Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta zoológica mexicana
versão On-line ISSN 2448-8445versão impressa ISSN 0065-1737
Acta Zool. Mex no.88 Xalapa Abr. 2003
Artículo
Comparative analysis of reproductive and nesting behavior in several species of Eurysternus Dalman (Coleoptera: Scarabaeinae: Eurysternini)
Carmen Huerta, Gonzalo Halffter, Violeta Halffter and Rosario López
Instituto de Ecología, A.C., Departamento de Ecología y Comportamiento Animal Km 2.5 Antigua Carretera Coatepec No. 351, Congregación El Haya CP 91070, Xalapa, Veracruz, MÉXICO. E- mail: carmen@ecologia.edu.mx
Recibido: 13 de diciembre 2001
Aceptado: 29 de julio 2002
Resumen
Este artículo es un trabajo de síntesis, pero también incorpora mucha información inédita (más de un 50% del total). Para dar una nueva visión global de la nidificación en Eurysternus, utilizamos los datos ya publicados, la información reunida entre 1967 y 2001, conservada en los cuadernos de laboratorio, así como observaciones sobre especies nunca antes estudiadas. El propósito es presentar una visión de conjunto de la nidificación y de los procesos a ella asociados, en todas las especies de Eurysternus en donde se tiene información (9 especies).
En un género muy homogéneo en su morfología, aparecen dos tipos de nidificación totalmente distintos (E. foedus que elabora masas-nido y las otras especies con nidificación conocida que fabrican bolas-nido) que corresponden a dos tendencias muy diferentes en la evolución de la nidificación dentro de los Scarabaeinae. Pero además, dentro de lo que sería el tipo de nidificación más representado: varias bolas-nido integrando un nido compuesto, aparecen especies con cuidados (subsociales) y sin cuidados post-oviposición. De la revisión de los datos obtenidos de 307 parejas mantenidas en el laboratorio y de cuatro especies cuyos nidos fueron observados en el campo, resalta la existencia en unas especies, pero no en todas, de dos tipos de nido (provisionales y definitivos), así como el infanticidio practicado por la madre, por el padre o por ambos progenitores. Sabemos realmente mucho de la nidificación de Eurysternus, pero la reunión de datos abre muchas preguntas nuevas.
Palabras clave: Nidificación, Eurysternus, Scarabaeinae, infanticidio.
Abstract
This work aims at achieving a synthesis, and for this reason incorporates much unpublished information (more than 50% of all the data presented here). The goal is a better overall vision of nesting behavior and associated processes of the nine Eurysternus species for which data are available. Thus, we used not only published information, but also information from laboratory notebooks (covering 1967 to 2001) and observations on species never formally studied to date.
Eurysternus, a morphologically quite homogeneous genus, shows two distinct types of nesting behavior (i.e., that of E. foedus, which makes brood-masses; and that of other Eurysternus species, so far as they are known, which make brood-balls), representing two directions in the evolution of Scarabaeinae nesting behavior. Beyond this, among the species showing the most common nesting behavior pattern—with several brood-balls integrated in a compound nest—some species care for their young after oviposition (subsocial species) while others do not. A review of data on a total of 307 Eurysternus pairs (representing seven species) maintained and studied in the laboratory, and four Eurysternus species (E. deplanatus, E. inflexus, E. jessopi, and E. magnus) whose nests were observed in the field demostrated that some species (though not all) develop two different types of nests (provisional or experimental, and definitive). Infanticide has also been observed, by the mother, by the father, and by both parents (for five of the nine species studied). While much is known in general about Eurysternus nesting behavior, synthesizing the data available raises new questions.
Key Words: Nidification, Eurysternus, Scarabaeinae, infanticide.
Introduction
Even within a group like the Scarabaeinae, which shows elaborate and varied nesting behavior, Eurysternus behavior is exceptional. It is exceptional for its complexity, for its seemingly extravagant use of a food source as valuable as dung, for its display of behavior not seen in other Scarabaeinae, and for the strong differences seen among morphologically similar species.
At the beginning of the nesting season, Eurysternus forms balls of excrement. Because of the form of the beetles, notably the disposition of their coxae and legs, they are unable to roll the dung balls that they prepare. Eurysternus is the only well known member of Scarabaeinae that remains outside the two principal branches of the subfamily's evolutionary tree (for recent taxonomic reviews of Eurysternus, see Jessop [1985] and Martínez [1988]). Scarabaeinae are distinguished by the way they relocate food for adults and nests and by morphological characteristics associated with this process: diggers and rollers (see Halffter and Edmonds 1982). Like Eurysternus, some other Scarabaeinae also do not relocate their food (see Halftter and Edmonds [1982] on nesting pattern VII), although there is clearly a relationship between these species and other Oniticellines which do show relocation in the form of burial (see Cambefort, 1982, Cambefort and Lumaret, 1983). Eurysternus is the only Scarabaeinae in which there appears to be no relationship between adult feeding and nesting behavior. The beetles simply feed directly from the food source. In nesting, there is no clear relocation of food.
Halffter (1977) caracterized the nesting behavior of Eurysternus, as pattern VI reproductive behavior. This pattern has been revised by Halffter and Edmonds (1982), together with other previously characterized patterns, as one demonstrating the following: "Larval provision supplied as brood balls, which may be covered after fabrication; nests endocoprid or superficial, compound, established after a complicated sequence of brood ball construction, abandonment or consumption and oviposition; brood balls grouped in nesting cavity; provisioning direct; egg chamber superior and not isolated; male-female cooperation absent or slight; brood care present."
This definition covers all Eurysternus nesting processes known to date, except those of E. foedus (Halffter et al. 1980).
Up to the present, nesting and reproductive behavior have been studied in the following species: E. magnus Laporte, E. balachowskyi Halffter and Halffter, and E. mexicanus Harold (Halffter 1977); E. magnus, E. balachowskyi, E. caribaeus (Herbst) and E. foedus Guérin-Méneville (Halffter et al. 1980). Halffter and Edmonds (1982) offer a critical review of known nesting behavior, comparing it with other Scarabaeinae species. Halffter (1997) provides a similar review with a focus on subsocial behavior.
Clearly, the published data on Eurysternus behavior raise a number of questions: Why is the nesting and reproductive behavior so complex, with various cycles and more than one type of nest? Why is infanticide practiced? Why are there major differences in nesting behavior among Eurysternus species? Our research team has long focused on the study of Eurysternus , yielding a wealth of observations, although many remain unpublished. Additionally, we have studied species other than those covered in the literature. We have maintained all the observational and metholodological records for the work carried out by G. Halffter and colleagues since 1967. All of this material is used here to produce a comparative study, with the goals of clarifying species differences and to determine whether the nesting pattern VI definition continues to be valid. Infanticide is also studied here through a comparative and synthetic approach. Experimental results are covered in other work. As in all syntheses, we sought commonalities and differences among previously studied characteristics, but we also sought to identify gaps in understanding and to propose ways to fill these gaps.
To present state-of-the-art knowledge on Eurysternus nesting patterns, we analyzed the nesting of nine species, five of which have been studied by Halffter and his group, including the authors. In adition to this previously published information, we included laboratory observations of E. foedus Guérin-Méneville, whose nesting behavior was very summarily covered in Halffter et al. (1980), along with recent laboratory observations of E. mexicanus Harold, E. marmoreus Laporte and E. jessopi Martínez, and observations of José Ramón Verdú (CIBIO, Universidad de Alicante) on E. deplanatus Germar and E. inflexus Germar. No information on the last four species has been presented in the literature to date. Additionally, we include results of field studies carried out by Julio N. C. Louzada on E. jessopi Martínez (Universidade de Lavras, M. G. Brasil).
Material and methods
a) Data Sources
For four species, we have used information from laboratory notebooks covering the work of Halffter and colleagues between 1967 and 1983. This includes observations of seven terraria of E. balachowskyi (1969-1970), three of E. magnus (1967-1968), two of E. foedus (1967), and 37 of E. caribaeus (1977-1978 and 1983).
E. mexicanus has been the species best studied, perhaps because of its relatively ample distribution in tropical Mexico. Beyond our group's published papers, we also have data on this species from 1970 to 1972 for nine terraria, from 1976 to 1977 for ten terraria, from 1986 to 1987 for nine terraria, and from 1995 to the present for about 100 terraria.
We obtained information on the biology of E. marmoreus for 15 terraria that were followed from 1995 to 1996. E. jessopi was studied in 34 terraria maintained from August 1999 to August 2000. Dr. Verdú gathered data on E. deplanatus in September 1996 in the Estación Biológica de Boraceira, Salesopolis, São Paulo, Brazil, and data on E. inflexus during this same month in Rio Claro, São Paulo, Brazil.
