Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.2 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1509-2
Scientific articles
Phytophthora cinnamomi Rands. pathogenicity tests in Pseudotsuga mensiezii
1 Programa de Fitopatología, Colegio de Postgraduados, km 36.5 carr. México-Texcoco. Montecillo, Texcoco, Edo. de México. 56230
2 Departamento de Parasitología Agrícola, Universidad Autónoma Chapingo, km 38.5 carr. México-Texcoco. Chapingo, Texcoco, Estado de México. 56230
Phytophthora cinnamomi Rands is a soil microorganism that has caused large economic and environmental losses worldwide in a wide array of hosts. For this reason, P. cinnamomi was identified and its pathogenicity was evaluated in five isolations from five different regions in Mexico on Pseudotsuga mensiezii plants. The isolations were taken from the soil, roots, and cankers of Quercus salicifolia trees, from El Arrayanal, Col. (COL-A); Quercus elliptica from Tecoanapa, Gro. (GRO-P), Quercus peduncularis from Manántlan, Jal. (JAL-C), Pseudotsuga mensiezii from Edo. de México (EDO-T), and Persea americana, defrom Peribán Mich. (MICH-P). Through a morphological analysis on genus and molecular analysis on species (accession number: KP773290, KP773291, KP773292, KP773293, KP773294) the identification of the isolations was corroborated with the molecular analyses that indicate a homology of 99 % identity with P. cinnamomi. Pathogenicity was tested on healthy three year old P. menziesii plants. Five plants were used for each isolation with its respective control of five uninoculated plants; the plants were kept under greenhouse conditions and the observations of symptoms were carried out weekly for eight months. The results obtained indicated that the five P. cinnamomi plants are capable of infecting the P. menziesii plants.
The isolation COL-A, from the area of Arrayanal Col. Behaved as the most pathogenic, causing the death, in the shortest period, of the plants after 120 ddi, followed by the isolations GRO-P at 180 ddi, JAL-C and EDO-T at 210 ddi, and MICH-P at 240 ddi. The data obtained in the test was analyzed with the Kruskall-Wallis test, finding significant differences of the isolations on the host. The control presented no symptoms. This is the first report on P. cinnamomi isolated from Q. salicifolia, Q.elliptica, Q.peduncularis, P. mensiezii, and P. americana evaluated in P. mensiezii plants, where the pathogenicty of the pathogen is corroborated.
Key words: P. cinnamomi; identification; morphological; molecular; Pseudotsuga mensiezii; Persea americana; Quercus spp
Phytophthora cinnamomi Rands es un microorganismo del suelo que ha causado grandes pérdidas económicas y ecológicas a nivel mundial, en una amplia gama de hospedantes. Es por esto que se identificó y evaluó la patogenicidad de cinco aislados de P. cinnamomi, procedentes de cinco regiones de México, en plantas de Pseudotsuga mensiezii. Los aislados se obtuvieron de suelo, raíz y cancros de árboles de Quercus salicifolia, de El Arrayanal, Col. (COL-A); Quercus elliptica de Tecoanapa, Gro. (GRO-P), Quercus peduncularis de Manántlan, Jal. (JAL-C), Pseudotsuga mensiezii del Edo. de México (EDO-T) y Persea americana, de Peribán Mich. (MICH-P). A través de un análisis morfológico a género y molecular a especie (No Accesión: KP773290, KP773291, KP773292, KP773293, KP773294) se corroboró la identificación de los aislados con los análisis moleculares que indican una homología de 99 % de identidad con P. cinnamomi. La patogenicidad se probó en plantas sanas de P. menziesii de tres años de edad. Para cada aislado se utilizaron cinco plantas con su respectivo testigo de cinco plantas sin inocular, las plantas se mantuvieron bajo condiciones de invernadero y las observaciones de síntomas se realizaron semanalmente durante ocho meses. Los resultados obtenidos indicaron que los cinco aislados de P. cinnamomi son capaces de infectar a las plantas de P. menziesii.
El aislado COL-A, de la región del Arrayanal Col., se comportó como el más patogénico, causando la muerte en un periodo más corto de las plantas a los 120 ddi, le siguieron los aislados GRO-P a los180 ddi, JAL-C y EDO-T a los 210 ddi, y MICH-P a los 240 ddi. Para analizar los datos obtenidos en el ensayo se llevó a cabo la prueba de Kruskall-Wallis detectando diferencias estadísticas significativas de los aislados sobre el hospedante. El testigo no presentó síntoma alguno. Este es el primer reporte de P. cinnamomi aislado de Q. salicifolia, Q.elliptica, Q.peduncularis, P. mensiezii y P. americana evaluado en plantas de P. mensiezii, donde se corrobora la patogenicidad del patógeno.
