroxyl-type metabolites that provide a higher tolerance to copper, and which may be related to its different action mechanisms.
Table 2 Effect of Trichoderma spp. on the inhibition of phytopathogenic fungi related to diseases under in vitro conditions.
| Especie | Porcentaje de Inhibición (%)y | ||||
|---|---|---|---|---|---|
| Fusarium oxysporum | Fusarium solani | Rhizoctonia solani | Alternaria alternata | Colletotrichum gloeosporioides | |
| T. asperellum | 70 bz | 64 c | 56 b | 69 b | 63 b |
| T. harzianum | 60 d | 63 c | 40 d | 56 d | 50 d |
| T. koningii | 75 a | 73 a | 62 a | 73 a | 69 a |
| T. longibrachiatum | 69 b | 68 b | 56 b | 61 c | 62 b |
| T. atroviride | 63 c | 68 b | 55 b | 68 b | 62 b |
| Trichoderma sp. | 63 c | 64 c | 50 c | 68 b | 63 b |
| T. viride | 64 c | 64 c | 51 c | 69 b | 62 b |
| Tricho-Sin® | 64 c | 54 d | 57 b | 69 b | 56 c |
yPI = Percentage of inhibition
zDifferent letters represent significant differences between treatments according to Duncan’s test p≤0.05.
Cheng et al. (2012) reported that the efficiency of most Trichoderma species (including T. asperellum, T. harzianum, T. koningii, T. longibrachiatum, T. atroviride and T. viride) consists in inhibiting the growth of the hyphae of phytopathogenic fungi by causing cytosolic vacuolization and lysis in the hyphae, whereas Kashyap et al. (2020) associates it with the reduction in the sporulation of fungi. Other antagonistic mechanisms exerted by Trichoderma spp. in the in vitro and in vivo inhibition of the phytopathogens is the production of hydrolytic enzymes (chitinase and β-1,3-glucanase) that degrade the cell wall of the fungus (Ruangwong et al., 2021), volatile organic compounds (azetidine, 2-phenylethanol and ethyl hexadecanoate) with antimicrobial activity (Dini et al., 2021), the production of antibiotics (Bae et al., 2016), competition for space and nutrients such as sucrose and glucose (Liu et al., 2021), induction of the systemic resistance of the host (Li et al., 2018), and others. Due to the survival, the establishment and the antagonistic activities of Trichoderma species in the field are still inconsistent, it is crucial to carry out studies aimed at understanding the ecology and the dynamics of the Trichoderma populations in the soil to protect the crops in an efficient manner.
Conclusions
Seven Trichoderma species were identified: T. asperellum, T. atroviride, T. harzianum, T. koningii, T. viride, T. longibrachiatum and Trichoderma sp. Las Pocitas presented the largest population and frequency of occurrence of Trichoderma spp., and in El Saltito, a greater variability of species was observed, since T. harzianum, T. atroviride, T. asperellum, T. longibrachiatum, T. viride and Trichoderma sp. were identified. In the antagonism, T. koningii presented the best inhibition response in the growth of F. oxysporum, F. solani, R. solani, A. alternata and C. gloeosporioides, in comparison with the rest of the species and the T. harzianum commercial product Tricho-Sin® . A knowledge of the different species of Trichoderma present in the region will be essential for future studies related to the selection of native strains from semiarid areas that can be used in extreme environmental conditions against root pathogens in plants of agricultural interest.