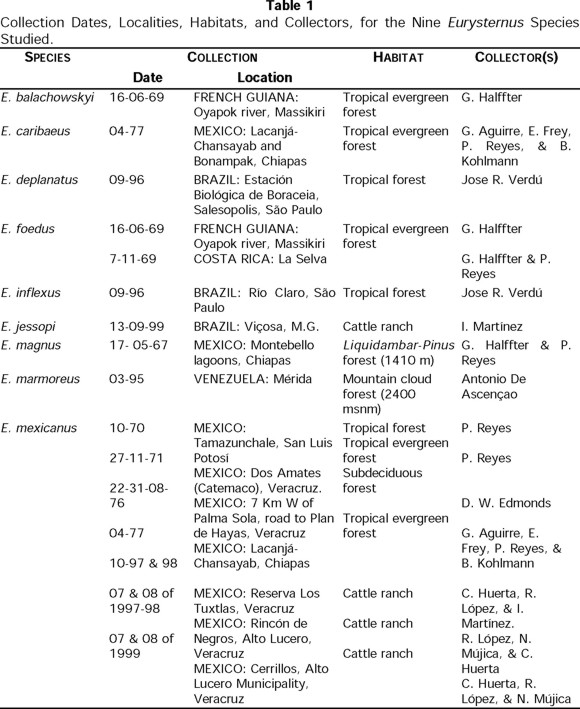
Table 1 shows the origin, collection dates, and collectors of the adults and larvae of various species.
b) Laboratory Breeding Studies
The same methodology was used to maintain and breed E. balachowskyi, E. caribaeus, E. magnus, E. foedus, and E. mexicanus studied between 1969 and 1977. Pairs were formed at random and distributed in terraria that held a soil layer 10 to 15 cm deep. Food was supplied once a week in the form of cow dung. Terraria maintained in Mexico City were in closed rooms, but without special arrangements for climate control (mean annual temperature of 20 / C; mean relative humidity of 40%; and a photoperiod of 13 to 14 hours in summer and 9 to 10 hours in winter). Observations were made every day, at different times. This methodology was also used for the 1986-87 studies of E. mexicanus.
Beginning in 1995, investigations were conducted in the laboratory of the Instituto de Ecología in Xalapa, Veracruz, México. In the case of E. marmoreus, both adults and young were maintained in an insectarium subject to ambient environmental conditions (mean annual temperature of 19 oC; mean relative humidity of 70%, and a photoperiod from 12 to 13 hours in summer and from 10 to 11 hours in winter). Pairs were formed at random and distributed in plastic terraria with dimensions of 20 x 15 x 7 cm, and with a soil layer 5 cm deep. They were fed once a week with cow manure. Observations were made daily, at 8-hour intervals such that one observation was made in the morning and one in the afternoon.
For observations of E. mexicanus, both adults and young were maintained in an insectarium with a constant temperature of 27 oC and constant 70% relatively humidity, and with a photoperiod of 14 hours in summer and 12 hours in winter. Pairs formed at random were maintained in plastic terraria under the same conditions as the terraria for E. marmoreus, and again, fed once a week with cow dung. Observations were made every day, such that one observation was made in the morning and one in the afternoon.
Adult E. jessopi were obtained from brood-balls representing eight nests. Once emerged, adults were paired at random and maintained in terraria under the same conditions used for E. marmoreus.
Ethograms for each species were developed on the basis of the daily observations. Means, standard deviations, and error estimations were determined for the duration, in days, of each behavioral period recorded for the adult life of each species, as well as for the durations of copulations observed.
A number of brood balls were selected at random from each type of nest, for both provisional (or experimental) and definitive nests, to be measured (length x width in mm) and weighed. To identify any significant differences between the two types of brood-balls, their volume was determined with the formula for a spheroid (V = 4/3 B ab2). The results were analysed using the Student t Test (Zar 1996). The same test was used to test for significant differences in the weights of brood-balls elaborated in each type of nest, and for differences in the time females of each species dedicated to each of the two types of nest. For E. jessopi, the Student t Test was also used to analyze differences between the final volume of the elaborated brood balls and the volume of pupal cocoons that third-stage larvae prepared in the same brood ball once the larvae had concluded feeding.
To analyze fecundity, 11 pairs of subsocial species ( E. marmoreus and E. mexicanus, for which the best data are available) were chosen at random, along with 11 pairs of the non-subsocial E. jessopi. The number of nests and brood balls in each nest were then counted, and counts were subjected to the Kruskal-Wallis test to determine significant differences between species (Zar 1976). These same species were chosen to study survival. 20 to 25 nests were randomly chosen, and survival percentages were measured for each developmental stage (egg; L1, L2 and L3, pupae; and adult), and analyzed using chi square tests to determine survival differences between species (Zar 1996).
Results
I. Feeding Period
Eurysternus are coprophagous beetles. Under natural conditions, they eat various kinds of excrement, adapting well to conditions in which cattle dung is found. Because dung is a resource highly fought over in the tropics, the feeding behavior of Eurysternus is surprising. They do not relocate food, neither by burying nor rolling. Instead, they eat directly from the surface of dung, or introduce themselves in it. This approach leaves them exposed to competition from faster and more aggressive species. An example of Eurysternus feeding behavior was observed by G. Halffter (pers. obs.) near the Oyapock River, French Guiana, where various species arrive at a dung pat on the forest floor in the late afternoon. The first beetles to arrive —just a few minutes after the dung was deposited— was Eurysternus, which flew directly to the dung. Other beetles then followed: rollers, which made a ball of food and then carried it away and diggers, which buried a portion of the food. Among the diggers was an enormous Coprophanaeus lancifer (Linneaeus), which, after pushing in and scattering the other beetles, left. At about two hours later, nothing was left of the excrement and all the beetles had left, with the exception of various Eurysternus, the first to arrive and the only beetles that did not separate a portion of the food; they fed from the surface and simply remained at the place where the excrement was.
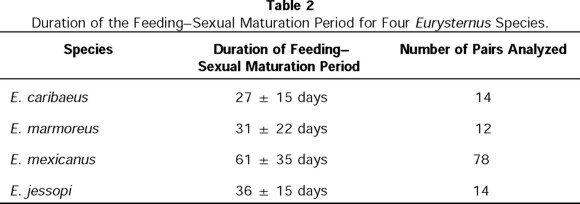
After emergence, all the Scarabaeinae studied passed through a stage of alimentation and sexual maturation (Halffter & Matthews 1966). In all Eurysternus species studied, adults fed directly from the excrement during the period in which both females and males develop to sexual maturity. Halffter (1977) and Halffter et al. (1980) reported that in all species studied to date, adults could feed directly from the dung for long periods of time, up to 200 days, without elaborating balls. This observation was confirmed in our recent studies of E. mexicanus, E. marmoreus, and E. jessopi. Table 2 shows mean duration of this stage for each species under laboratory conditions. Considering that environmental and feeding conditions were optimal, the maturation periods observed are quite long compared to other Scarabaeinae species.
II.- Sexual Encounter and Copulation
In Eurysternus, the pair is established at the beginning of nesting, either for a provisional or definitive nest (Table 3). In laboratory, mating was observed in four of the nine species studied: E. deplanatus, E. caribaeus, E. mexicanus, and E. marmoreus. The behavior of the last two was very similar to that described by Halffter et al. (1980) for E. caribaeus, although the context in which the behavior has been observed differed for all of them.
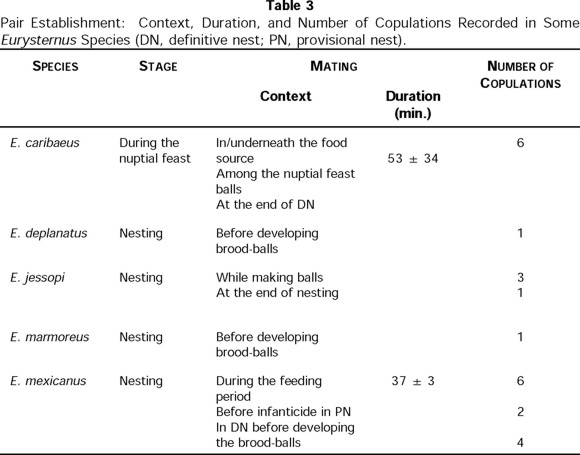
Eurysternus caribaeus was very frequently observed to form pairs after the nuptial feast had begun. Copulation was observed on various occasions alongside a mass of excrement, during the elaboration of balls that constitutes the feast, or at the end of the stage of caring for a definitive nest (Table 3). In E. marmoreus, copulation was observed in just one occasion: the adults encountered each other beside the food source, before the formation of balls. Copulation of E. mexicanus was seen in 12 occasions. Based on dissections of pairs of known age, copulation in at least one occasion, took place between 20 and 30 days after emergence and was not linked to elaboration of balls but rather to ovarian maturation (C. Huerta and I. Martínez, pers. obs.). This latter is the normal pattern in Scarabaeinae, in which first copulation stimulates or is indispensable for maturation of the oocytes (see López-Guerrero & Halffter 2000). The second copulation took place in the chamber where the brood-balls were to be lodged, before their elaboration. For those pairs that developed provisional nests, a third copulation occurred during the care of these nests. These cases were followed by infanticide or abandonment of the brood balls (Table 3).
No copulation of E. jessopi was observed, but we estimated from laboratory records that copulations should take place during the elaboration of the brood balls and at the end of any type of nesting period (Table 3). Copulation in E. deplanatus was observed by Verdú (pers. com.) before developing brood-balls (Table 3).