Palabras clave: P. cinnamomi; identificación; morfológica; molecular; Pseudotsuga mensiezii; Persea americana; Quercus spp
Phytophthora cinnamomi is a soil microorganism that causes large economic and environmental losses; it causes rotting of roots, butt, trunk, and branches. It affects many plant species in agriculture, vegetable production, and forest species, including over 1000 species and has a wide geographic distribution throughout the world (Crone et al., 2013; Erwin and Ribeiro, 1996; Garbelotto et al., 2006; Philip et al., 2009; Zentmyer, 1980). The first description of Phytophthora cinnamomi was by Rands in 1922 as the cause of canker in cinammon tree trunks, in Sumatra (Erwin and Ribeiro, 1996). Since then, its damage has been reported in different hosts. In Mexico, the first reports of P. cinnamomi were made by Zentmyer in 1952 as causing severe damages in avocado production areas, and losses of up to 90 % (Pérez, 2008; Téliz and Mora, 2007; Tamayo, 2007). Likewise, it has been reported in natural Quercus spp forests at the beginning of the year 2000, in El Arrayanal, Col., causing the death of, and damaging, red oak trees (Tainter et al., 2000). Later, it was found to affect trees of the same genus in the Reserva de la Biosfera, in Jalisco (Davidson et al., 2003) and in Tecoanapa, Gro. (Alvarado et al., 2007 and 2008).
It has also been reported to affect Pseudotsuga menziesii trees in the states of Jalisco, Edo. de México, and Veracruz (García, 2007). The symptoms caused by the pathogen are chlorosis, defoliation, death of branches, cankers on the bark, and rotting of roots, leading to the death of the tree (Dos Santos et al., 2011; Erwin and Ribeiro, 1996). The pathogenicity of the Phytophthora genus is influenced by temperature, humidity, soil texture, pH and nutrient availability (Shew and Benson, 1983; Balci et al., 2008; Jonsson et al., 2005; Sánchez et al., 2005; Thompson et al., 2014). Pathogenicity depends on the type of strain and adaptation of pathogens in the plant, and can be calculated by the speed at which the pathogen damages the host (Pariaud et al., 2009; Robin and Desprez 1998).
Pathogenicity tests have been performed on P. cinnamomi in Mexico on avocado varieties, although there are no reports of these with P. cinnamomi isolations from different regions in Mexico, taken from forest species. This pathogen is a threat to both commercial and natural forest species found in other areas of the country, since the lack of awareness of this information can bring as a consequence the economic and environmental importance of the species affected. This pathogen has been identified with the use of taxonomic keys, selective media and has, very recently, has been characterized using different molecular techniques using PCR (Polymerase Chain Reaction), with specific indicators for each species, obtaining important results on the taxonomy, phylogeny, and variability of the fungus (Gallegly and Hong, 2008; Tsai et al., 2006; Martín et al., 2000). Due to this, the aim of this study was to identify and evaluate the pathogenicity of five P. cinnamomi isolations from five regions of Mexico taken from Quercus elliptica, Q salicifolia, Q. peduncularis, Pseudotsuga mensiezii, and Persea americana trees, in P. mensiezii plants.
Materials and methods
Isolation of the pathogen
The isolations used in this investigation were obtained from soils, roots or cankers on Quercus salicifolia trees from El Arrayanal, Col. (COL-A); Quercus elliptica from Tecoanapa, Gro. (GRO-P), Quercus peduncularis from Manantlán, Jal. (JAL-C), Pseudotsuga mensiezii from Edo. de México (EDO-T), and Persea americana, from Peribán Mich. (MICH-P), which were identified with a morphological analysis on the genus, and a molecular analysis on the species.
The trees displayed characteristic symptoms caused by P. cinnamomi, including regressive death, wilting, chlorosis, premature defoliation, cankers, and a dark colored exudate in the bark. In order to obtain the pathogen directly in the field from cankers, we used the selective medium PARPH (pimaricin-ampicilin-rifampicin-PCNB e hymexazol) (Jeffer and Martín, 1986; Erwin and Ribeiro, 1996. In the lab, the root samples were disinfected and planted in a PARPH selective medium. For the soil samples, a method of trapping was used with camellia discs, a water soil + root and selective medium suspension was used (Almaraz et al., 2013). The avocado isolation was provided by Dr. Salvador Ochoa Ascencio of the Department of Agrobiology "Presidente Juárez" of the UMSNH. The avocado tree isolation was obtained by the characteristic symptoms of the avocado tristeza disease by P. cinnamomi.