Literatura citada
Alfiky A, and Weisskopf L. 2021. Deciphering Trichoderma-plant-pathogen interactions for better development of biocontrol applications. Journal of Fungi 7(1): 61. https://doi.org/10.3390/jof7010061. [ Links ]
Al-Mekhlafi NA, Abdullah QY, Al-Helali MF and Alghalibi SM. 2019. Antagonistic potential of native Trichoderma species against tomato fungal pathogens in Yemen. International Journal of Molecular Microbiology 2(1): 1-10. https://psmpublishers.org/issues/antagonistic-potential-of-native-trichoderma-species-against-tomato-fungal-pathogens-in-yemen/. [ Links ]
Al-Ani LKT. 2018. Trichoderma from extreme environments: Physiology, diversity, and antagonistic activity. In Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications 8: 389-403. https://doi.org/10.1007/978-981-13-0329-6_14. [ Links ]
Asis A, Shahriar SA, Naher L, Saallah S, Fatihah HNN, Kumar V and Siddiquee S. 2021. Identification patterns of Trichoderma strains using morphological characteristics, phylogenetic analyses and lignocellulolytic activities. Molecular Biology Reports 48: 3285-3301. https://doi.org/10.1007/s11033-021-06321-0. [ Links ]
Ayele TM, Gebremariam GD and Patharajan S. 2021. Isolation, identification and in vitro test for the biocontrol potential of Trichoderma viride on Fusarium oxysporum f. sp. lycopersici. The Open Agriculture Journal 15: 10-20. https://doi.org/10.2174/1874331502115010010. [ Links ]
Bae SJ, Mohanta TK, Chung JY, Ryu M, Park G, Shim S, Hong S-B, Seo H, Bae D-W, Bae I, Kim J-J and Bae H. 2016. Trichoderma metabolites as biological control agents against Phytophthora pathogens. Biological Control 92: 128-138. https://doi.org/10.1016/j.biocontrol.2015.10.005 [ Links ]
Barnett H and Hunter B. 1972. Illustrated genera of imperfect fungi. EE. UU. Burgess Publ. Co. 241p. [ Links ]
Bissett JW, Gams W and Jaklitsch GJ. 2015. Trichoderma names in the year 2015. IMA Fungus 6: 263-295. https://doi.org/10.5598/imafungus.2015.06.02.02. [ Links ]
Bononi L, Chiaramonte JB, Pansa CC, Moitinho MA and Melo IS. 2020. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Scientific Reports 10: 2858. https://doi.org/10.1038/s41598-020-59793-8. [ Links ]
Brito RAS, Cavalcante GP, Stock VM, Colman AA, dos Santos DP, Sermarini RA and Maffia LA. 2020. Trichoderma species show biocontrol potential against Ceratocystis wilt in mango plants. European Journal of Plant Pathology 158(3): 781-788. https://doi.org/10.1007/s10658-020-02095-6. [ Links ]
Camacho-Aguiñiga DG, Hernández-Montiel LG, López-Aburto MG y Romero-Bastidas M. 2016. Identificación y caracterización del agente causal de la marchitez en esparrago en Baja California Sur. Memoria de congreso. Suplemento de la Revista Mexicana de Fitopatología 34 (Suplemento): S49. https://www.smf.org.mx/rmf/suplemento/Suplemento342016.html [ Links ]
Carrillo P, Woo SL, Comite E, El-Nakhel C, Rouphael Y, Fusco GM, Borzacchiello A, Lanzuise S and Vinale F. 2020. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 9(6): 771. https://doi.org/10.3390/plants9060771. [ Links ]
Cheng CH, Yang CA and Peng KC. 2012. Antagonism of Trichoderma harzianum ETS 323 on Botrytis cinerea mycelium in culture conditions. Phytopathology 102(11): 1054-1063. https://doi.org/10.1094/PHYTO-11-11-0315. [ Links ]
Dini I, Marra R, Cavallo P, Pironti A, Sepe I, Troisi J, Scala G, Lombari P and Vinale F. 2021. Trichoderma strains and metabolites selectively increase the production of volatile organic compounds (VOCs) in olive trees. Metabolites 11(4): 213. https://doi.org/10.3390/metabo11040213. [ Links ]