III.- Reproductive Stage
Halffter and Matthews (1966) provided the first reports on nesting behavior in Eurysternus, for an E. magnus Laporte nest encountered by Prof. H. F. Howden under natural conditions, in La Mesa del Chipinque, Nuevo León, Mexico. In September 1996, J. R. Verdú, (pers. com.) found E. deplanatus nests in Estación Biológica de Boraceia, Salesopolis, São Paulo, Brazil, under partially dehydrated excrement, and E. inflexus nests near Rio Claro, São Paulo, Brazil, under or at the border of partially dry dung pats. Julio N. C. Louzada (pers. com.) studied E. jessopi nests under natural conditions in Viçosa, M. G., Brazil, as did I. Martínez in the same location in 1999 (Fig. 1). Until now, no nests occurring under natural conditions have been found for the other five species examined in this paper; thus, the data on these species represent provisional or definitive nests seen under laboratory conditions.

To facilitate analysis of the reproductive stage of the nine species, we refer to the scheme encompassed under nesting pattern VI (Halffter & Edmonds 1982), which includes the following phases:
a) The nuptial feast, consisting of the elaboration of numerous balls, always by the female, at the beginning of the nesting process. These balls are not rolled, but pushed, and remain on the surface beside the food source.
b) The consumption or abandonment of these balls, some of which are used in nesting.
c) Elaboration of a compound nest of one or two different types, for most species.
d) Care of the young, principally by the female, but also with the participation of the male.
e) The formation of a stable pair that defends the nest while the nest is in formation.
f) Destruction of some or all of the brood balls (with eggs) after an initial period of care.
g) Various nesting cycles that can include provisional and definitive nests, with intermediate periods of feeding directly from the food source, periods during which balls are not elaborated.
E. foedus nesting does not fall under pattern VI. Nesting of E. deplanatus, E. inflexus, and E. jessopi differs from that of other species because no care is provided to the young. In the discussion below, we present a new general scheme of nesting in Eurysternus, based in part on the new information presented here.
a) Elaboration of the Balls.
Consistent with Halffter et al. (1980), our studies confirm that in all Eurysternus species the process of elaborating balls is associated with reproduction; however, in this as in other ways, E. foedus nesting is distinct. The elaboration of balls has always been associated with the process of nesting. The process is carried out only by the female, although on two occasions one E. jessopi female and one male were both observed to develop a brood-ball. Beyond the species studied by the above-mentioned authors, our observations confirm that when E. marmoreus and E. jessopi females (Huerta, pers. obs.), as well as E. mexicanus females (Huerta and López, pers. obs.) are isolated from the males they do not elaborate balls. However, when these females have copulated, they do elaborate balls and even nest, even when the male is absent (this has also been observed among other studied Scarabaeinae).
The way in which Eurysternus develops balls is quite distinct from species that roll the elaborated balls (Fig. 2a). As observed by Halffter et al. (1980), E. balachowskyi forms balls from both superior and inferior parts of the dung pat. The female crumbled the food into small bits, which she then compacted with both anterior and posterior legs. Anterior legs were used to incorporate and compact the dung, while the posterior legs rotated the ball to give it a spherical form. Completed balls formed from the surface of the heap were then pushed down and back such that the fully elaborated balls were pushed to the periphery. In E. magnus balls were separated from the bottom of the dung where it reached the ground, and was more moist (Fig. 2b). Ball fabrication was very similar to that observed in E. balachowskyi. E. caribaeus females began elaborating the balls in a different manner. The female placed herself underneath the excrement, with her back to the ground and both anterior and posterior legs grasping the excrement. The excrement was displaced, cut, and molded little by little into balls (Fig. 2c, d). The middle legs were also placed above and used as oars to move in the excrement. Once a ball was complete, the female continued with another, introducing it into the cavity left by the previous ball. Each ball took about 50 minutes to be elaborated.
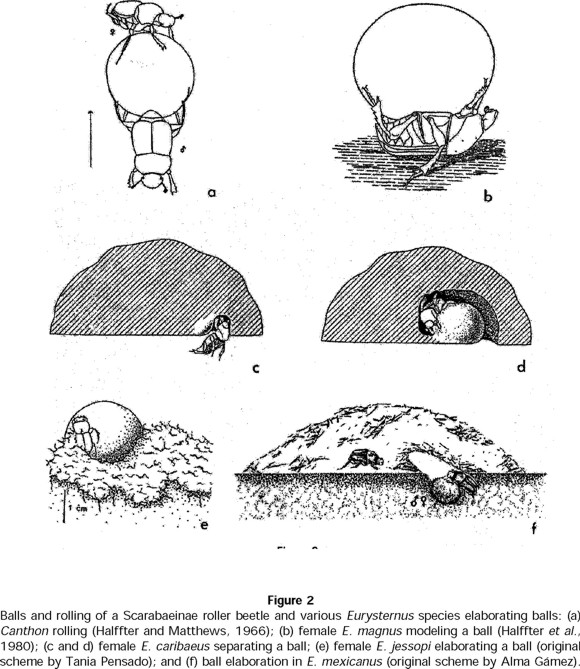
In their formation of balls, E. jessopi shredded the supply of dung, so that it carpeted virtually the entire surface of the terrarium. Using this shredded dung, the female, sometimes accompanied by the male, elaborated balls one at a time much like E. balachowskyi (C. Huerta, pers. obs.). Balls at first were small (13.4 ± 0.23 mm high x 13.06 ± 0.22 mm wide). After oviposition, each ball was further elaborated until it reached a size of 23.86 ± 0.40 mm in height x 23.96 ± 0.43 mm in width (n = 30 balls) (Fig 2e). As balls were finished, they were pushed away from the shredded dung, with all balls remaining on the surface. Mean time for elaboration of a ball was 48 hours (n = 10 balls, for those observed from the beginning until the end of the process). Underneath each ball, the female added a base of compacted soil, so that the balls remained perfectly erect and isolated from the soil surface. E. mexicanus shredded the excrement from underneath the dung and hauled it through a gallery approximately 2-3 cm in diameter by 3-5 cm in depth that the pair had elaborated in the ground just beneath the excrement (G. Halffter, S. Anduaga, C. Huerta, and R. López, pers. obs.). Once the shredded food had been moved, the female elaborated balls in a similar manner to E. balachowskyi (Fig. 2f). E. marmoreus used the same procedure (C. Huerta, pers. obs.). In both cases, balls were always found in a cavity or crater underneath or at the side of the food source.
b) Nuptial Feast
The nuptial feast does not occur in E. deplanatus, E. foedus, E. inflexus, E. jessopi, or E. marmoreus (Table 4). Consistent with Halffter (1977) and Halffter et al. (1980), our observations indicate that E. caribaeus, E. magnus, and E. balachowskyi conduct the feast at the end of the feeding period. In these three species, the feast has a sudden onset, with the female elaborating numerous balls beside the food source (Fig. 3). Both duration of ball elaboration and number of balls elaborated by these species varied. The number of feasts at the previous nesting stage also differed (Table 4). Most of the balls elaborated during this stage did not contain eggs or larvae and were later consumed or abandoned. Among E. caribaeus, only three cases were seen in which one of the balls contained eggs, but in none of these cases the female cared for them, and they did not complete larval development.
Regarding E. mexicanus, Halffter (1977) noted that a great nuptial feast occurs with the formation of about 14 balls which are stored in a superficial crater prepared underneath or at the side of the dung pile. Similarly, in eight of the terraria maintained for this species during 1986-1987 and in the nearly 100 terraria observed between 1995 and 2000, pairs prepared a large number of balls, with and without eggs, which, unlike those of E. balachowskyi, E. caribaeus, and E. magnus, were always found in a clearly delimited crater underneath or beside a dung pat. The balls were always cared for by the female. For these reasons, we believe that what Halffter (1977) described as feasts in E. mexicanus would be better considered as provisional nests. Up to three of these provisional nests may be prepared before preparation of a definitive nest.
In all species that conducted nuptial feasts, the feasts always took place after a period of direct feeding from the food source and preceded provisional or definitive nesting.
c) Provisional (or Experimental) Nesting
Provisional nests refer to nesting in which the female elaborates a compound nest and in which most of the balls contain eggs, but the eggs generally do not reach the hatching stage. The female cares for the balls during a period that depends on the species, after which the balls (including the eggs or larvae found within them), are eaten by the female or male parent, or by both or simply abandoned.
Eurysternus deplanatus, E. foedus, E. inflexus, E. jessopi, and E. magnus were not observed to develop provisional nests. In E. caribaeus pairs, 65% prepared experimental nests, but the remaining 35% did not. In E. marmoreus, 60% of the pairs observed produced provisional nests, while the other 40% did not (n = 15 pairs observed). In E. mexicanus, of 89 pairs, 40% elaborated provisional nests; the remaining 60% developed only definitive nests. Because of the variability in the number and sequence (over the reproductive period) of provisional nests, and because during provisional nesting the male, female, or both may commit infanticide, or the nests may be simply abandoned (Table 5), we analyze these phenomena separately for each species.