Morphological identification
The colonies obtained from Phytophthora were performed at hypha point, transferred to Petri dishes with a PDA (potato-dextrose-agar) medium and incubated for 7 days in the dark at a temperature of ± 25 °C (Zentmyer, 1980). Later, were identified to genus based on the morphology of the colony and reproductive structures. The development of the colonies was measured on a daily basis from each of the isolations, until the oomycete filled the Petri dish. For the production of sporangia, a soil extract was suspended in sterile distilled water, and mycelia discs with a diameter of 5 mm in Petri dishes were placed inside them (Romero, 1996); these Petri dishes were kept at a temperature of ± 25 °C under continuous light for six days. The sporangia were observed under the microscope and each isolation was described, along with their measurements for height and length; 100 sporangia were measured from each isolation.
Once the genus of each isolation was identified, they were placed in test tubes containing PDA and mineral oil was added. All this was carried out in the Forest Pathology lab of the Colegio de Postgraduados.
Molecular characterization
The DNA of each isolation used for the identification of species was extracted from mycelial colonies developed in a PDA medium with a growth of approximately seven days. For the extracion of DNA, the technique used was Sambrook and Russell (2001). The total DNA obtained was observed by electrophoresis in agarose gel at 0.8 % and quantified in a Nanodrop 1000 (Termoscientific) spectrophotometer.
PCR. To perform the analysis by PCR of the DNA extracted, we amplified the ITS regions with the primers ITS'5 (GGAAGTAAAAGTCGTAACAAGG) and ITS'4 (TCCTCCGCTTATTGATATGC) of rRNA genes of the subunits 18S-5.8S and 5.8S-28S (White et al., 1990). For the PCR reaction, we used a final volume of 25 μL with the following formulation: 13.22 μL of ultrapure sterile water, 2.5 μL with a buffer solution 5X, 2.08 μL of MgCl2 at 2.5 mM, 2 μL dNTPs at 2.0 mM, 2 μL of each primers at 20 ρMol, 0.2 μL of Taq-DNA polymerase at 1.5 U and 1 of an 80 ng DNA sample. The amplification of the primers was performed in a Perkin-Elmer (Mod. CT 2400) thermocycler, with the following program: one initial DNA denaturation cycle at 95 °C for 2 min; 30 cycles at 95 °C for 1 min, aligning at 50 °C for 30 s and extension at 72 °C for 2 min; the final extension was at 72 °C for 10 min. The amplified fragment was purified using the KIT Quiagen® and the quality was verified by electrophoresis in agarose gel at 1 % stained with ethidium bromide. La banda se visualizó en un transilluminator (GelDoc 2000, BIO RAD®), and analyzed with the program Quantity One 4.0.3 (Sambrook and Russel, 2001). The PCR product was sent to the company Macrogen, U.S.A. for sequencing (Automatic Sequencer 3700xl DNA Analyzer).
The sequences obtained with the primers ITS5-ITS4 were aligned with those available in the genebank of the National Center for Biotechnology Information (NCBI), in the U.S.A. (http://www.ncbi.nlm.nih.gov). Of the quantitative values generated, only the sequences with the highest values were reduced in order to compare them with those obtained in this study. The sequences were aligned with Clustal W version 1.6. and were deposited in the database of the Gen Bank to obtain their accession number.
Pathogenicity tests
Once the colonies were developed in the seven-day old PDA medium, the pathogenicity tests began. For each one of the isolations obtained, the tests were carried out under greenhouse conditions on three-year old Pseudotsuga menziesii trees planted in pots, with a height of 45 to 47 cm. The inoculation was carried out with the method of mycelium discs in the stem (O'Gara et al., 1996). In the point of inoculation, the stems of the plants were cleaned with sterile distilled water, using a dissection needle disinfected with alcohol at 70 % and heated with a burner, ten punctures were performed, from each isolation, disks were taken with a diameter of 0.5 mm with a mycelium of the pseudofungus grown in a PDA medium, which were placed in the area and covered with moist sterile cotton, and covered with a gauze and parafilm tape around the stem in order to retain humidity. All this was carried out under aseptic conditions.
Five plants were inoculated for each of the isolations (repetitions), and there was a control which was only applied PDA madium, giving a total of 30 inoculated plants. Finally, each plant was covered with a plastic bag to produce conditions of relative humidity. They remained in this way for one week, and were kept at a temperature of ± 25 °C. The plants were distributed at random on tables in the greenhouse and watered every three days at field capacity. The number of days before the appearance of symptoms and death of each plant was evaluated on a weekly basis for eight months, and the results obtained underwent a statistical analysis (Kruskall-Wallis test) using the program SAS (SAS, 2012) to determine the behavior of the P. cinnamomi isolations.
