Introduction
Bats (Chiroptera) are the second most abundant order of mammals on Earth (Simmons, 2005). Although most bat species are insectivorous, this order exhibit a high diversity in diet types including frugivores, nectarivores, carnivores, piscivores and sanguinivores, providing different ecological services such as insects pest control, seed dispersal and pollination (Kunz et al., 2011; Voight & Kingston, 2016). In Chiroptera, only two families (Pteropodidae in Paleotropics and Phyllostomidae in Neotropics) contain nectarivorous bats morphologically specialized for flower visiting, representing about 3.7% of bat species worldwide (Fleming et al., 2009).
The nectarivorous bats in Phyllostomidae comprise two subfamilies: Glossophaginae (Long-nosed bats) and Lonchophyllinae (Nectar bats), these species present morphological adaptations for the consumption of nectar and pollen (Simmons & Cirranello, 2023). These include an elongated rostrum, teeth reduced in size and number, and a long tongue with hair-like papillae (Freeman, 1995). In Neotropics, about 360 plant species are pollinated by bats of the Phyllostomidae family (Fleming et al., 2009; Kunz et al., 2011). Most of these plant species exhibit characteristics of bat pollinated flowers such as nocturnal anthesis, pale coloration, musty smell, exposed flowers and tubular or radially symmetrical flowers (Howe & Westley, 1988).
In Mexico, among the plant families pollinated by bats is Cactaceae, which stands out for its economic and cultural importance (Parra et al., 2008; Fleming et al., 2009). Cactaceae is endemic to America, and its diversification center is in Mexico, where many species, many of them endemic, thrive. Of the 850 species found in the Mexican territory, 84% are exclusive to the country (Becerra, 2000). At the national level, the northeastern region and the southern portion of the Chihuahuan Desert stand out for their large number of species and endemic taxa (Martínez-Ávalos & Jurado, 2005). The state of Tamaulipas stands out for the large number of genera of cacti, being the municipality of Jaumave the one with the largest number of species. This lends this municipality a priority status in conservation efforts (Martínez-Ávalos & Jurado, 2005).
One of these species is Stenocereus huastecorum, which, like many other species of columnar cacti, features flowers with characteristics indicative of bat pollination (Parra et al., 2008). This species is relevant for its nutritional value for humans since the whole plant can be eaten, and its fruit is the most appreciated part. Though in Mexico various species of Stenocereus of great economic value are cultivated, these plants are mainly harvested in the wild (Campos-Rojas et al., 2011). The floristic lists of cacti register S. huastecorum as being most abundant in the state of Tamaulipas, mainly the municipalities of San Carlos, Victoria, Jaumave, and Tula (Martínez-Ávalos, 1998; Martínez-Ávalos & Jurado, 2005). However, very little is known about their populations and the bats that could be pollinating them.
Bats of the Glossophaginae subfamily are the main pollinators of columnar cacti species in the desert regions of northern Mexico (Valiente-Banuet et al., 2004). In the state of Tamaulipas, five species of bats of this subfamily have been reported: Anoura geoffroyi Gray, 1838, Choeronycteris mexicana, Glossophaga mutica Merriam, 1898 sensu (Calahorra-Oliart et al., 2021), Leptonycteris yerbabuenae, and L. nivalis. These bats may be visiting the flowers of S. huastecorum during the night and contributing to their pollination (Moreno-Valdez & Vásquez-Farías, 2005; Medellín et al., 2008). Therefore, this study seeks to identify the bat species that visit the flowers of these columnar cacti, describe their nocturnal activity patterns, and compare their effectiveness as pollinators to that of diurnal pollinators.