- E. balachowskyi
Consistent with Halffter et al. (1980), a review of the laboratory notebooks indicates that E. balachowskyi begins provisional nesting about 60 days after the nuptial feast. Provisional nesting consists of elaboration of a variable number of balls (6 ± 1; n = 14 nests), around which the female excavates a cavity. The female oviposits in some of these balls, and the pair cares for the balls for some days (Table 5). The balls are then attacked and partially eaten, and the nest is abandoned. The females construct up to three successive provisional nests, repeating the entire process just described (Table 5). In these cases, it was not determined which member(s) of the pair carried out infanticide, but it could be determined that the consumed balls contained eggs and first-instar (L1) larvae.
- E. caribaeus
About 30 to 50 days after the nuptial feast, E. caribaeus began elaborating experimental nests (Fig. 4). The process consisted of excavating a cavity underneath or at the side of the food source, a crater in which a variable number of balls were introduced (7 ± 1; n = 12 nests). The duration of the construction phase, the duration of care provided, and the number of provisional nests constructed all varied (Table 5). In all cases, the brood-balls were destroyed and/or abandoned. Destruction and infanticide were carefully carried out for eight of the nine provisional nests, with the female destroying the brood nest in four cases, the male in one case, and the responsible part unknown in the other cases. In one case, the nest was simply abandoned without being destroyed. Some of the attacked balls were empty and the others contained eggs. In no cases were destroyed balls observed to contain first-instar (L1) or further developed larvae.
- E. marmoreus
For E. marmoreus pairs that developed provisional nests, nests were constructed after a feeding period in various sequences over the reproductive period. In 23% of the reproductive periods observed, at least one experimental nest was constructed prior to one or more definitive nests, after which another feeding period and the elaboration of another provisional nest followed (sequence A1, Fig. 5). For most pairs (44%), a single provisional nest was prepared before the preparation of various definitive nests (sequence A2, Fig. 5). In sequence A3 (Fig. 5), seen in 22% of the pairs, various provisional nests preceded the formation of a single definitive nest. Sequence A4 (Fig. 5), in which a single definitive nest was followed by various provisional nests, was seen in just 11% of the pairs.
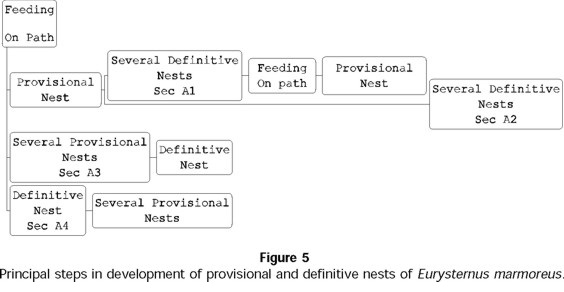
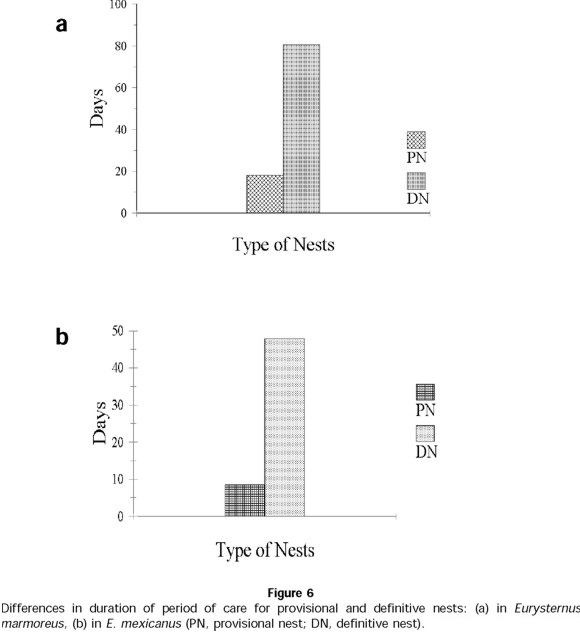
The number of provisional nests elaborated by pairs was 1 ± 1 (n = 15 pairs). Nests initially contained 7 ± 2 balls (n = 15 nests) and a final number of 2 ± 2 balls (n = 15 nests). Duration of care for provisional nests was 21 ± 17 days (n = 15 nests), significantly less time than those dedicated to the care of definitive nests ( t = 12.47; t0.001(2), 40 = 3.551; P<0.001) (Fig. 6a).
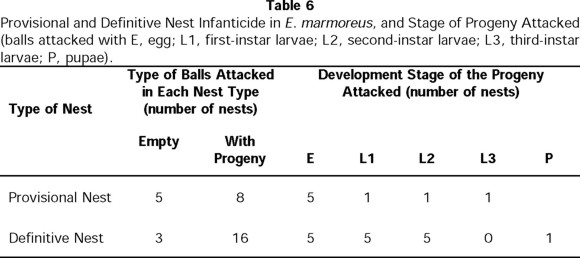
With regard to infanticide (Table 6), in seven cases the female was observed consuming balls that contained eggs and L1 larvae. In five nests, the female consumed balls that contained no eggs or larvae. Three nests were simply abandoned.
- E. mexicanus
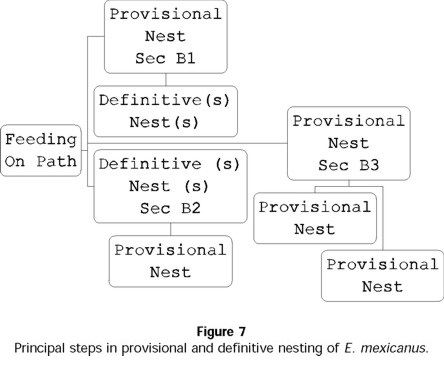
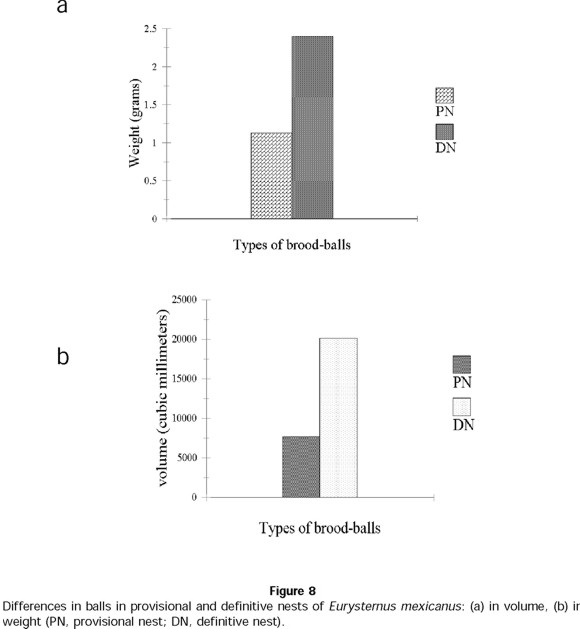
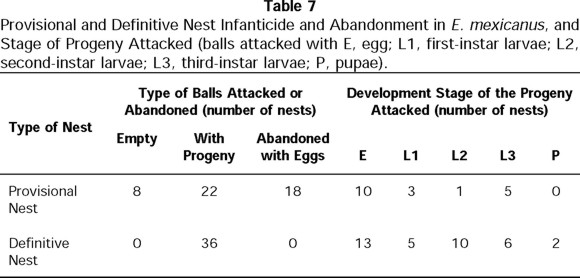
In E. mexicanus, provisional nesting begins immediately after the period of feeding and development to sexual maturity. Of 89 E. mexicanus pairs, only 36 made provisional nests. Just as in E. marmoreus, the development of provisional nests follows different sequences over the reproductive period: for 27% of pairs, a provisional nest always preceded a definitive nest(s) (sequence B1, Fig. 7). For 6% of pairs, a definitive nest was followed with a provisional nest (sequence B2, Fig. 7); 7% of pairs only developed provisional nests and never developed definitive nests (sequence B3, Fig. 7). The number of provisional nests developed per pair was 1 ± 1 (n = 89 pairs in total), containing an initial number of 5 ± 2 balls (n = 76 provisional nests) and a final number of 2 ± 2 balls (n = 76 provisional nests). The duration of care given to this type of nest was 8 ± 7 days (n = 49 nests), significantly less than the care given to definitive nests ( t = 22.59; t0.001(2),110 = 3.381; P<0.001) (Fig. 6b). Two significant differences between the provisional and definitive nests were that the former contained smaller elaborated balls ( t = 12.08; t0.001(2),126 = 3.370) (Fig. 8a) and lighter balls ( t = 14; t0.001(2),126 = 3.370) (Fig. 8b).