Results and Discussion
The isolations obtained from the soil, root, and cankers of the trees in different locations are shown in Table 1.
Table 1 Isolations obtained from P. cinnamomi, used in the pathogenicity tests on Pseudotsuga menziesii plants.
| Clave | Localidad | Hospedante | Origen Suelo/Raíz/cancro |
|---|---|---|---|
| COL - A | El Arrayanal, Col. | Quercus salicifolia. | cancro |
| GRO - P | Tecoanapa, Gro | Quercus elliptica. | cancro |
| JAL - C | Manantlán, Jal. | Quercus peduncularis. | Suelo |
| MICH- P | Peribán, Mich. | Aguacate | Raíz |
| EDO -T | Tres Encinos. Edo. de México | Pseudotsuga menziesii | Suelo |
Morphological identification
The five isolated colonies of Q. salicifolia, Q. elliptica, Q. peduncularis, Pseudotsuga mensiezii and Persea americana trees showed a uniform growth in a PDA medium, displaying a mycelial growth with a cotton-like aspect, white colored, camellia-shaped (Figure 1) due to the depressed growth; coenocytic mycelium, torulose, with coralloid hyphea and abundant swellings, all at the genus level. This pathogen was observed to produce spherical, oval, piriform, terminal or intercalated chlamydospores, frequently in bunches. Some authors mention that they persist in the soil for years, which constitute an organ of conservation and survival as reported by Erwin and Ribeiro (1996); Jung et al., (2013) and Zentmyer et al., (1996).
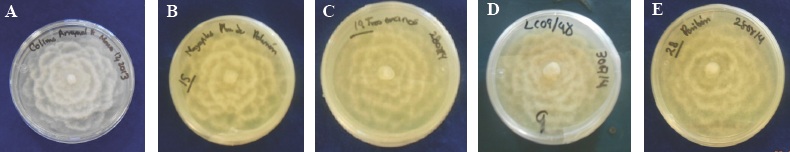
Figure 1 A) Colonies of the six-day old heterothallic P. cinnamomi isolations coded: A) COL-A and seven days old with the codes: B) GRO-P, C) EDO-T encinos, D) JAL-C and E) MICH-P , camellia-shaped, developing in PDA medium.
The measurements of the average length and width of 100 sporangia from each isolation were also taken, and they are as follows: COL-A 40.49 x 28.19, JAL-C 40.81 x 28.63 μm, GRO-P 48.24 x 31.47 μm, EDO-T 43.2 x 32.9 μm and MICH 56.23 x 33. 8 μm. On the other hand, Erwin and Ribeiro (1996) report values of 75 x 40 μm, and therefore they do not coincide with those obtained in this study. Regarding the size of the sporangia, these vary depending on the weather conditions and are only produced in the soil extract (Sánchez et al., 2002b). The morphological characteristics of the P. cinnamomi sporangia, elongated ovoids and a non-papillated apex, agree with those reported by Erwin and Ribeiro (1996), Gallegly and Hong (2008) and Waterhouse (1963).
Molecular characterization
The band of the product of PCR was approximately 1200 to 1500 pb (Figure 3). The results of the sequencing of each isolation were compared with the sequences reported in the gene bank (NCBI). The sequences by isolation had a 99 % similarity index for the species P. cinnamomi. When comparing the nucleotides of each isolation and morphologically identified with those reported in the gene bank (NCBI), they corresponded to the same species. This corroborated the morphological identification of each isolation (Table 3).

Figure 2 A) Development of symptoms in three-year old Pseudotsuga menziesii plants inoculated with the different P. cinnamomi isolations, coded (COL-A, GRO-P, EDO-T, JAL-C, and MICH-P). B) Canker in plants. C) Sap on the base of the stem. D) Progress of the disease in the plant E) Light to dark brown canker caused by the isolation COL-A. F) Control and plants inoculated during the pathogenicity tests. G) Tissue infected with the isolation COL-A and mycelia of the organism in the selective medium PARHP. H) Reaisolation COL-A of seven-day old P. cinnamomi in PDA.
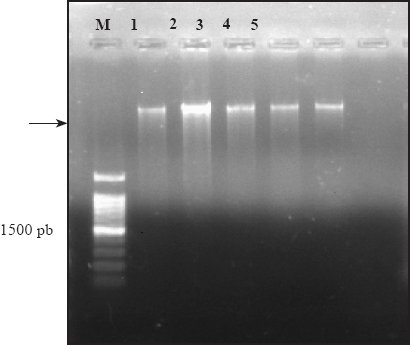
Figure 3 Electrophoresis in 1% agarose gel that shows a band of approximately 1500 pb of the product amplified by PCR with the primers ITS4 and ITS5; M. 1 kb molecular marker, lane 1. COL-A., 2. GRO-P., 3. JAL-C., 4. MICH-P, and 5. EDO-T.