Materials and methods
Study area: The Jaumave valley is in the municipality of Jaumave, Tamaulipas, Mexico, at 23.578889° N, and -99.363889° W, which spans over an area of 452.71 km2 (Gobierno del Estado de Tamaulipas, 2011). We conducted this study at Ejido las Pilas in the municipality of Jaumave. The Jaumave Valley, as this entire area surrounded by the Sierra Madre Oriental is known, includes a series of low-lying plateaus in its interior, as well as hills in the highest parts on the border with the Sierra Madre Oriental. This area of southwestern Tamaulipas is home to different plant communities adapted to its rugged landscape (González-Medrano, 2004). For example, in the highlands of the Sierra Madre Oriental, oak and pine forests are dominant mainly represented by Quercus polymorpha Schlecht. & Cham., 1830, Quercus canbyi Trel., 1924 and Pinus teocote
(Schiede ex Schltdl. & Cham.,1830), while in slopes and plateaus, microphile desert scrub and submontane scrub are often found. Species like Flourensia laurifolia DC., 1836, Acacia rigidula Benth., 1842, and Pilosocereus leucocephalus (Poselg.) Byles & G.D. Rowley, 1957 tend to be dominant in these plant formations, while on flat parts and plains of the Valley it is common to find large stands of mesquite along with patches of rosetophil shrublands. Among the most common species of the latter are: Prosopis leaevigata ((Humb. et Bonpl. ex Willd) M.C. Johnston), Forestiera angustifolia (Torr.), Agave lechuguilla Torr., 1859 and Hechtia hernandez sandovalii (I. Ramírez, C.F. Jiménez & J. Treviño), as well as many endemic cacti, such as Mammillaria baumii Boedeker, 1926, M. roseoalba (Boed.) and Obregonia denegrii (Fric.) (Martínez-Ávalos & Jiménez-Pérez, 1993). In addition, there are some columnar species such as Stenocereus huastecorum (Alvarado-Sizzo et al., 2018), which is very abundant; Myrtillocactus geometrizans (Mart. ex Pfeiff.) Console, 1897, Pilosocereus leucocephalus (Poselg.) Byles & G.D. Rowley, Cact. Succ. J. Gr. Brit. 19: 67. 1957 and Cephalocereus euphorbioides (Haw.) Britton & Rose, Cact. 2: 33. 1920 (Tapia et al., 2017), all of them of great importance for diurnal fauna such as some microarthropods (Islas-Barrios et al., 2021). Bat potential visitors of columnar cacti flowers at the study area include five Glosophaginae species (Moreno-Valdez & Vásquez-Farías, 2005), as well as bat species that occasionally consume pollen and nectar, for example, some frugivorous species such as Artibeus jamaicensis, A. lituratus and Sturnira parvidens (Valiente-Banuet et al., 1997a; Rocha et al., 2019), and Antrozous pallidus a gleaning insectivorous bat (Frick et al., 2009).
Stenocereus huastecorum has two flowering periods throughout the year, the first one from March to May and the second one from August to October (Cortés-Díaz, 1997). A population density of 0.048 individuals/m² of S. huastecorum was estimated at the study site, other characteristics recorded in the individuals were an average height of 5.22 m, the average number of branches was 17, while the average of flowers/individual and flower buds/ individual were 3, finally, an average of 6 fruits/ individual.
Effectiveness of Pollinators: During the two flowering periods in 2018, 19 cacti individuals located at Ejido las Pilas were selected and marked: 13 for the first period and six for the second period. From each individual, three flowers were chosen for the application of the following field treatments: nocturnal exclusion where the flower was covered at night with an organza bag and exposed during the day, diurnal exclusion where it was exposed at night and covered with an organza bag during the day, and control where the flower was exposed all the time (Fig. 1) (Valiente-Banuet et al., 1997b). The number of fruits obtained from each treatment was recorded.

Figure 1 Day and night exclusion treatments applied to individuals of Stenocereus huastecorum during their flowering periods.