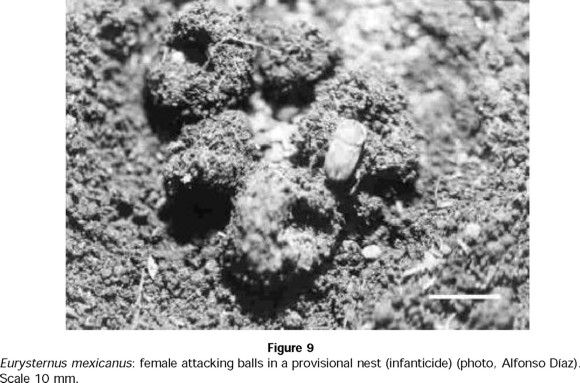
Regarding infanticide, in 8 out of 45 nests, the female was observed attacking brood balls that contained eggs or L1 or L3 larvae (Fig. 9). In other eight nests, the male attacked balls that contained eggs or L1 or L3 larvae. For other six nests, the balls attacked showed no sign of oviposition and neither member of the pair was actively involved in the destruction at the time of observation. The attacker was unknown for other five nests, but in these cases the balls contained eggs or L1 or L3 larvae. In 18 nests, balls were abandoned with no sign of having been attacked (Table 7).
d) Definitive Nesting
In general, definitive nesting is characterized by the female elaboration of a compound nest in which all balls contain eggs. In most Eurysternus species, females care for the balls during the entire period of larval development. The duration varying with the species. During this period, some of the balls that make up the nest (including the eggs or larvae found within the balls) may be eaten, although this does not generally occur. In subsocial species, the female abandons the nest when the young emerge. Because definitive nesting differs among Eurysternus species (differences in form, number of nests developed per female, nest sequence over the entire reproductive period, care of young, and infanticide by the male, female, or both) we analyze definitive nesting of each species separately, just as we did for provisional nesting.
SUBSOCIAL SPECIES
- E. balachowskyi
As Halffter et al. (1980) observed, the definitive and provisional nests of E. balachowskyi differ in the number, form and structure of the balls. From the last balls elaborated during the last provisional nest, two balls in which the female has oviposited, are selected for the definitive nest. These balls measured about 27 mm in diameter and were deposited on the surface in contact with one another (Fig. 10a) and then covered with a layer of soil. Thereafter, the female dug a groove around them (Fig. 10b).
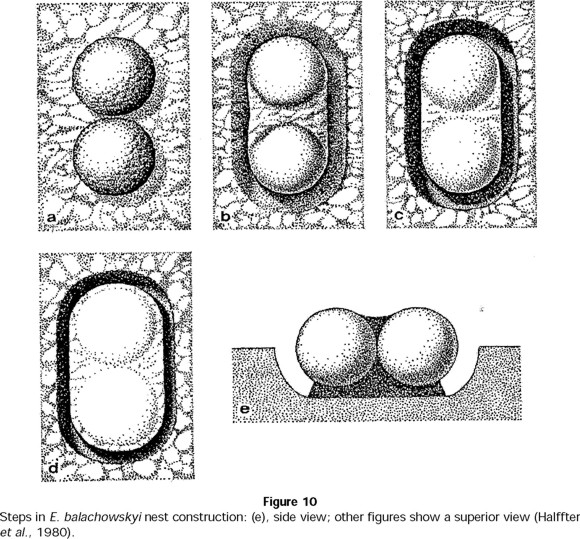
The balls were joined together by compacted soil and separated from the cavity floor by a soil support, also elaborated by the female (Fig. 10c, d and e). The female cared for these brood balls, maintaining the structure of the nest intact. Development from egg to imago had a mean duration of 50 days, and the female abandoned the nest without attacking the balls 10 days before the young emerged (Table 8).
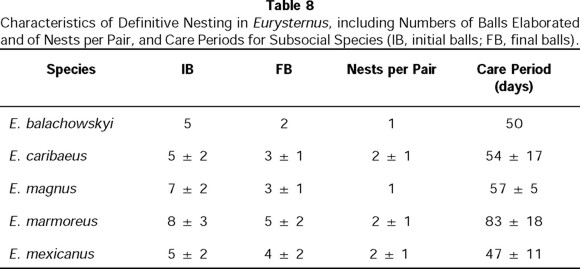
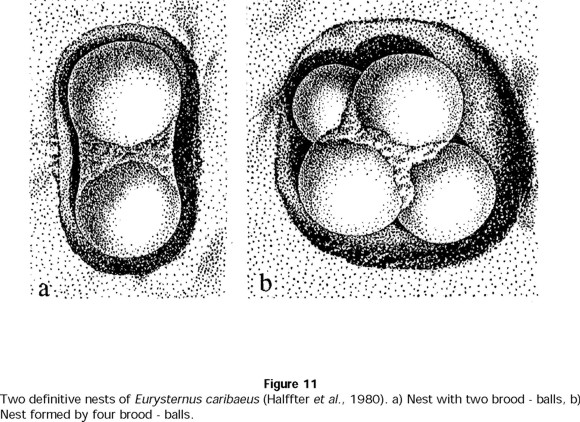
- E. caribaeus
As with E. balachowskyi, provisional and definitive nests of E. caribaeus differed in form and structure. The female cared for the nest without attacking any balls for 54 ± 17 days (n = 13 nests), and she abandoned the nest when the brood balls contained pupae or teneral adults (Table 8). To prepare a definitive nest, the female used some of the balls developed during the nuptial feast, selecting and remodeling the balls after oviposition. Later, she excavated a cavity around the balls, covered them with a soil layer, and joined them with soil mixed with her own excrement (Fig. 11). Balls found in the definitive nests were 5 ± 2 (n = 13 nests) and measured 24.4 ± 3 mm in diameter (n = 20).
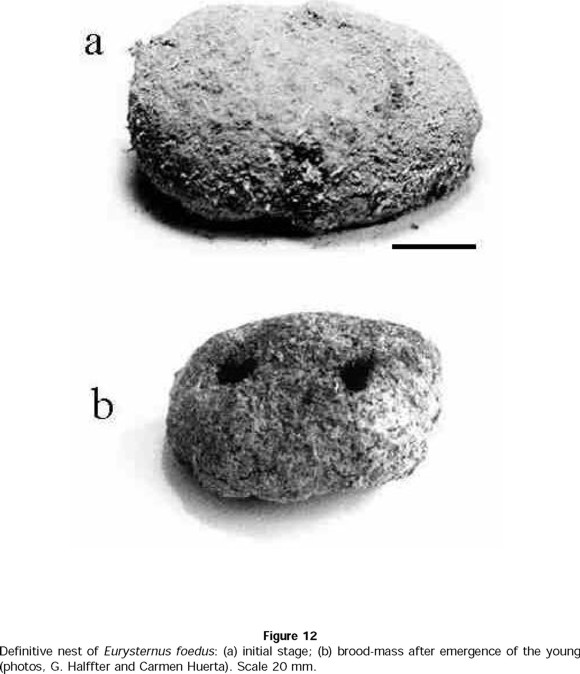
- E. foedus
As Halffter et al. (1980) observed, E. foedus does not form brood-balls. The adults dig a cavity underneath or at the side of the food source. Into this cavity, they introduce dung, which they compact, into a sort of cake approximately 80 ± 10 mm in length, 60 ± 10 mm in width, and 65 ± 8 mm in height (n = 3 nests) (Fig. 12a). The female oviposits two or three eggs in this mass, which is thereafter covered by soil. The larvae develop in the mass without disturbing each other, and the female cares for them until the young emerge (Fig. 12b). Regarding infanticide, the original laboratory notebooks do not indicate any aggression toward the nest by the adults. This nest is very similar to that developed by Cyptochirus distinctus (Janssens) (see Cambefort, 1982), a beetle that belongs to a different taxonomic group (Oniticellini: Drepanocerina) within the Scarabaeinae. This is the only other nest of this type that we know of among the Scarabaeinae; these beetles also sharing subsocial care of the progeny and a similarity in facies.
- E. magnus
In E. magnus, as Halffter (1977) and Halffter et al. (1980) observed, definitive nesting takes place immediately following the nuptial feast. Just as for E. balachowskyi and E. caribaeus, some of the balls elaborated during the feast are used by the female to form the nest. Around them, the female digs a cavity with a mean diameter of 5 cm. After ovipositing in some of these balls, the female remodels and augments them. Two days after the nests were initiated, the female began to add a soil cover to each ball containing egg. Initially, nests contained 7 ± 2 brood-balls (n = 4 nests), but at the end of the process it contained only 3 ± 1 (n = 4 nests) (Fig. 13). Balls with no eggs were used as food or as additional material to add to the balls that did contain eggs. The nest was cared for during the entire development period, which lasted 57 ± 5 days (n = 4 nests) (Table 8). No infanticide was observed in any of these nests.

- E. marmoreus
In 40% of the pairs (n = 15 pairs observed), the definitive nest was elaborated at the end of the feeding-maturation period. In the remaining 60% of cases, the definitive nest was prepared after the development of one or more provisional nests. To develop the definitive nest, the female or the pair shredded some of the excrement at the base of the dung pile found in contact with the soil, and dug a tunnel underneath or at the side of the food source. The tunnel's dimensions were 2 to 3 cm in diameter by 3 to 5 cm in depth. The female or the pair transported the shredded food through the tunnel, where the female developed the brood-balls and a cavity around them (Fig. 14). The initial number of balls was 8 ± 3 (n = 26 nests), while the final number of balls was 5 ± 2 (n = 26). Nests were cared for over a period of 83 ± 18 days (n = 26 nests) (Table 8). During this stage, when the brood-balls contained more developed second-instar larvae or less developed third-instar larvae, the female added a soil cover to each of the brood-balls, with the brood-balls remaining apart from each other. The number of definitive nests elaborated per pair was 2 ± 1 (n = 15 pairs).