Elnashar A, Abbas M, Sobhy H and Shahba M. 2021. Crop water requirements and suitability assessment in arid environments: A new approach. Agronomy 11(2): 260. https://doi.org/10.3390/agronomy11020260. [ Links ]
Elshahawy IE, Saied N, Abd-El-Kareem F and Morsy A. 2017. Biocontrol of onion white rot by application ofTrichodermaspecies formulated on wheat bran powder. Archives of Phytopathology and Plant Protection, vol. 50(3-4): 150-166. https://doi.org/10.1080/03235408.2016.1276423. [ Links ]
Gal-Hemed I, Atanasova L, Komon-Zelazowska M, Druzhinina I, S, Viterbo and Yarden O. 2011. Marine isolates of Trichoderma spp. as potential halotolerant agents of biological control for arid-zone agricultura. Applied and Environmental Microbiology 77 (15): 5100-5109. doi:10.1128/AEM.00541-11. [ Links ]
Gamarra MAF, Ojeda MM and Maldonado GAE. 2017. Identificación molecular y tasa de crecimiento de cepas nativas de Trichoderma spp. aisladas de la Región Norte del Paraguay. Investigación Agraria 19(2): 127-132. https://doi.org/10.18004/investig.agrar.2017.diciembre.127-132. [ Links ]
Garnica-Vergara A, Barrera-Ortiz S, Muñoz-Parra E, Raya-González J, Méndez-Bravo A and Macías-Rodríguez L. 2016. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytologyst 209 (4): 1496-1512. https://doi.org/10.1111/nph.13725. [ Links ]
Gherbawy Y, Druzhinina I, Shaban GM, Wuczkowsky M, Yaser M, El-naghy MA, Prillinger HJ, and Kubicek CP. 2004. Trichoderma populations from alkaline agricultural soil in the Nile valley, Egypt, consist of only two species. Mycological Progress 3(3): 211-218. https://doi.org/10.1007/s11557-006-0091-y. [ Links ]
Harman GA, Howell CR, Viterbo A, Chet I and Lorito M. 2004. Trichoderma species-opportunistic, avirulent plant symbionts. Nature Reviews Microbiology 2: 43-56. https://doi.org/10.1038/nrmicro797. [ Links ]
Harman G, Obregon M, Samuels G and Lorito M. 2010. Changing models for commercialization and implementation of biocontrol in the developed and developing world. Plant Disease 94: 928-939. https://doi.org/10.1094/ PDIS-94-8-0928. [ Links ]
Hewedy OA, Abdel LKS, Seleiman MF, Shami A, Albarakaty FM and M El-Meihy R. 2020. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 9(8): 189. https://doi.org/10.3390/biology9080189. [ Links ]
Katyayani KKS, Bindal S, Prakash Singh J, Rana M and Srivastava S. 2020. In vitro evaluation of Trichoderma spp. against chickpea wilt. International Archive of Applied Sciences and Technology 11(3): 1-4. https://doi.org/10.15515/iaast.0976-4828.11.3.14 [ Links ]
Karthikeyan BC, Jaleel A., Lakshmanan GA and Deiveekasundaram M. 2008. Studies on rhizosphere microbial diversity of some commercially important medicinal plants. Colloids and Surfaces B: Biointerfaces 62 (1): 143-45. https://doi.org/10.1016/j.colsurfb.2007.09.004. [ Links ]
Kashyap PL, Solanki MK, Kushwaha P, Kumar S and Srivastava AK. 2020. Biocontrol potential of salt-tolerant Trichoderma and Hypocrea isolates for the management of tomato root rot under saline environment. Journal of Soil Science and Plant Nutrition 20(1): 160-176. https://doi.org/10.1007/s42729-019-00114-y. [ Links ]
Kim K, Heo YM, Jang S, Lee H, Kwon SL, Park MS, Lim YW and Kim JJ. 2020. Diversity of Trichoderma spp. in marine environments and their biological potential for sustainable industrial applications. Sustainability 12, 4327: 1-12. https://doi.org/10.3390/su12104327. [ Links ]
Li N, Alfiky A, Wang W, Islam M, Nourollahi K, Liu X and Kang S. 2018. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum .Frontiers in Microbiology 9: 2-16. https://doi.org/10.3389/fmicb.2018.02614. [ Links ]
Liu CM, Liu SY, Liao CK, Lo CT, Lin KC and Peng KC. 2021. Cabbage defense response provoked by Trichoderma Th-LAAO. Archives of Microbiology 203(4): 1641-1647. https://doi.org/10.1007/s00203-020-02174-6. [ Links ]