Table 2 Kruskal-Wallis test to evaluate the number of days in symptoms and the deaths of plants inoculated with the five Phytophthora cinnamomi isolations.
| Tratamiento | Hongo | Medias de rangos | Grupos |
| Testigo | P. cinnamomi | 31.50 | A |
| MICH-P | P. cinnamomi | 60.68 | B |
| EDO-T | P. cinnamomi | 64.85 | B |
| JAL-C | P. cinnamomi | 64.85 | B |
| GRO-P | P. cinnamomi | 66.52 | B |
| COL-A | P. cinnamomi | 97.00 | C |
Same letters do not differ significantly between treatments.
Table 3 Morphological and molecular identification of Phytophthora cinnamomi isolations, number of nucleotides, species aligned with 99 % reliability and accession number in the National Center for Biotecnology Information (NCBI) database.
| Clave de Aislamiento | Identificación Morfológica | No. de nucleótidos | Especie alineada | Núm. de acceso a especie alineada NCBI |
| Col-A | P. cinnamomi | 1046 | P. cinnamomi | KP773290 |
| GRO-P | P. cinnamomi | 925 | P. cinnamomi | KP773291 |
| JAL-C | P. cinnamomi | 940 | P. cinnamomi | KP773292 |
| MICH-P | P. cinnamomi | 926 | P. cinnamomi | KP773293 |
| EDO-T | P. cinnamomi | 952 | P. cinnamomi | KP773294 |
Pathogenicity tests
The Pseudotsuga mensiezii plants that were given the pathogen began showing symptoms of sprout wilting, flaccid tips, chlorosis of the needles, death of leaves on the base of the plants, along with the growth of cankers on the stem, which was characterized by the presence of necrosis on its epidermis and detachment of the bark, giving a light to dark brown color.
The isolations presented a different pathogenicity in the inoculated host. The isolation COL-A from Arrayanal, Col., found in Quercus salicifolia, was the first to cause visible symptoms at 67 ddi, and the death of plants at 120 days after inoculation. It was also observed that the isolation from El Arrayanal, Col. (COL-A) was the first to cover the Petri dish after 5 days, as well as the first to form sporangia in six days in the soil solution. Isolation GRO-P, taken from Q. elliptica, caused the symptoms 93 ddi and death at 180 days after inoculation; in the case of EDO-T, in Pseudotsuga mensiezii, 135 days were required, as with JAL-C, taken from Q. peduncularis and for the death of plants, 210; forMICH-P, isolated from Persea Americana, 160 ddi and 240 days for death (Figure 2). These results show that all the isolations were able to cause a disease, yet the isolation COL-A behaved the most pathogenic in Pseudotsuga menziesii plants, therefore it was evident that there is a variation in pathogenicity amongst the P. cinnamomi isolations from the different regions in Mexico.
The above data, specifically determine death based on the pathogenicity of the isolations. These results underwent a statistical analysis using the KrusKall-Wallis test. Significant differences between isolates (P 0.0001) were obtained (Table 2). The isolation COL-A was statistically different to the others, showing an average with a higher range than the others.
The pathogen P. cinnamomi was re-isolated from the infected tissue of the plants inoculated with each isolation. The Pseudotsuga menziesii plants used as a control presented no symptoms. No similar studies on forest species were found, although these results coincide with reports by Ceja et al., (2000) on avocado plants inoculated with P. cinnamomi, who observed that the pathogen caused wilting in the foliage, dark colored cankers in the stem with a brown internal lesion, and finally the death of the plants.
This study related the symptoms caused by for the isolations in Pseudotsuga mensiezii plants, which coincides with reports by Erwin and Ribeiro (1996).
On the other hand, Jordan and Tainter (1996) observed that inoculating white oak or red oak trees, these species are very vulnerable to P. cinnamomi, and that symptoms may take months or years to become evident.
Likewise, Robin and Desprez-Loustau (1998), who inoculated P. cinnamomi mycelium agar discs in chestnut and red oak trees, tested its pathogenicity and found symptoms of yellowing, defoliation, cracking, brown coloring, and cankers, but with different levels of virulence. However, Chastagner (1997), mention that the formation or development of the canker does not always take place, since it will depend on the mechanism of the pathogen and the environment.
