Introduction
GPR35 was initially discovered in the rat intestine.1 This receptor is coupled to Gαi/o and Gα13 proteins.2-5 Kynurenic acid (KYNA) remains the most likely endogenous agonist ligand for GPR35;3,5 interestingly, it also seems to negatively regulate dopamine levels in the ventral striatum,6 as well as glutamate levels in hippocampus.7 However, the action mechanisms for these effects remain unclear. Previous reports have involved actions on: i) alpha 7 nicotinic acetylcholine receptor (alpha7nAChR); ii) N-methyl-D-aspartic acid receptor (NMDAR); iii) GPR35; iv) among others (e.g., GABAA channels).8-10 Furthermore, recently, important participation of the kynurenine pathway in the addiction phenomena was proposed.11 There is a plethora of evidence for the roles of alpha7nAChR, NMDA, and GABAA channels in addictions, but not for GPR35.
In the nervous system, GPR35 was reported in dorsal root ganglion neurons,9 and moderate expression in the brain and spinal cord.12 In sensory neurons, its functions are related to pain inhibition, whereas its role in the central nervous system (CNS) is less clear, although it has been demonstrated that the loss of GPR35 may underlie at least one kind of mental retardation (i.e., Albright hereditary osteodystrophy-like syndrome).13 This pilot study was set up to explore the pharmacological effects of intra-ventral-striatum injections of lodoxamide (a GPR35 full agonist) on the conditioning place preference paradigm.
Methods
Animals
Fifty male Wistar rats (250-300 g) in their young adult life (~ 8 weeks)14 were obtained from the vivarium of the Autonomous University of Aguascalientes. All experimental protocols and manipulation were approved by our Institutional Ethics Committee (CE-UAA) and followed the Mexican Guidelines for Animal Care (NOM-062-ZOO-1999) in severe harmony with the ARRIVE guide15 and the Guide for the Care and Use of Laboratory Animals in the USA.16 Animals were kept under a 12-hour light/dark cycle with free access to water and food.
Stereotaxic surgery
All rats were anesthetized with 1 mL/kg (i.p.) of the cocktail: Zoletil®50 (35 mg/mL + Xylazine 8 mg/mL). A two-sided guide cannula was stereotaxically placed into the ventral striatum (P: 1.28 mm, L: 3 mm, and V: 7 mm) based on Paxinos and Watson.17 The guide cannula was held in place to the skull with two screws and dental acrylic, and a stylet was inserted into the guide. After surgery, the animals were in a post-surgery recovery phase of at least seven days, as previously reported.18,19
Microinjections were performed over a period of 60 s using a syringe pump (Sage Instruments, model 355); the injection cannula was left undisturbed for another 60 s to prevent drug reflux. 2,2′-[(2-Chloro-5-cyano-1,3-phenylene)diimino]bis2-oxoacetic acid (lodoxamide), and CID-9581011 (ML194) were dissolved in 10% dimethyl sulfoxide (10% DMSO). Fresh solutions were prepared each experimental day, and the free base was considered to calculate the reported concentrations. The drugs used in the present study were purchased from Sigma Aldrich® (Saint Louis, Missouri, USA).
Behavioral procedure
The conditioned place preference (CPP) apparatus consisted of two large side chambers (41.5cm X 32.5cm X 30.5cm) and one middle chamber between both compartments (27.5cm X 15cm X 12.5cm). The two side chambers differed in floor textures (rough versus fine) and somatosensory cues (black versus white). The CPP paradigm consisted of three different phases: pre-conditioning, with a duration of 3 continuous days, conditioning, with a duration of 5 continuous days, and the day of the test. The CPP apparatus was placed in an isolated quiet room that was illuminated with red light, and every procedure was video-recorded for its posterior analysis.
During the conditioning phase, animals were treated and placed in the opposite chamber that was preferred during the pre-conditioning phase. The difference between the time spent in the drug-paired compartment throughout the test day and the time spent in the drug-paired compartment during preconditioning (lateral preference shift) was taken as a degree of conditioning. Therefore, a statistically significant increase in time spent in the drug-paired chamber suggests a conditioning effect induced by the treatments, as previously reported.20,21
Pre-conditioning phase
The baseline and compartment preference were determined by placing each animal in the middle aisle of the CPP apparatus, then removing the barriers to allow free access to the entire apparatus for 15 min on days 1 to 3.
The mean time spent in the non-preferred chamber (i.e., the chamber where animals spent less time) during these days was used as a baseline.20,22
Counterbalance conditioning phase
This phase consisted of 5 days of conditioning sessions. Five min before performing each paired compartment session, the pretreatments vehicle (10% DMSO), lodoxamide (56 pmol), lodoxamide (100 pmol), ML-194 (300 pmol), or ML194 (300 pmol) + lodoxamide (100 pmol) was microinjected through the cannulas into the ventral striatum (Figure 1). Conditioning was carried out twice a day (7:00 a.m and 11 a.m) with a 4-hr interval between each 15-min session. A counterbalance scheme was applied by injecting treatment/vehicle in the paired and unpaired chamber, respectively. Access to other compartments was blocked with guillotine doors during this phase.