Activity of floral-visiting bats: Six sampling expeditions were conducted per each flowering period, monitoring during two nights (24 nights in total). Three 12 x 2.6 meter mist nets were placed in proximity (50-150 centimeters apart) to individuals of S. huastecorum, left open for four hours after sunset and checked every 20 minutes, because the highest number of reported captures of Stenocereus pollinating bats occurs between 20-24 hours (Casas et al., 1999), but also because security reasons. Captured bats were identified using the field key guide by Medellín et al. (2008). To determine whether captured bats had visited the flowers of the cacti, a sample of pollen was taken with a moist cotton bud rubbing the animal’s face and body (Jiménez, 2008).
Identification of pollen samples obtained from bats: Pollen samples were processed by the acetolysis technique (Erdtman, 1969), mounted on glycerol gelatin-fixed slides, and identified using an optic microscope (Carl Zeiss PRIMOSTAR, 100x), with reference samples collected in the field (Jiménez, 2008).
Data analysis: The activity patterns of the bat species that visit the flowers of S. huastecorum per night were described in alluvial graphs with the RStudio 2022.02.2+485 "Prairie Trillium", ggallivual package (Brunson, 2020). A Generalized Linear Model (GLM) with Poisson error was run to compare the effectiveness of nocturnal and diurnal pollinators (Badii & Castillo, 2009), with the number of fruits obtained by individual cactus per treatment in each flowering period as the response variable.
Results
A total of 67 bats belonging to three species of the Glossophaginae subfamily (Leptonycteris nivalis, L. yerbabuenae, and Choeronycteris mexicana) were captured during the two flowering periods.
Throughout the first flowering period (March to May), 39 individuals were captured, most of which belonged to the L. yerbabuenae species (Table 1). The largest number of L. nivalis (three individuals) was captured in March, and the largest number of C. mexicana (four individuals) in April. Most bats captured in this season (58.97%) presented pollen from S. huastecorum (Fig. 2).
Table 1 Bat species recorded as floral visitors of Stenocereus huastecorum during flowering periods in 2018.
| Bat species | 1st Flowering Period | 2nd Flowering Period | ||
| Captured Individuals | Individuals with pollen | Captured Individuals | Individuals with pollen | |
| Leptonycteris yerbabuenae | 28 | 18 | 11 | 8 |
| Leptonycteris nivalis | 5 | 3 | 7 | 1 |
| Choeronycteris mexicana | 6 | 2 | 10 | 7 |
| Total | 39 | 23 | 28 | 16 |
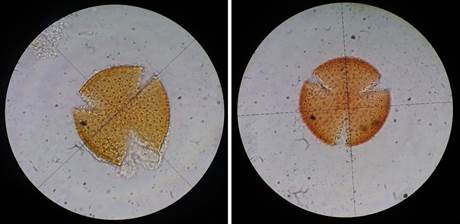
Figure 2 Pollen samples of Stenocereus huastecorum obtained from Glossophaginae bats and observed using an optic microscope Zeiss Primostar, 100X.
During the second flowering period, 28 bats of the three species of bats were captured (Table 1). In this period, L. yerbabuenae and C. mexicana registered a similar number of individuals. August was the peak month for L. yerbabuenae (seven individuals), September for C. mexicana (six individuals) and October for L. nivalis (five individuals). More than 70% of captured individuals of L. yerbabuenae and C. mexicana presented pollen from S. huastecorum in their body (Fig. 2).
Leptonycteris yerbabuenae was the most active of the species at night, its activity began a little after sunset and continued until past midnight (Fig. 3). The activity of L. nivalis and C. mexicana were concentrated in the early hours of the night, with very few individuals around midnight.
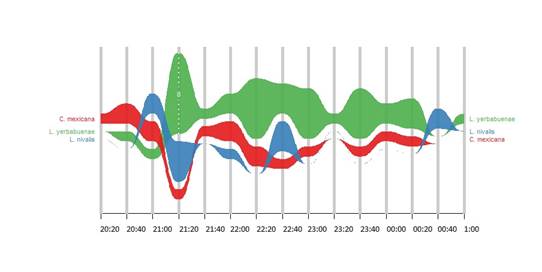
Figure 3 Activity patterns of floral visiting bats of Stenocereus huastecorum in both flowering periods
During the first flowering period, 13 flowers were used per treatment, the diurnal treatment yielded eight fruits, the nocturnal treatment eight fruits, and the control treatment 11 fruits. In the second period, six flowers were used per treatment. Six fruits were obtained from diurnal exclusion, five from nocturnal exclusion, and four from control. The GLM showed no significant differences in the number of fruits obtained from treatments in the two flowering periods under evaluation (Tables 2-3).