Podger (1989), meanwhile, mentions that when he used 14 Australian P. cinnamomi isolations, taken from 10 species of host plants, they diseased dicotyledonous species, and the main symptom consisted of an abnormal coloring in the root, and finally death. The isolates showed no difference in their pathogenicity.
The results obtained in this study on the pathogenicity of the P. cinnamomi isolations coincide with those reported by Huberli et al., (2001), who found that the pathogenicity of Australian isolations on Eucalyptus marginata and Corymbia calophylla plants displayed three types of responses: the first, with a high capacity to kill plants after 59 days after inoculation; the second, of 182, which were considered intermediate, and the third, which do not lead to the death of plants, but are considered the least pathogenic.
At the same time, Dudzinski et al., (1993) found differences between the P. cinnamomi isolations collected in Australia inoculated in Eucalyptus marginata stems. These authors found a variation of the pathogenicity of the manifestation of symptoms, from inoculation until the death of the plant. Another study, by Brasier et al., (1993) mentions that P. cinnamomi is an aggressive pathogen on oak trees, and that the introduction and spreading of forest soil with the pathogen may be an important factor in declining and reducing cork in the Iberian Peninsula.
On the other hand, Tippett et al., 1985 evaluated the inoculation method by injury in 21 Eucalytus marginata trees, and with a treatment of P. cinnamomi they discovered there is no variation in the pathogenicity of P. cinnamomi in inoculated plants.
Conclusions
All Phytophthora isolations corresponded to P. cinnamomi and proved to be pathogenic on Pseudotsuga mensiezii , out of which the isolation COL-A was the most pathogenic.
This is the first study in Mexico to report the pathogenicity of P. cinnamomi isolations from different places and hosts on P. menziesii.
Acknowledgements
To the National Science and Technology Council (CONACYT) and the Colegio de Postgraduados for the funding and acceptance to carry out my graduate studies.
REFERENCES
Alvarado-Rosales D, L de L Saavedra Romero, A Almaraz-Sánchez, B. Tlapal-Bolaños, O Trejo-Ramírez, J. M Davidson, J T Kliejunas, S W Oak, J G O'Brien, F Orozco-Torres y Quiroz-Reygadas D. 2007. Agentes asociados y su papel en la declinación y muerte de encinos (Quercus, Fagacaeae) en el centro-oeste de México. Polibotánica 23: 1-21. http://www.redalyc.org/articulo.oa?id=62102301 [ Links ]
Alvarado-Rosales-D, L de L Saavedra y A Almaraz-Sánchez . 2008. Primer reporte de Phytopohthora cinnamomi Rands. asociado al encino Quercus spp., en Tecoanapa, Guerrero, México. Agrociencia 42: 565-572. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952008000500008 [ Links ]
Almaraz-Sánchez-A, Alvarado-Rosales-D, Saavedra-Romero L de L. 2013. Trampeo de Phytophthora cinnamomi en bosque de encino con dos especies ornamentales e inducción de esporulación. Revista Chapingo. Serie Ciencias Forestal y del Ambiente 19: 5-12. http://dx.doi.org/10.5154/r.rchscfa.2011.09.062 [ Links ]
Balci Y, S Balci, W L MacDonald, and Gottschalk K W. 2008. Relative susceptibility of oaks to seven species of Phytophthora isolated from oak forest soils. For. Pathol. 38:394 409. http://dx.doi.org/10.1111/j.1439-0329.2008.00559.x [ Links ]
Brasier C M, Sobredo F Ferraz J F P. 1993. Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant. Pathol. 42, 140-145. http://dx.doi.org/10.1111/j.1365-3059.1993.tb01482.x [ Links ]
Crone M McComb, J A O'Brien P A, Hardy G E St J 2013. Assessment of Australian native annual/herbaceous perennial plant species as asymptomatic or symptomatic hosts of Phytophthora cinnamomi under controlled conditions. For. Pathol. 245-251. http://dx.doi.org/10.1111/efp.12027 [ Links ]