Long Y, Yang X, Cao Y, Lv G, Li Y, Pan Y and Liu Y. 2021. Relationship between soil fungi and seedling density in the vicinity of adult conspecifics in an arid desert forest. Forests 12(1): 92. https://doi.org/10.3390/f12010092. [ Links ]
Ma J, Tsegaye E, Li M, Wu B and Jiang X. 2020. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 10(8): 1-13. https://doi.org/10.1007/s13205-020-02301-6. [ Links ]
Michaud JP. 2018. Challenges to conservation biological control on the high plains: 150 years of evolutionary rescue. Biological Control 125: 65-73. https://doi.org/10.1016/j.biocontrol.2018.07.001. [ Links ]
Miguel-Ferrer L, Romero-Arenas O, Andrade-Hoyos P, Sánchez-Morales P, Rivera-Tapia JA and Fernández-Pavía SP. 2021. Antifungal activity of Trichoderma harzianum and T. koningiopsis against Fusarium solani in seed germination and vigor of Miahuateco chili seedlings. Mexican Journal of Phytopathology 39(2): 228-247. https://doi.org/10.18781/R.MEX.FIT.2101-5 [ Links ]
Muniappan V, and Muthukumar TV. 2014. Influence of crop species and edaphic factors on the distribution and abundance of Trichoderma in Alfisol soils of southern India. Acta Botánica Croatica 73(1): 37-50. https://doi.org/10.2478/botcro-2013-0004. [ Links ]
Montoya-González AH, Quijano-Vicente G, Morales-Maza A, Ortiz-Uribe N, Hernández-Martínez R. 2016. Isolation of Trichoderma spp. from desert soil, biocontrol potential evaluation and liquid culture production of conidia using agricultural fertilizers. Journal of Fertilizers and Pesticides 7: 163. https://doi.org/10.4172/2471-2728.1000163. [ Links ]
Naeimi S, Khosravi V, Varga A, Vágvölgyi C and Kredics L. 2020. Screening of organic substrates for solid-state fermentation, viability and bioefficacy of Trichoderma harzianum AS12-2, a biocontrol strain against rice sheath blight disease. Agronomy 10(9): 1258. https://doi.org/10.3390/agronomy10091258. [ Links ]
Nykiel-Szymańska J, Bernat P and Słaba M. 2018. Potential ofTrichoderma koningiito eliminate alachlor in the presence of copper ions. Ecotoxicology and Environmental Safety 162(30):1-9. https://doi.org/10.1016/j.ecoenv.2018.06.060. [ Links ]
Nuangmek W, Aiduang W, Kumla J, Lumyong S and Suwannarach N. 2021. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Frontiers in Microbiology 12: 410. https://doi.org/10.3389/fmicb.2021.634772. [ Links ]
Núñez-Madera CA, Hernández-Montiel LG, López-Aburto MG y Romero-Bastidas M. 2016. Identificación morfológica del agente causal de la marchitez en garbanzo (Cicer arietinum) en Baja California Sur. Memoria de congreso. Revista Mexicana de Fitopatología 34(suplemento): S49 https://www.smf.org.mx/rmf/suplemento/Suplemento342016.html [ Links ]
Osorio-Concepción MFS, Casas PC, Cortés 2013. Efecto de la limitación de fosfato sobre la conidiación de Trichoderma atroviride y mutantes ciegas a la luz. Revista Mexicana de Micología 37: 41-40. [ Links ]
Otadoh JA, Okoth SA, Ochanda J and Kahindi JP. 2011. Assessment of Trichoderma isolates for virulence efficacy on Fusarium oxysporum F. sp. phaseoli. Tropical and Subtropical Agroecosystems 13(1): 99 - 107. http://www.scielo.org.mx/pdf/tsa/v13n1/v13n1a15.pdf [ Links ]
Rifai MA. 1969. A revision of the genus Trichoderma. Mycology Papers 116: 1-56. https://doi.org/10.1139/b91-298. [ Links ]
Rodríguez-Macías KM, Hernández-Montiel LG, López-Aburto MG y Romero-Bastidas M, Chiquito-Contreras R. 2016. Aislamiento e identificación del agente causal de la mancha foliar en albahaca (Ocimum basilicum) en Baja California Sur, México. Revista Mexicana de Fitopatología 34 (suplemento): S50. https://www.smf.org.mx/rmf/suplemento/Suplemento342016.html [ Links ]
