Plant pathogenic fungi have developed resistance mechanisms to synthetic fungicides (Dooley et al., 2016), which has promoted the search for new fungicide agents; moreover, fungicide agents isolated from natural sources are especially valued (Mishra et al., 2010). Compounds that exert fungicidal action on a target site are of particular interest because they affect the essential processes involved in fungal growth, specifically those ones required to maintain pathogenic cell viability (Arroyo et al., 2016).
The fungal cell wall is one of the most important target sites because it is a fundamental structure for the viability and pathogenicity of fungi (Arroyo et al., 2016). Antagonistic microorganisms produce enzymes that degrade the components of the cell wall of other microorganisms (Mouyna et al., 2013). Consequently, agents that inhibit the synthesis of fungal cell wall components must be very selective (Balasubramanian et al., 2012). The cell wall of ascomycetes, basidiomycetes, deuteromycetes and some oomycetes is composed of 1,3-β-glucans, 1,6-β-glucans, chitin and proteins (Kagimura et al., 2015) in a multilayered structure. 1,3-β-glucans are the most abundant polysaccharide of the fungal cell wall (Gastebois et al., 2010; Papaspyridi et al., 2018). The dynamic structural reorganization of the fungal cell wall during morphological changes, such as cell growth (branching), cell division and germination in filamentous fungi or budding of yeast, are morphogenetic events in which endo-β-1,3-glucanases play an essential role in the synthesis of the cell wall (Gastebois et al., 2010; Hartl et al., 2011). Because all of these important morphogenetic events involve the β-1,3-glucans of the fungal wall, a strategy to discover antifungal agents of natural origin is to identify substances that inhibit endo-β-1,3-glucanases at specific target sites of fungi whose cell walls contain β-glucans, specifically at the β-1,3-glucan linkage (Balasubramanian et al., 2012; Mouyna et al., 2016). During the search for antifungal substances in plant extracts that selectively inhibit the synthesis of the fungal cell wall, it has been previously reported that the crude extracts of Turnera diffusa, effectively inhibited the activity of endo-1,3-β-glucanase (Vargas-Arispuro et al., 2017). Therefore, this work was focused on the identification of the components from crude extract of T. diffusa that have the inhibitory effect on the fungal endo-1,3-β-glucanase activity.
To reach this objective, leaves and small stems of T. diffusa (Passifloraceae), were collected from natural populations located near to Tonichi Sonora (28° 35’ 56” N, 109° 33’ 56” W).
Plant samples were identified the Botanical Herbarium of the Sonora University (Mexico), and a voucher specimen was deposited at the herbarium. The leaves and small stems were separated and air-dried at room temperature for two weeks. Dried material (500 g of leaves or 300 g of small steams) was macerated in methanol (1 L), at room temperature (25 °C) for 7 days in darkness. The filtrates of each extract were evaporated to dryness at 40 °C under reduced pressure (450 mmHg). The viscous residue resulted, were called the crude extract of leaves or crude extract of stems of T. diffusa. The effect of the crude extract on endo-1,3-β-glucanase (Trichoderma sp, SIGMA) activity was determined using laminarin (Laminaria digitata, SIGMA) by coupled procedure previously described by Vargas-Arispuro et al. (2017). The procedure was carried out in 250 μL of a reaction mixture containing 100 mM potassium acetate buffer (pH 5.0), 0.5% (w/v) laminarin, 10 μL of endo-β-1,3-glucanase (0.05 U) and 50 μL of plant crude extract (3 mg mL-1) or pure compounds (10 and 25 mM). The reaction was run for 40 min at 37 °C and then stopped by boiling for 5 min. The amount of reducing sugars released was measured with a spectrophotometer (BioSpec-1601) at 540 nm. Enzyme and substrate blanks were also included. A calibration curve with glucose was used. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the release 1 µmol of glucose equivalent per minute under the reaction conditions used. The following equation was used to calculate the enzyme activity. Units mL-1 of enzyme = (μM of glucose released) (0.410) /(40)(0.01)(0.01), where 0.410 is the total volume of the sample in mL; 40 is the reaction time in min; 0.01 is the volume of the enzyme used in mL and 0.01 is the adjustment volume of the colorimeter in mL. Protein concentrations were determined by the Bradford method, with bovine serum albumin as the standard.