Ceja T L F, Téliz O D, Osada K S, Morales G J L. 2000. Etiología, distribución e incidencia del cancro del aguacate Persea Americana Mill. en cuatro municipios del estado de Michoacán, México. Rev. Mexi. Fitopatol. 18:79-86. [ Links ]
Chastagner G A, (Eds). 1997. Christmas tree diseases, insects, and disorders in the Pacific Northwest: identification and management. Washingtn State University Cooperative, Pullman 156. [ Links ]
Davidson J M, O'Brien, J G, S W Oak and J Kliejunas. 2003. Report on a site visit to México-Muerte del Encino. USDA Forest Service. 9 p. [ Links ]
Dos Santos Á F, D J Tessmann, T C A, Alves J B Vida, and R Harakava. 2011. Root and crown rot of Brazilian pine (Araucaria angustifolia) caused by Phytophthora cinnamomi. J. Phytopathol. 159:194-196. http://dx.doi.org/10.1111/j.1439-0434.2010.01741.x [ Links ]
Dudzinski M L, Old K M, and Gibbs R.J. 1993. Pathogenic variability in Australian isolates of Phytophthora cinnamomi. Aust. J. Bot 17: 35-37. http://dx.doi.org/10.1071/BT9930721 [ Links ]
Erwin D C, and O K Ribeiro.1996. Phytophthora diseases worldwide. APS. USA. 562 Gallegly M.E, and Hong C. 2008. Phytophthora: identifying species by morphology and DNA Fingerprints. APS Press.ST. Paul, MN USA. 158. [ Links ]
Gallegly M E, and Hong M. 2008. Phytophthora: identifying species by morphology and DNA Fingerprints. APS Press. ST. Paul, MN USA. 150 p. [ Links ]
Garbelotto M, Huberli D. 2006. First report on an infestation of Phytophthora cinnamomi in natural oak woodlands of California and its differential impact on two native oak species. Plant Dis. 90:685 http://dx.doi.org/10.1094/PD90-0685C [ Links ]
García D S E. 2007. Pudriciones de raíz por Phytophthora / Root rot by Phytophthora. En: Cibrián T D, Alvarado R.D, y García D S E (Eds). 2007 Enfermedades forestales en México/ Forest Disesases in México. Universidad Autónoma Chapingo y CONAFOR-SEMARNAT, México; Forest Service USDA, E.U.A.; NRCAN Forest Service, Canadá y Comisión Forestal de América del Norte, CONAFOR, FAO Chapingo, México. 587. [ Links ]
Huberli D, Tommerup I C, Dobrowolski M P, Calver M C, and Hardy G E St J . 2001. Phenotypic variation in a clonal lineage of two Phytophthora cinnamomi populations from Western Australia. Mycol. Res. 105:1053-1064. http://dx.doi.org/10.1016/S0953-7562(08)61967-X [ Links ]
Jordan A P and Tainter F H. 1996. The susceptibility of Southern Appalachian Oaks to Phytopthora cinnamomi. Department of Forest Resources, Cleson University,USA. Castanea 61(4): 348-355. http://www.jstor.org/stable/4033859?seq=1#page_scan_tab_contents [ Links ]
Jonsson U Jung T, Sonesson K, and Rosengren U. 2005. Relationships between health of Quercus robur, occurrence of Phytophthora species and site conditions in southern Sweden. Plant Pathol. 54: 502-511. http://dx.doi.org/10.1111/j.1365-3059.2005.01228.x [ Links ]
Jeffer N, Martín JB.1986. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 70, 1038-1043. http://dx.doi.org/10.1094/PD-70-1038 [ Links ]
Jung T, Colquhom I J, Hardy G E St J. 2013. New insights into the survival strategy of invasive soilborne pathogen Phytophthora cinnamomi in indifferent natural ecosystems in Wester Australia. Forest Pathol. 43: 266-288. http://dx.doi.org/10.1111/efp.12025 [ Links ]
Martin R R, James D, Levesque. 2000. Impacts of molecular diagnostic technologies on plant disease. Annu Rev Phytopathol. 38: 207-239. http://dx.doi.org/10.1146/annurev.phyto.38.1.207 [ Links ]
Pariaud B, Ravigne V, Halkett F, Goyeau H, Carlier J, y Lannou C. 2009. Aggressiveness and its role in the adaptation of plant pathogens. Plant Pathology, 58:409-424. http://dx.doi.org/10.1111/j.1365-3059.2009.02039.x [ Links ]
O'Gara E, Hardy G E S, and Mc Comb J A. The ability of Phytophthora cinnamomi to infect trough wounded periderm tissue of Eucalyptus marginata. Plant Pathology 45: 955-963. http://dx.doi.org/10.1111/j.1365-3059.1996.tb02906.x [ Links ]