Ruangwong OU, Pornsuriya C, Pitija K and Sunpapao A. 2021. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. Journal of Fungi 7(4): 276. https://doi.org/10.3390/jof7040276. [ Links ]
Siddiquee S. 2017. Practical handbook of the biology and molecular diversity of Trichoderma species from tropical regions. Springer International Publishing. P. 17. https://doi.org/10.1007/978-3-319-64946-7. [ Links ]
Shamurailatpam D and Kumar A. 2020. Selected fungicides and biocontrol agents for managing early blight of tomato caused by Alternaria solani. Indian Journal of Plant Protection 48(4): 474-481. [ Links ]
Silva JBT, Marques E, Menezes JE, Silva JP and Mello SCM. 2020. Population density of Trichoderma fungi in natural environments and agrosystems of a Cerrado area. Biota Neotropica 20(4): 1-9. https://doi.org/10.1590/1676-0611-BN-2020-1048. [ Links ]
Singh SK and Jadon KS. 2019. Biocontrol efficacy of Trichoderma viride against fungal pathogens of cumin, groundnut and castor. Indian Phytopathology 72(3): 537-543. https://doi.org/10.1007/s42360-019-00156-3. [ Links ]
Sharma S, Kour D, Rana KL, Dhiman A, Thakur S, Thakur P, Thakur S, Thakur N, Sudheer S, Yadav N, Yaday AN, Rastegari AA and Singh K. 2019. Trichoderma: Biodiversity, ecological significances, and industrial applications. In: Yadav AN, Mishra S, Singh S and Gupta A (eds). Recent advancement in white biotechnology through fungi. Springer 85-120. https://doi.org/10.1007/978-3-030-10480-1_3. [ Links ]
Su D, Ding L, He S. 2018. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini-Rev. Medicinal Chemistry 18(20):1702-1713. https://doi.org/10.2174/1389557518666180727130826 [ Links ]
Tegene S, Dejene M, Terefe H, Tegegn G, Tena E and Ayalew A. 2021. Evaluation of native Trichoderma isolates for the management of sugarcane smut (Ustilago scitaminea) in sugar plantations of Ethiopia. Cogent Food and Agriculture 7(1): 1872853. https://doi.org/10.1080/23311932.2021.1872853. [ Links ]
Torres-De la Cruz M, Ortiz-García CF, Bautista-Muñoz C, Ramírez-Pool JA, Ávalos-Contreras N and Cappello-García S. 2015. Diversidad de Trichoderma en el agroecosistema cacao del estado de Tabasco, México. Revista Mexicana de Biodiversidad 86: 947-961. https://doi.org/10.1016/j.rmb.2015.07.012. [ Links ]
Wang C and Zhuang WY. 2020. Carbon metabolic profiling of Trichoderma strains provides insight into potential ecological niches. Mycologia 112(2): 213-223. https://doi.org/10.1080/00275514.2019.1698246. [ Links ]
Wu Q, Sun R, Ni M, Yu J, Li Y, Yu C, Dou K, Ren J and Chen J. 2017. Identification of a novel fungus, Trichoderma asperellum GDFS1009, and comprehensive evaluation of its biocontrol efficacy. Plos One 12(6): e0179957. https://doi.org/10.1371/journal.pone.0179957. [ Links ]
Yang H, Chen Y and Zhang F. 2019. Evaluation of comprehensive improvement for mild and moderate soil salinization in arid zone. Plos One 14(11): e0224790. https://doi.org/10.1371/journal.pone.0224790. [ Links ]
Yang X, Long Y, Sarkar B, Li Y, Lü G, Ali A and Cao YE. 2021. Influence of soil microorganisms and physicochemical properties on plant diversity in an arid desert of Western China. Journal of Forestry Research 1-15. https://doi.org/10.1007/s11676-021-01292 [ Links ]
Zhou Y, Wang Y, Chen K, Wu Y, Hu J, Wei Y, Li J, Yang H , Ryder M and Denton MD. 2020. Near-complete genomes of two Trichoderma species: A resource for biological control of plant pathogens. Molecular Plant Microbe Interactions 33(8): 1036-1039. https://doi.org/10.1094/MPMI-03-20-0076-A. [ Links ]
Received: June 29, 2021; Accepted: August 15, 2021











 texto em
texto em