As in E. mexicanus, infanticide was seen in some definitive nests of E. marmoreus, but the adults did not attack all the balls that made up the nest, only some. Of the 26 definitive nests that were observed, infanticide was observed in 16, always by the female. Table 6 shows the number of definitive nests in which some balls were consumed that contained eggs, L1, L2 or pupa stage. In the remaining nests, all balls were cared for until the new generation emerged.
- E. mexicanus
E. mexicanus was observed to elaborate nests in a manner much like E. marmoreus. The only difference was that, in E. mexicanus, the female or the pair compacted the excrement once it had been separated into a type of cake. Balls were made using material that was found in the middle of this cake, so that after oviposition the wall of the chamber that contained the brood- balls was completely covered with the remaining excrement. This excrement was eaten by the adults during the first stages of care-providing. The number of balls containing eggs initially was 5 ± 2; the final number of balls with young adults in their interiors was 4 ± 2 (138 nests). The brood-balls were covered one by one with a layer of soil when they contained advanced L2 or L3 larvae (Fig. 15).

The female cared for all or some of the balls in the nest during the development of the new generation, a process that lasted 47 ± 11 days (n = 138 nests). Of the 89 pairs observed, 60% made only definitive nests, while the others developed provisional as well as definitive nests. A total of 138 definitive nests were observed, of which 55 were developed at the end of the feeding-maturation period. The rest were made days after destroying or abandoning provisional nests or after having concluded another definitive nesting period. The number of definitive nests prepared per pair was 2 ± 1 (n = 89 pairs).
Eurysternus mexicanus also showed infanticide during definitive nesting. In 65% of the nests observed, no infanticide was observed and the females cared for all of the balls that the nests contained. In the remaining 35%, infanticide was observed, principally by females, but also by males, mainly on balls that contained eggs or L2 larvae (Table 7). In some nests, infanticide was committed in balls that contained eggs, but it was not possible to determine whether the adult female or adult male was the responsible. Infanticide was committed only for some balls in definitive nests, but in all cases the female continued caring for the other balls that had not been attacked.
NON-SUBSOCIAL SPECIES
- E. deplanatus
According to J. R. Verdú (pers. com.), after E. deplanatus has copulated, females elaborate brood-balls that they put in compound nests underneath partially dehydrated dung deposits. Nests contain a maximum of five brood-balls. It is still unclear whether this species provides post-oviposition care or not. The nest is very similar to that seen in subsocial species. J. R. Verdú noted that E. deplanatus brood-balls are often encountered abandoned under partially dehydrated dung. In an insect as cryptic as Eurysternus, in which soil and dung stick to ocellar spots and setae of the back, it is not uncommon to be unable to find the female (on various occasions, we had difficulty finding the female in our terraria). Moreover, in the nests of many subsocial species, the females fail to provide care. For these reasons, it is best to leave questions of female care in this species as issues that remain to be resolved.
- E. inflexus
José Verdú observed eight nests of E. inflexus, a species closely related to E. deplanatus according to Jessop's (1985) taxonomy. Verdú found that the female constructed three or four isolated brood-balls with cow or horse dung, constructions that were left abandoned underneath or at the border of semi-dry dung, without excavation or lodging. Larval development was very rapid. Larvae took two weeks to reach the L3 stage, and only seven days later, sclerotized adults were observed.
Despite that this species, like E. deplanatus, is now considered non-subsocial, we believe the more prudent judgment is to consider the issue of post-oviposition care to be unresolved. This and the previous species are the only ones included in this study that we did not raise several times under laboratory conditions.
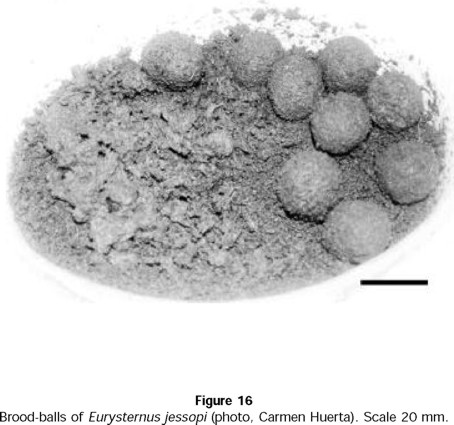
- E. jessopi
N. C. Louzada (pers. com.) observed that E. jessopi nests are found on the surface during the dry, cold period of the year, always at the side of old cow excrement or fresh horse excrement. These nests contain 5 ± 3 balls and are abandoned by the females once the balls have been constructed.
In the laboratory, reproduction begins once the feeding-maturation period is complete (Fig. 16). A total of 14 E. jessopi pairs produced 4 ± 1 nests per pair. The number of balls in each nest was 7 ± 3 (n = 49 nests). Females were observed elaborating brood-balls, but were never observed to care for the balls.
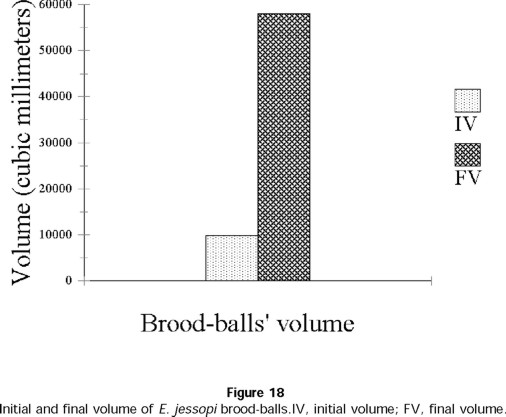
These balls are extremely large for the size of the species (Fig. 17). Larvae develop in the medial-inferior part of the balls and feed only on the dung from the center of the ball. A little before pupation, L3 larvae fashion a cocoon whose walls are very resistant. The cocoon remains encircled with dry excrement, which forms the exterior wall of the ball. A significant difference was seen between the total volume of the brood-balls and the volume of the cocoon in which pupation occurred ( t = 16.73; t0.001(2),58 = 3.237) (Fig. 18).
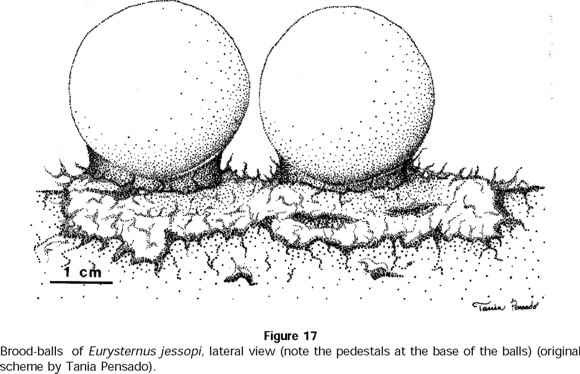
In only three of the terraria containing E. jessopi were the females observed to consume the eggs that had been deposited in balls, regardless of the fact that sufficient food was available. We believe this behavior may have to do with the laboratory conditions—the terraria were saturated with the elaborated balls, and the female may no longer have had any space to fabricate new ones. Under field conditions, females may abandon brood-balls to colonize a different dung pat and elaborate new balls. In any event, an interesting fact is that in all but three out of the 34 terraria, E. jessopi females did not attack the brood-balls.
e) Fertility and Survival
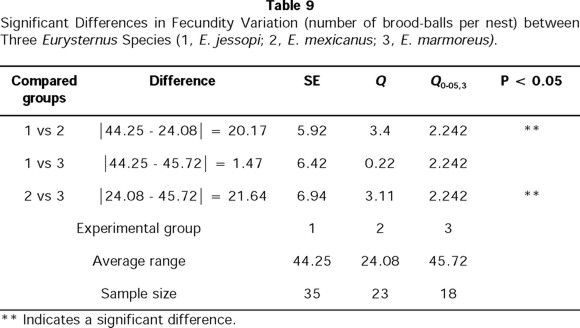
We have more extensive data on fertility for three species: E. mexicanus, E. marmoreus, and E. jessopi. These data shown a significant difference among the species in the number of brood balls found in each nest (X 2= 14.05; X20.01,2,= 9.210, P >0.01). Of the three species, E. mexicanus elaborates the least number of brood-balls per nest (Table 9). No significant differences in the number of brood-balls per nest were found between E. marmoreus and E. jessopi.
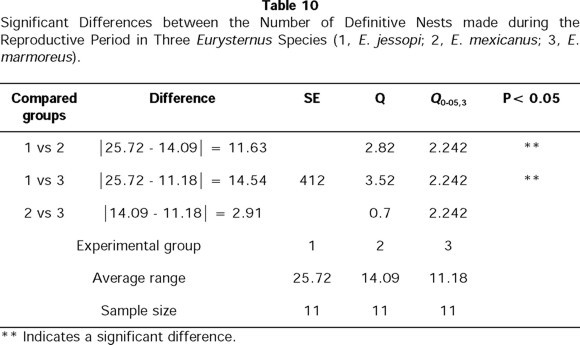
We also found a clear difference in number of nests elaborated by the three species, (X2= 19.93; X20.01,2 = 9.210, P >0.01). The non-subsocial species, E. jessopi, is the one that develops the greatest number of nests, while the two subsocial species develop a similar number of nests (Table 10).