Table 2 Comparison of exclusion treatments during the flowering periods of Stenocereus huastecorum.
| Flowering period | Treatments | Estimate | Std. Error | Z value | Pr(>|z|) |
|---|---|---|---|---|---|
| First | Diurnal vs. Control | -1.2347 | 0.957 | -1.29 | 0.399 |
| Nocturnal vs. Control | -1.2347 | 0.957 | -1.29 | 0.399 | |
| Nocturnal vs. Diurnal | 0 | 0.8062 | 0 | 1 | |
| Second | Diurnal vs. Control | -4.89E-16 | 1.23E+00 | 0 | 1 |
| Nocturnal vs. Control | 9.16E-01 | 1.40E+00 | 0.656 | 0.788 | |
| Nocturnal vs. Diurnal | 9.16E-01 | 1.40E+00 | 0.656 | 0.788 |
Table 3 Coefficients of comparison of exclusion treatments during the flowering periods of Stenocereus huastecorum.
| Flowering period | Treatments | Estimate | Std. Error | Z value | Pr(>|z|) |
|---|---|---|---|---|---|
| First | Intercept | 1.7047 | 0.7687 | 2.218 | 0.0266 |
| Diurnal exclusion | -1.2347 | 0.957 | -1.29 | 0.197 | |
| Nocturnal exclusion | -1.2347 | 0.957 | -1.29 | 0.197 | |
| Second | Intercept | 9.93E-16 | 8.66E-01 | 0.8 | 0.423 |
| Diurnal exclusion | -4.89E-16 | 1.23E+00 | 0 | 1 | |
| Nocturnal exclusion | 9.16E-01 | 1.39E+00 | 0.656 | 0.512 |
Discussion
We identified three species of Glossophaginae bats that visit S. huastecorum flowers. The abundance of L. yerbabuenae, especially in the first flowering period, which coincides with the results of Cajas et al. (2008) in Guatemala, where this bat was the most abundant during S. huastecorum flowering period. Since several individuals of this species presented pollen from S. huastecorum in both seasons, it is likely that this bat species plays a relevant role in the nocturnal pollination of this species of cacti.
Though in their study Moreno-Valdez & Vásquez-Farías (2005) reported five species of Glossophaginae bats in Tamaulipas, the fact that two of them (Glossophaga mutica and Anoura geoffroyi) were absent in our study area may be due to its location at the northernmost limit of distribution of these two bat species (Álvarez et al., 1991; Ortega & Alarcón, 2008). These bat species have been reported as floral visitors of other cacti, G. mutica bats have been reported as visitors to the flowers of Pterocereus gaumeri (Britton & Rose) Th. MacDoug. & Miranda in Mexico (Méndez et al., 2005), whereas A. geoffroyi has been reported as flower visitor of Espostoa frutescens (J.E. Madsen) in Ecuador (Simon et al., 2019).
The high abundance of L. yerbabuenae in the first flowering period (March-May) could be due to a foraging strategy in small groups (up to four individuals) adopted by this species (Horner et al., 1998), or to their migration to the United States during spring (Rojas-Martinez et al., 1999), which contributes to their numbers being larger in the northern part of Mexico, where they benefit from the food resources available (mostly cacti) along their route (Burke et al., 2019). These authors also suggest that S. huastecorum has a great influence on the distribution pattern of L. yerbabuenae during winter, which may be due to the consumption by these bats of the fruits more than the flowers at the second flowering period.