The active compounds in the plant extract were purified by chromatographic techniques. For this procedure, plant crude extract (100 mg) was dissolved in 5 mL of methanol for analysis by preparative thin-layer chromatography (TLC). Preparative TLC plates coated with a GF-254 fluorescent silica gel 60 (5 x 20 cm, 250 µm, EM, Science, Germany) and spotted with plant crude extract in methanol were developed in dichloromethane. The TLC plate was marked under ultraviolet (UV) light at 254 nm, and each band was carefully scraped off the plate. The scrapings were dissolved in methanol, filtered through filter paper (Whatman N° 1) and centrifuged at 5,000 rpm for 10 min. The methanol filtrates of each band were evaporated to dryness at 40 °C under reduced pressure. A portion of each residue from the separated TLC bands was dissolved (1:1 w/v) in methanol and subjected to an enzyme assay. The fraction that retained the compounds with inhibitory activity was subjected to high pressure liquid chromatography (HPLC) purification process (1260 AgilentTechnology, USA) on an instrument coupled to a photodiode array detector (HP-1260, Germany), with a Nucleosil 120 C18 (250 x 4 mm, 5 μ, Cronus) reverse-phase column. HPLC separation was performed using a gradient program with the mobile phase of 1% formic acid (solvent A) and 100% acetonitrile (Solvent B) and beginning with 80% A in 40 min, 50% A in 60 min, 10% A in 80 min and 0% A in 85 min. The flow rate was 0.3 mL min-1, detection was performed at 254, 280 and 320 nm, and spectral data were collected from 240 to 550 nm. Subfractions were collected under 254 nm and subjected to the enzyme assay. The compounds were identified by comparing the data with the UV spectrum of pure compounds from a database of phenolic compounds.
The antifungal evaluation of both plant extracts and the isolated compounds was carried out on spores from Botrytis cinerea (a fungus isolated from grape, GenBank accession number AY568636) grown at 25 °C on potato dextrose agar (PDA, Difco, Sparks, MD, USA) in 9-cm diameter petri dishes until sporulation. The spore suspension was prepared by adding 5 mL of sterile water with 0.01% Tween 20 to each plate, and the surface was scraped gently with a glass rod to release spores. The water-spore suspension was filtered through glass wool to remove the mycelium. The collected filtrate was centrifuged at 5000 x g for 15 min and adjusted to a density of 1 x 104 spores mL-1 using a hematocytometer. The spore germination assay was performed on microwell strips (Nalge, Nunc, Naperville, IL, USA). Sabouraud dextrose broth (SDB) (Difco, Sparks, MD, USA) (900 µL) containing HPLC fractions (50 and 100 mM) or water (control) was pipetted into each well of a strip of 8 wells per treatment. A spore suspension (100 µL) containing ~100±15 spores of B. cinerea was added, the well contents were gently mixed, and the strips were incubated at 25 °C. The germinated spores in each well were counted by microscopic examination until the control spores reached 100% germination. These bioassays were conducted four times. The data were analyzed by analysis of variance of one way using NCSS software (version 2007). The means were compared by Tukey’s test (p≤0.01).
The effect of the plant crude extracts on fungal endo-1,3-β-glucanase is shown in Figure 1. The stem extract showed greater inhibition of enzymatic activity than the leaf extract. Thus, by preparative thin layer chromatography, the crude extract from stems was separated, and four fractions were obtained (Table 1). After evaluating the effect of the fractions on the enzyme, it was determined that the inhibitory compounds of the enzyme were retained in fraction 2 (Table 1). The inhibitory effect of this fraction against fungal endo-1,3-β-glucanase displayed the same inhibition percentage to that of the crude extract of stems (Figure 1).
Based on these results, were separated the compounds from fraction 2 using HPLC. Fifteen identified compounds were classified as phenolic compounds. The evaluation of the effect of each isolated compound on the activity of the fungal endo-1,3-β-glucanase revealed that only 2 compounds, identified as luteolin and apigenin, evaluated at 25 mM, were able to inhibit the fungal enzyme by 60 and 89 %, respectively. The remaining 13 compounds had no effect on the activity of the fungal enzyme (Table 2). Apigenin and luteolin showed synergistic inhibitory effects on the enzyme activity, when were evaluated together at the same concentration, they showed an inhibitory effect of the enzyme of 100 % compared to the control (Table 2). Previous works have reported that the antifungal activity of extracts of some plants is due to the content of endogenous inhibitory phenolic compounds (Kaushik et al., 2015); some of these compounds inhibit the activity of cellulases. Endo-1,3-β-glucanase is a member of this group of enzymes (Takeda et al., 2015; Menget et al., 2016).

Figure 1. Effect of the leaf and stem crude extracts of Turnera diffusa on the activity of fungal endo-1,3-β-glucanase evaluated at 3 mg mL -1 expressed as a percentage of inhibition related to control (means ± SD, n=6).