Pérez M, 2008. Significant Avocado Diseases Caused by Fungi and Oomycetes. The European J Plant Sci Biotechnol 2(1):1-24. http://www.globalsciencebooks.info/Online/GSBOnline/images/0806/EJPSB_2(1)/EJPSB_2(1)1-24o.pdf [ Links ]
Philip A, O'Brien Nari Williams, and Giles E St J Hardy. 2009. Critical Review in Microbiology 35(3): 169-181. [ Links ]
Podger F D. 1989. Comparative pathogenicity of fourteen Australian isolates of Phytophthora cinnamomi determined on transplants of Tasmanian temperate heathland. Aust. J. Bot. 37: 491-500. http://dx.doi.org/10.1071/BT9890491 [ Links ]
Robin C, and Desprez-Loustau M L. 1998. Testing variability in pathogenicity of Phytophthora cinnamomi. European Journal of Plant Pathology (Buscar abreviatura) 104: 465-475. http://dx.doi.org/10.1023/A:1008649806620 [ Links ]
Romero C S. 1998. Hongos fitopatógenos. Universidad Autónoma Chapingo. Dirección General de Patronato Universitario. 347 p [ Links ]
Sánchez M E, Andicoberry S, and Trapero A. 2005. Pathogenicity of three Phytophthora spp. causing late seedling rot of Quercus ilex ssp. ballota. Forest Pathol 35: 115-125. http://dx.doi.org/10.1111/j.1439-0329.2004.00392.x [ Links ]
Sánchez M E, Caetano P, Ferraz J, Trapero A. 2002b. Phytophthora disease of Quercus ilex insouthwestern Spain. For. Path. 32, 5-18. http://dx.doi.org/10.1046/j.14390329.2002.00261.x [ Links ]
Sambrook J, and Russell D W. 2001. Molecular Cloning: A Laboratory Manual Volumen 1, 3rd. edition. Cold spring Harbour Laboratory Press, NY. USA. 234 p. [ Links ]
Shew H D, and Benso D M. 1983. Influence of soil temperature and inoculum density of Phytophthora cinnamomi on root rot of Fraser fir. Plant Dis. 67:522-524. http://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1983Articles/PlantDisease67n05_522.PDF [ Links ]
SAS Intitute. 2012. GLM Procedure. SAS Institute. Cary, NC, USA. [ Links ]
Tainter F H, O'Brien J G, Hernández A, Orozco and O Rebolledo. 2000. Phytophthora cinnamomi as a cause of oak mortality in the state of Colima, Mexico. Plant Dis. 84(4):394-398. http://dx.doi.org/10.1094/PDIS.2000.84.4.394 [ Links ]
Tamayo P J. 2007. Enfermedades del aguacate. Revista Politécnica 4:52-71. [ Links ]
Tsai H L, Huang L C, Ann P J, and Liou R F. 2006. Detection of orchid Phytophthora diseases by nested PCR. Bot Bull. Acad. Sin. 47: 379-387. http://ejournal.sinica.edu.tw/bbas/content/2006/4/Bot474-03.pdf [ Links ]
Téliz O D y Mora A J A. 2007. El manejo integral del aguacate. In Téliz,O D; Mora A. El aguacate y su manejo integral 2a. ed(Eds).Editorial Mundi-Prensa, Méx.D.F., p 287-306. [ Links ]
Tippett J T, Hill T C, and Shearer B L.1985. Resistance of Eculalyptus spp. to invasion by Phytophthora cinnamomi. Australian Journal of Botany 33, 409-18. http://dx.doi.org/10.1071/BT9850409 [ Links ]
Thompson S E, Levin S, and Rodriguez-Iturbe I. 2014. Rainfall and temperatures changes have confounding impacts on Phytophthora cinnamomi occurrence risk in the southwestern USA under climate change scenarios. Glob. Chang. Biol. 20 1299-1312. http://onlinelibrary.wiley.com/doi/10.1111/gcb.12463/abstract [ Links ]
Waterhouse G H. 1963 Key to the species of Phytophthora de Bary. Commonw Mycol. Inst. Kew. UK. 92 p. [ Links ]
White JCH, Bruns, TLee S, Taylor J W. 1990. Amplification and direct sequencing of fungal ribosomaol RNA genes for phylogenetics. P315-322 In: Innis, ma, Gel fand, D.H., Sninsky, J. J., White, J. J. PCR protocols A guide to methods and apllications. Eds. Academic Press, Inc., New York. Pp:315-322 [ Links ]
Zentmyer G A. 1952. Phytophthora cinnamomi on avocado in México and Costa Rica and others avocado diseases in México. Plant. Dis. Rep. 36: 31 p. [ Links ]
Zentmyer A G. 1980. Phytophthora cinnamomi and the diseases it causes. Monograh. 10 APS.Press. St. Paul, MN. USA. 96 pp. [ Links ]
Zentmyer G A, Mircetich S M.1966. Saprophytims and persistence in soil by Phytohthora cinnamomiPhythopathology 56:710-112. [ Links ]
Received: October 13, 2015; Accepted: May 05, 2016











 text in
text in 