In the case of C. mexicana, there was a slight increase in the number of individuals visiting the flowers of S. huastecorum in the second flowering period at the end of summer and the beginning of fall. Since L. yerbabuenae returns to Mexico in autumn (Rojas-Martínez et al., 1999), lower competition with this species may have favored the presence of C. mexicana. A territorial and aggressive behavior has been reported for both L. yerbabuenae and C. mexicana around their food resources, which may help to explain the presence of a larger number of individuals of these species with pollen from S. huastecorum, as compared to L. nivalis (Arias-Cóyotl et al., 2006).
Various authors have suggested that different daily activity patterns among species using the same resources could facilitate their coexistence in a given area (Delaval et al., 2005; Adams & Thibault, 2006). We found a clear overlapping in the activity patterns of the Glossophaginae bat species, especially during the two first hours after sunset. Since flowers of S. huastecorum present nocturnal anthesis (Cortés-Díaz, 1997), as do other species of the same genus (S. queretaroensis (F.A.C.Weber ex Mathes.) Buxb., S. thurberi (Engelm.) Buxb.), they may produce more nectar with a higher concentration of sugars during the first hours after anthesis (Ibarra-Cerdeña et al., 2005; Bustamente et al., 2010). This would help explain the larger number of bats visiting their flowers in this period of the night. However, further studies on the flowering biology of this plant species will be needed to confirm this, since in this study the production of nectar and pollen by S. huastecorum could not be assessed.
The species with the highest activity around S. huastecorum plants during the four hours of monitoring was Leptonycteris yerbabuenae, with a reduction of individuals captured after midnight. It has been observed that the activity pattern of this species can vary depending on the type of vegetation and the availability of food. For example, in a tropical deciduous and semi-deciduous forest in Michoacán, Mexico, Leptonycteris yerbabuenae individuals show a bimodal pattern, with one activity peak three hours after sunset and another one three hours before dawn (Chávez-Estrada, 2019), while in a bay in an arid area in the state of Sonora, Mexico, where columnar cacti are predominant, the peak of activity was found to be between 23:00 and 01:00 h around Pachycereus pringlei cacti (S.Watson) Britton & Rose (Horner et al., 1998). Both C. mexicana and L. nivalis individuals were captured during the first two hours after sunset. As for C. mexicana, a bimodal activity pattern has been reported, namely one a little after sunset, following pollen liberation, and another one after 24:00 h, coinciding with the nectar production peak of Stenocereus stellatus (Pfeiff.) Riccob. in the Tehuacan Valley (Casas et al., 1999). Though information about the activity patterns of L. nivalis is scarce, a study on the seasonal and nocturnal activity of this species in the state of Texas found that most of the nocturnal activity followed a bimodal pattern, with a peak after sunset and another one a few hours before dawn (Adams, 2015). In this study, due to security concerns, it was not possible to conduct the monitoring of bats throughout the entire night, but we suspect that a similar bimodal pattern would be found for both species if the monitoring periods were extended.
Though bats were expected to be the most effective pollinizers of S. huastecorum, given that its flowers present the flowering chiropterophily syndrome, we found similar fruit numbers in the diurnal, nocturnal and control treatments. Other columnar cacti species whose flowers present nocturnal anthesis, pale colors, and strong and unpleasant smell, also have both nocturnal and diurnal visitors (such as S. stellatus and S. queretaroensis) and are successfully pollinized by bats (Casas et al., 1999; Ibarra-Cerdeña et al., 2005). Several authors suggest that the activity of bats as pollinators of columnar cacti varies geographically, and that in extra tropical areas, at the limits of distribution of these cacti, pollination can be successfully carried out by diurnal and nocturnal pollinators (Sahley, 1996; Valiente-Banuet et al., 1997b; Fleming et al., 2001), which coincides with the results obtained in this study.
In our study area, pollination ecology of S. huastecorum is important as these cacti are key to the survival of the fauna as well as the economy of local rural communities.











 nueva página del texto (beta)
nueva página del texto (beta)