Table 1. Residual activity and inhibition of fungal endo-1,3-β-glucanase by fractions of Turnera diffusa stems extract obtained from preparative thin layer chromatography evaluated at 3 mg mL -1 expressed as a percentage of germination related to control.
| TLC Fractionz | Retention factor (Rf) | Residual activity of fungal endo-1,3-β-glucanase (%) | Inhibition of fungal endo-1,3- β-glucanase (%) |
|---|---|---|---|
| 1 | 0.7 | 100 | 0 |
| 2 | 0.5 | 15 | 85 |
| 3 | 0.3 | 97 | 3 |
| 4 | 0.2 | 100 | 0 |
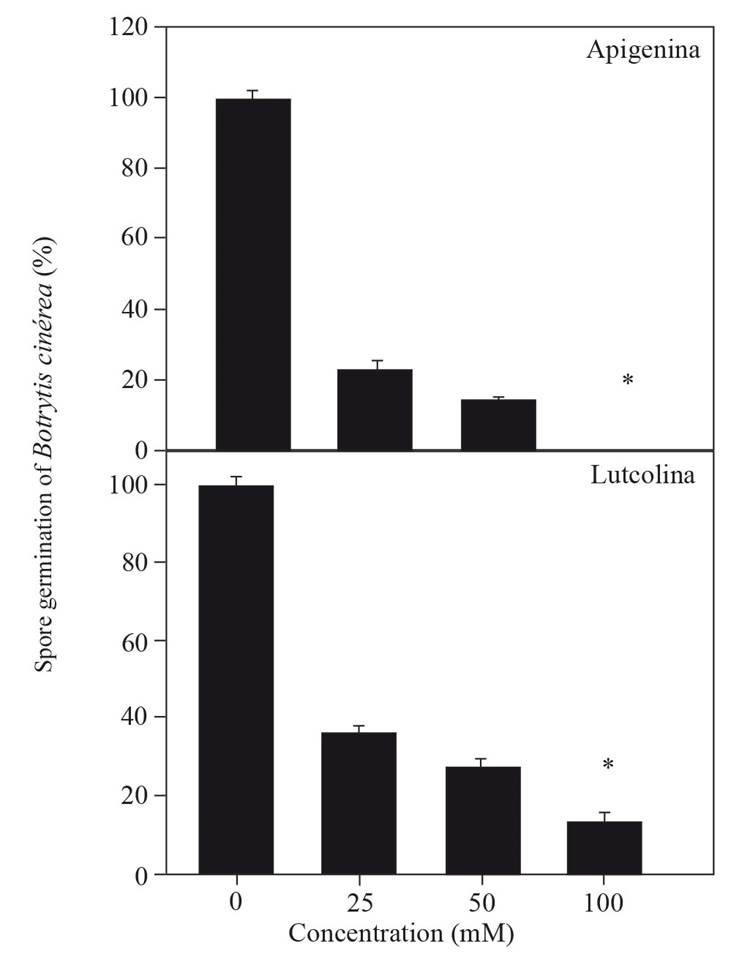
The fungicidal potential of the flavones was calculated based on their effect on the germination of B. cinerea spores. The results show that apigenin completely inhibited spore germination at a concentration of 100 mM, while luteolin inhibited 90 % of spore germination at the same concentration (Figure 2). These results correlated with the effect of flavones on the inhibition of the activity of fungal endo-1,3-β-glucanase (r= 0.98), presented in Table 2; thus, it is possible to infer that apigenin has a greater fungicidal effect than luteolin. Szewczyk and Zidorn (2014) and Sarris et al. (2013) reported the isolation of apigenin from an extract of T. diffusa, identifying apigenin as an active compound that protects against anxiety. Additionally, Willer et al. (2019) reported that among the 24 phenolic compounds isolated from T. diffusa, luteolin and apigenin were found, and the latter exerted estrogenic activity. Apigenin is recognized in traditional and alternative medicine to have diverse pharmacological activities (Si et al., 2009), including the inhibition of the 17-β-hydroxysteroid dehydrogenase enzyme (Choi and Kim, 2009; Hasegawa et al., 2013). There have been no published reports on the inhibitory effect of apigenin on fungal endo-1,3-β-glucanase enzymes; hence, this work is the first report of this activity. Based on these research findings, it is concluded that the phenolic compound apigenin isolated from stem extracts of T. diffusa inhibits the fungal enzyme endo-1,3-β-glucanase and consequently decreases the germination of B. cinerea spores.
Table. 2. Phenolic compounds identified in the stem extract of Turnera diffusa and their effects on fungal endo-1,3-β-glucanase activity evaluated at 25 mM expressed as a percentage of germination related to control.
| Compound | Name | Inhibition of fungal endo-1,3- β-glucanase (%) |
|---|---|---|
| 1 | Hydrated catechin | 0 |
| 2 | Epicatechin | 0 |
| 3 | Luteolin | 60 |
| 4 | Kaempferol | 0 |
| 5 | Cinnamic acid | 0 |
| 6 | Quercetin | 0 |
| 7 | Trans-4-hydroxy-3-methoxycinnamic acid | 0 |
| 8 | P-coumaric acid | 0 |
| 9 | 3,4-Dihydroxycinnamic acid | 0 |
| 10 | Chlorogenic acid | 0 |
| 11 | Ferulic acid | 0 |
| 12 | Sodium 4-hydroxybenzoate | 0 |
| 13 | Trans-cinnamic anhydrous acid | 0 |
| 14 | Apigenin | 89 |
| 15 | Gallic acid | 0 |
| 3+14 | Luteolin + apigenin | 100 |











 texto en
texto en