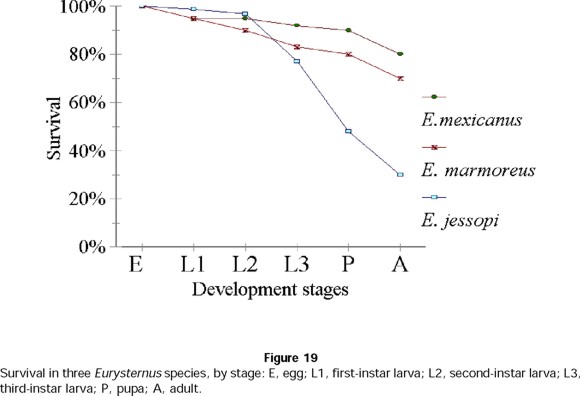
Differences were also seen in survival (X2= 8.70; X 20.05,2 = 5.991, P >0.05). Non-subsocial species showed the lowest survival, particularly in the passage from L3 larva to pupa (P), and from pupa to adult (A) (Fig. 19). The subsocial species showed no difference in survival.
Discussion
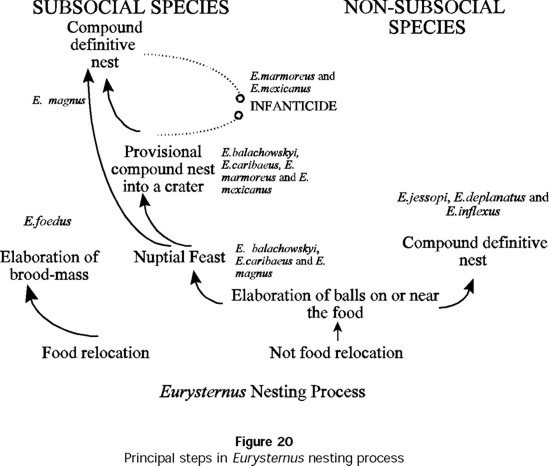
The diversity of nesting behavior in Eurysternus is even greater than previously recognized (Fig. 20). E. foedus in particular is quite distinct, failing to fall under nesting pattern VI. We have already pointed out the strong resemblance to nesting in Cyptochirus distinctus. We have studied the nesting behavior of nine species in a genus of at least 30 species (Jessop 1985, Martínez 1988, Gill 1990). Beyond the unusual nesting of E. foedus, how many other nesting patterns are seen in this genus that is already known to be heterogeneous for this behavior?
The presence or absence of post-oviposition care is not the only major behavioral difference seen in Eurysternus. Although balls are always made by the female, there are at least two methods of cutting and molding them, as E. balachowskyi and E. caribaeus illustrate.
Having found species that fail to care for the nest is not surprising. In Canthon, Malagoniella, Scarabaeus and Kheper, beetles that roll their balls, there are both subsocial species and non-subsocial species, with the latter failing to provide post-nesting care (Halffter 1997).
Post-oviposition care provided by subsocial species is one of the two major protective behaviors for progeny. The other is to augment the external cover with earth or dry excrement (Halffter 1997), as seen in E. jessopi. In this species, the thick layer of dry excrement that surrounds the more humid excrement wherein the larvae develop protects them from dehydration and from possible Diptera attack, though not from fungi (C. Huerta, pers. obs.).
Subsocial care includes protection of brood-balls, and in some species ( E. balachowskyi, E. caribaeus, and E. magnus ) of the entire nest. The female can make a compact layer of soil that may unite the different balls or separate and isolate them from the ground. Coverage of the brood-balls with a layer of soil or other special disposition of soil is a characteristic of definitive nests, although in some species (e.g., E. balachowskyi ) it can be seen in incipient form in provisional nests. The layer of soil protects larvae and pupae. Recall that in E. jessopi, which shows no maternal care, it is the larvae that make a strong cocoon before pupating. Moreover, in a very marked way in E. balachowskyi, but also in other species, the specially disposed earth and the excavation of a groove around the brood balls isolate them from the contiguous ground. In E. jessopi, the female also prepares a base of soil for each brood-ball before leaving it, which again isolates the ball from the ground and the humidity the ground may impart, but assures that humidity is adequate in the area where the larvae are feeding. Alternatively, when subsocial care is provided, the female keeps the external surface of the brood-balls and the surrounding space free of fungi.
Beyond the characteristics noted above that protect the brood-balls and nests of the various species, there are clearly inter-specific differences between subsocial and non-subsocial species in the fecundity and survival of the progeny. In E. jessopi (a non-subsocial species), high mortality of the young is offset by high fertility of the females (who produce a higher number of nests with a high number of brood-balls in each nest). In subsocial species, lower fertility of females is balanced by higher survival of young during the juvenile stages.
Despite the new knowledge provided by this work, there are questions remaining as: (1) Why is there heterogeneity of behavior in a genus that is morphological similar and in which the species studied feed on the same material? (2) How do we reconcile the economy and speed of nesting behaviors characteristic of the great majority of Scarabaeinae—behaviors that have the goal of separating and protecting a part of the food source—with the Eurysternus species' squandering of the food, as if there were no competitors?
How is it possible for Eurysternus to survive and be abundant in spite of the wasting of resources? First, we note that Eurysternus does not relocate food, neither as an adult nor in the process of nesting. Relocation is a nearly universal characteristic in Scarabaeinae (with a few notable exceptions). Different forms of relocation permit the coexistence of various, sometimes numerous, species in one pile of dung. Eurysternus operates like beetles of the subfamily Aphodiinae, which similarly eat and reproduce in the pile. But Aphodiinae are abundant in temperate regions, where competition is not so intense or rapid as in the tropics, where Eurysternus lives.
Why are the Eurysternus balls elaborated during the nuptial feast and brood-balls not stolen by rolling beetles whose balls are very similar? (Such inter-or intraspecific robbery is very common in Scarabaeinae rolling beetles). Two hypotheses seem plausible to explain this exception for Eurysternus , hypotheses that can each be tested with additional research. The first hypothesis is that Eurysternus is able to impregnate the balls with some substance unattractive to other beetles. Works on impregnation of balls (Bellés & Favila 1983, Favila 1992, Burger et al. 1983, Halffter 1997), suggests that possibility requires experimental test.
The second hypothesis of survival despite resource wasting and lack of relocation is that Eurysternus nesting takes place when fewer species are active or in excrement that is too dry to be attractive to most other Scarabaeinae. Our findings on nests in the field (for E. deplanatus, E. jessopi, and E. inflexus ) suggest the use of dryer excrement that is not attractive to most other Scarabaeinae species. Also Oniticellus and Tragiscus form compound nests in the interior of the dung mass and thus do not relocate (Davis 1977, Halffter & Edmonds 1982). The long pre-nesting feeding period seen in Eurysternus could be interpreted as part of a nesting strategy to ensure that environmental conditions will result in the least competition. Curiously, the Eurysternus ovary matures quite rapidly and does not justify the long time lapse seen prior to nesting (see Halffter et al. 1980). To test the delayed-nesting hypothesis it would be ideal to follow nesting under field conditions.
Among Scarabaeinae, Eurysternus is the only to commit infanticide. There have been some observations of Heliocopris attacks on its own brood-balls, which it has cared for until that point (Halffter & Edmonds 1982:107). However, these instances are associated with lack of food resources for the caring female, a factor that is never seen in cases of Eurysternus infanticide (Halffter et al. 1980).
In all four species we have observed with provisional nesting, in some moment the brood-balls (including progeny) may be attacked during nesting. In one species, the mother has been observed commiting infanticide; in the other three species, both sexes. Quite possibly in all four species both sexes may be actors in infanticide.
For all six species that present definitive nests, infanticide has been observed in three: E. marmoreus, E. mexicanus, and E. jessopi (we could not obtain information for E. deplanatus and E. inflexus nests in the field, and as we noted, E. foedus follows a different nesting pattern). Such infanticide was commited by the female, which in both subsocial species was caring for the nest and in the case of E. mexicanus also by males. The attack consisted of partial destruction of the balls that the nests contained. In E. jessopi, the infanticide might have resulted from crowding of brood- balls in the terraria, although it was observed only in three terraria.
It is straight forward to conclude that infanticide is much more frequent in provisional nesting. Infanticide appears to be one of the characteristics of provisional nesting.
What hypotheses have been put forth in the literature for infanticide in Eurysternus ? According to Halffter et al. (1980), the mother's destruction and abandonment of brood-balls in experimental nests is due to continuation of oogenesis. The mechanisms seen in other subsocial Scarabaeinae that inhibit ovarian development at the outset of nest care do not function in Eurysternus. Instead, oocytes continue to develop in the female, with oviposition of new brood-balls and simultaneous attack and abandonment of those brood-balls previously cared for. The process of vitellogenesis and corresponding destruction cease (for example, in E. balachowskyi ) at the outset of definitive nesting.
