BACKGROUND
Autologous hematopoietic stem cell transplantation (HSCT) has been given to persons with multiple sclerosis (MS) for over 20 years, and more than 3000 HSCTs have been done worldwide1,2. Transplant-related mortality in MS managed with HSCT, was considered a limiting factor but has decreased to <2%2. We have conducted autotransplants in patients with all phenotypes of MS since 2006 and found an overall response rate of around 80% after grafting 1000 patients2. The salient features of our HSCT method include collection and administration of stem cells from the peripheral blood on an outpatient basis; avoidance of cellular freezing/thawing to increase the viability of hematopoietic cells in the graft and reduce costs, and splitting the total dose of cyclophosphamide (Cy, 200 mg/Kg) into four doses (50 mg/Kg each), with a period of 7 days between the first two and the last two doses2.
Cy may induce cardiac toxicity, specifically when it is employed in high doses. In a group of 32 patients given Cy at a dose of 180 mg/kg, acute myocarditis was found in 9 (28.1%)3. Others reported the development of acute cardiotoxicity in 22%4,5. Here, we report data of cardiotoxicity observed in a group of 1,000 patients with MS, autografted following the “Mexican method” of conditioning, based on cyclophosphamide (Cy, 200 mg/Kg) and rituximab.
METHODS
Patients
All consecutive patients with MS referred to our center for a HSCT after June 2015 were prospectively enrolled in the study. Primary endpoints were the development of acute cardiotoxicity and transplant-related mortality; secondary endpoint was overall survival, defined as the time elapsed between the autograft and death from any cause. Study was approved by the Ethics Committee of the Clínica RUIZ (Conbioetica 21CEI00120130605, Registry No. 13 CEI 21 114 126). Patients signed a consent form after being fully informed on the procedure's benefits and potential complications. Protocol is registered in ClinicalTrials.gov, identifier NCT02674217.
Conditioning and autografting
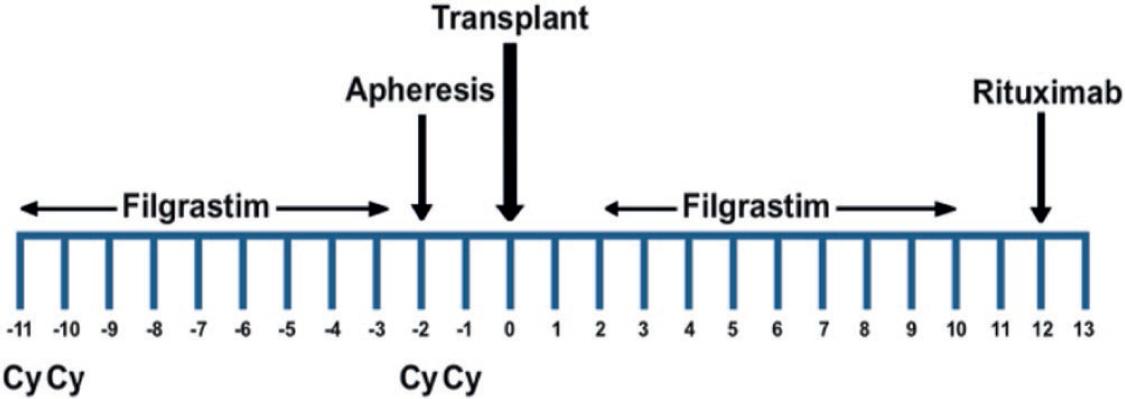
HSCT employing the “Mexican method” was conducted as described2. Total dose of Cy was 200 mg/kg; the initial two doses of Cy, each of 50 mg/kg, were delivered in 2 consecutive days (−11 and −10), whereas the last two were delivered 7 days later, also in 2 consecutive days (−2 and −1) (Fig. 1).
RESULTS
A total of 1,000 subjects were enrolled in the study after June 2015; 360 were male (36%) and 640, female, with a median age of 46 years (range 17–73).
Assessment of cardiotoxicity was done as follows. All patients had a cardiovascular consultation and an electrocardiogram done before HSCT. The following cardiac abnormalities were found: complete (4) and incomplete (4) right bundle branch block, left bundle branch block (1), paroxysmal auricular fibrillation (2), and hypertrophic cardiomyopathy with mild aortic obstruction (1). None of these findings was a reason for not conducting the HSCT. One patient developed acute myocarditis after receiving the four doses of Cy. There were no other adverse cardiovascular events and only two patients died as a result of transplant-related complications.
DISCUSSION
The mortality rate of autologous HSCT was considered a solid argument precluding it as therapy in various diseases, but since both have substantially decreased in recent years as a result of key changes done to the procedure, it appears to be gaining acceptance in the medical profession. Our method involves the use of high-dose Cy (200 mg/kg) delivered in four doses of 50 mg/kg each, the initial ones in 2 consecutive days (−11 and −10), and the last two 7 days later, also in 2 consecutive days (−2 and −1) (Fig. 1), whereas most schedules deliver the Cy in 4 consecutive days. The rationale of this method of delivering Cy lies on three points: to both mobilize cells and induce immunosuppression with Cy for the autograft; to allow the use of refrigerated rather than frozen cells, and to decrease toxicity.
In relation to cardiotoxicity induced by Cy, it has been reported that after receiving a single, unsplit dose above 180 mg/kg, acute myocarditis may develop in 22% of patients3, whereas early cardiac events post high-dose Cy were found in 19% of patients who received unsplit 50–100 mg/Kg of Cy during allogeneic transplants4. These figures contrast with the acute cardiotoxicity that we have observed in our study (1 out of 1000 patients, 0.1%). It is possible that, similar to what we have found in the decreased bone marrow toxicity of our method of delivering the Cy5, the difference in cardiotoxicity may stem from the way of delivering it. In conclusion, Cy delivered in two blocks apart resulted in less cardiotoxicity, at least when the “Mexican method” to conduct autotransplants in persons with MS was employed.











 text new page (beta)
text new page (beta)