This hypothesis of Halffter et al. (1980) is supported by histological studies of the E. caribaeus ovary. The development of the oocytes is inhibited during provision of care. At the end of this period a new series of oocytes matures, such that a physiological state is reached like that seen during the middle of the nuptial feast. New copulation occurs, and the nesting process begins again. The lack of synchronicity between ovarian maturity and care of the provisional nests is still more marked in E. balachowskyi. According to Halffter et al. (1980), for a period of 60 days after the nuptial feast the female oviposits in numerous balls and constructs three successive nests. There the balls are cared for a few days before being attacked, partially consumed, and abandoned. If there is still dung immediately available, the female continues making balls during the provisional nesting period. From 30 to 50 additional balls are made after the nuptial feast. This prolonged process of making balls and oviposition is associated, just like infanticide, with lack of interruption in vitellogenesis.
One hypothesis to explore is that the very existence of provisional nests, while perhaps ecologically "wasteful," may be associated with continued vitellogenesis. Vitellogenesis of this type is seen in Scarabaeinae showing more primitive nesting, without subsocial care. If this hypothesis is correct, the maturation of the oocytes would be slower and would pause during nesting in E. magnus, and would be faster and without pause in E. balachowskyi and E. mexicanus. In the last species, it would even continue during definitive nesting.
To explain male infanticide seen in provisional nesting, Halffter (1997) has proposed the following. The male is attracted by the female during the massive formation of balls, which coincides with the maturation of oocytes, while the female quite possibly has spermatozoids in the spermatheca from previous copulations with other males. The late-arriving male's destruction of brood-balls in the provisional nests, accompanied by copulation and formation of new brood-balls, would ensure that the surviving eggs would be fertilized by his own spermatozoids. The structure and function of the spermatheca (see López-Guerrero & Halffter 2000) favors this supposition, given that the most recent copulations will more likely produce the fertilizing spermatozoids. From the viewpoint of male fitness, representation of the male in the definitive nesting phase coincides with the exhaustion of spermatozoids from the pre-nesting copulations. This hypothesis has not been followed up experimentally. For example, if the male nests with a virgin female, will he be able to detect this and decrease his infanticide?
Scarabaeinae show behaviors to attract the other sex at the outset of nesting. To various degrees, nesting in most cases consists of a cooperative process. Thus, in digging beetles, the female begins excavating the gallery that will be the nesting site, an activity that attracts the male. Once the pair is established, the male helps in provisioning and defending the nest. In rolling beetles, it is the male that takes the initiative, elaborating and offering a ball, attracting a female with pheromones, and playing a major role in the cooperative rolling of balls. In Eurysternus, it is the female that attracts the male by fabricating balls during the nuptial feast or initiating nesting. In E. balachowskyi, the male remains in the nest and participates in the care of the provisional nests (Halffter et al. 1980). In E. magnus (Halffter 1977), the male was observed to occasionally participate in touching up the balls in the definitive nest. In E. caribaeus (Halffter et al. 1980), the male was present only during the nuptial feast.
In E. jessopi, on three occasions the male was observed cooperating with the female at the end of elaborating the brood-balls. In E. marmoreus and E. mexicanus, the male cooperated with the female during excavation of the cavity where the brood-balls were prepared and finally remained. In other Scarabaeinae, cooperation has clear advantages with regard to relocation, but it does not have an evident explanation in Eurysternus. What are the advantages to the female in attracting a male when quite possibly there are spermatozoids in the spermatheca from an earlier copulation? This is yet one more question that requires research to understand Eurysternus nesting behavior.
Acknowledgments
A number of colleagues have participated in the capture and care of Eurysternus: Pedro Reyes-Castillo, in capturing E. foedus and in following this species and E. balachowskyi in terraria; Sofía Anduaga, in following E. mexicanus in terraria; Imelda Martínez, in dessecting females and males and in collecting E. mexicanus as well as the nests of E. jessopi; and Yrma López-Guerrero, in dissecting E. caribaeus. To all, our deep gratitude. We also thank Magdalena Cruz, Alma Gámez, and Tania Pensado, along with the Instituto de Ecología A.C., for their help in diagramming brood-balls. We greatly appreciate José R. Verdú of the Universidad de Alicante, Spain, for his unpublished data on the nesting of E. deplanatus and E. inflexus, and Julio N. C. Louzada of the Universidad de Lavras, M. G., Brazil, for his original observations on nesting of E. jessopi under natural conditions. Thanks also to Antonio De Ascençao of the Universidad de Los Andes, Mérida, Venezuela, for his collection of E. marmoreus; and to Antonio González, director of the Reserva de la Biosfera Los Tuxtlas, Veracruz, Mexico, for his aid in collecting E. mexicanus. Finally, we give many thanks to Doña Virginia and Don Santos Tepoz, owners of the property in Los Tuxtlas, and Don Cándido López, owner of the property in Cerrillos, Alto Lucero, Veracruz, Mexico, for their aid in our collection of E. mexicanus on their cattle ranches, to Ann Covalt, for her translation of the manuscript into English and two anonymous reviewers whose comments are appreciated.
Literature cited
Bellés, X. & M. E. Favila. 1983. Protection chimique du nid chez Canthon cyanellus cyanellus LeConte (Coleoptera, Scarabaeidae). Bull. Soc. Entomol. Fr. 88: 602-607. [ Links ]
Burger, B.V., Z. Munro, M. Röth, H. S. C. Spies, V. Truter, G. D. Tribe & R. M. Crewe. 1983. Composition of heterogeneous sex attracting secretion of the dung beetle Kheper lamarcki. Z. Naturforsch. 38: 848-855. [ Links ]
Cambefort, Y. 1982. Nidification behavior of Old World Oniticellini (Coleoptera: Scarabaeinae). Pp. 141-145. In: G. Halffter & W. D. Edmonds. The Nesting Behavior of Dung Beetles (Scarabaeinae). An Ecological and Evolutive Approach. Instituto de Ecología, México, D.F. [ Links ]
Cambefort, Y. & J. P. Lumaret. 1983. Nidification et larves des Oniticellini afro-tropicaux. Bull. Soc. Entomol. Fr. 88: 542-569. [ Links ]
Davis, A. L. V. 1977. The endocoprid dung beetles of Southern Africa (Coleoptera: Scarabaeidae). M. Sc. Thesis. Rhodes University, Grahamstown, South Africa. [ Links ]
----------. 1989. Nesting of afrotropical Oniticellus (Coleoptera: Scarabaeidae) and its evolutionary trend from soil to dung. Ecol. Entomol. 14: 11-21. [ Links ]
Favila, M. E. 1988. Chemical labelling of the food ball during rolling by males of the subsocial Coleopteran Canthon cyanellus cyanellus LeConte (Scarabaeidae). Insectes Soc. 35: 125-129. [ Links ]
----------. 1992. Análisis del comportamiento subsocial de Canthon cyanellus cyanellus LeConte (Coleoptera: Scarabaeidae). Doctoral thesis. Escuela Nacional de Ciencias Biológicas, I. P. N., México. [ Links ]
Gill, B. D. 1990. Two new species of Eurysternus Dalman (Coleoptera: Scarabaeidae: Scarabaeinae) from Venezuela with notes on the genus. Coleopt. Bull. 44(3): 355-361. [ Links ]
Halffter, G. 1977. Evolution of nidification in the Scarabaeinae (Coleoptera, Scarabaeidae). Quaest. Entomol. 13: 231-253. [ Links ]
----------. 1997. Subsocial behavior in Scarabaeinae beetles. Pp. 237-259. In: The Evolution of Social Behavior in Insects and Arachnids. J.C. Choe & B.J. Crespi, (eds.) Cambridge University Press. [ Links ]
Halffter, G. & D. W. Edmonds. 1982. The Nesting Behavior of Dung Beetles (Scarabaeinae). An Ecological and Evolutive Approach.Mexico: Instituto de Ecología. 176 pp. [ Links ]
Halffter, G., V. Halffter & C. Huerta. 1980. Mating and nesting behavior of Eurysternus (Coleoptera: Scarabaeinae). Quaest. Entomol. 16: 599-620. [ Links ]
Halffter, G., & E. G. Matthews. 1966. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomol. Mex. 12-14: 1-312. [ Links ]
López-Guerrero, Y. & G. Halffter. 2000. Evolution of the spermatheca in the Scarabaeioidea (Coleoptera). Fragmenta entomologica. 32 (2): 225-285. [ Links ]
Jessop, L. 1985. An identification guide to Eurysternus dung beetles (Coleoptera, Scarabaeidae). J. Nat. Hist. 19: 1087-1111. [ Links ]
Martínez, A. 1988. Notas sobre Eurysternus Dalman (Coleoptera, Scarabaeidae). Entomologia Brasiliensis. 12: 279-304. [ Links ]
Zar, J. H. 1996. Biostatistical Analysis. 3rd ed. Englewood Cliffs, New Jersey: Prentice Hall. 770 pp. [ Links ]