Test day
The next day after the conditioning phase, the testing phase was carried out. Guillotine doors were removed, and the rats had access to the entire apparatus. The time each rat spent in the compartments over a 15-min period was video-recorded. The difference between the time spent in the drug-paired compartment during the test day and the time spent in the drug-paired compartment during pre-conditioning (lateral preference shift) was registered.
Histology
At the end of the behavioral procedure, the animals were overdosed with a lethal injection of sodium pentobarbital (70100 mg/kg, i.p.). Intracardiac perfusion was performed with 0.9% isotonic saline followed by 4% formaldehyde; the brain was removed, and it was preserved in 10% formaldehyde. Subsequently, the brain was mounted in a 30% sucrose bath and coronally sectioned (60 μm) using a cryostat. The slices were stained with cresyl violet (Sigma®, St Louis, MO) and examined with microscope to identify the microinjection site, in consultation with Paxinos and Watson.17 Only rats cannulated in the ventral striatum were included in this study.
Data analysis
For all tests, the measure of central tendency was the mean value, and the measure of dispersion was the standard error of the mean (SEM). All data showed normal distribution. Comparisons for the CPP protocol were obtained with a two-way analysis of variance with repeated measures (two-way-RM-ANOVA). If pertinent, this analysis was followed, by the Bonferroni post hoc test. For all experiments, statistical significance was considered p ˂ 0.05.
Results
Figure 2 shows the time spent in the CPP paradigm according to different lodoxamide concentrations. The concentration of 100 pmol lodoxamide increased the time spent in the drugconditioning chamber during the test [F(1, 27) = 6.328, p = 0.018] compared with baseline, whereas no changes in this value were detected in vehicle and 56 pmol of lodoxamide groups ( p >0.05; Figure 2) .
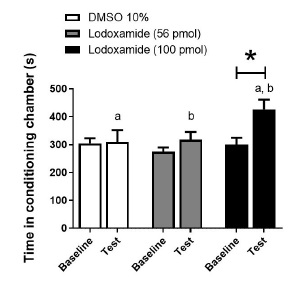
Figure 2 Effect of intra-ventral-striatum injections of vehicle (DMSO 10%) or lodoxamide (56 and 100 pmol) on time spent in the drug-paired chamber. *, p < 0.05 vs. respective baseline; a, p < 0.05 lodoxamide (100 pmol, during test) vs. vehicle (during test); b, p < 0.05 lodoxamide (100 pmol, during test) vs. lodoxamide (56 pmol, during test)
Figure 3 shows the effects of a selective GPR35 receptor antagonist per se and on the reinforcing actions of 100 pmol lodoxamide. By itself ML194 did not modify time spent in the drug-conditioning chamber during the test compared with baseline. Moreover, it failed to prevent the increment in this value during the test with the animals that received 100 pmol lodoxamide [F(1, 18) = 5.198, p = 0.035] (Figure 3). Additionally, time spent in the drug-conditioning chamber during the test was greater in ML194 (300 pmol) + lodoxamide (100 pmol) compared with ML194 (300 pmol) (p <0.05, Figure 3).
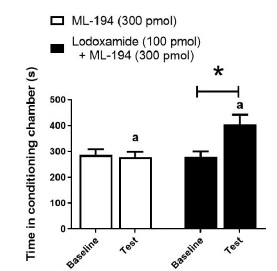
Figure 3 Effect of intra-ventral-striatum injections of ML-194 (selective GPR35 antagonist) per se and on the change in place preference induced by lodoxamide (100 pmol intra-ventral-striatum). *, p < 0.05 vs. respective baseline. a, p < 0.05 lodoxamide (100 pmol) + ML-194 (300 pmol; during test) vs. ML-194 (300 pmol; during test).
Discussion
General
In previous reports, systemic injections of kynurenic acid (a GPR35 full agonist) attenuated the rewarding effects of morphine in the CPP paradigm.23 A low dose of kynurenic acid produced a tendency to increase the time spent in the drugpaired arm in the CPP paradigm (not reported with higher doses), suggesting a possible reinforcing effect of this acid.23
Interestingly, the preventive effects of the kynurenic acid on the reinforcing actions of morphine has been linked to its role as an antagonist of the NMDA channels.23 This premise can be supported by the fact that the blockade of NMDA receptors prevented both the reinforcing and aversive effects of diverse substances in the CPP paradigm.24 However, the evaluation of the potential participation of GPR35 had been omitted. In our study, a low dose of lodoxamide (100 pmol) - a full GPR35 receptor agonist with not reported activity on NMDA channels - in the ventral striatum produced an increase in place preference in the CPP paradigm (Figure 2). This reinforcing effect was highly reproducible (Figures 2 and Figure 3), but resistant to the blockade with a GPR35 selective antagonist (i.e., ML-194; Figure 3). Thus, the mechanism of action probably involves GPR35-independent pathways that remain undetermined.
Hypothetic mechanisms involved in the reinforcing actions of lodoxamide
Clinically, the main use of lodoxamide in Ophthalmology is related with its anti-inflammatory actions via decrease of mastcells, T-lymphocytes and other immune cells.25 Lodoxamide has also been described as an anti-fibrogenic drug in a hepatic damage model.26 Similarly, other GPR35 agonists such as pamoic acid have been reported mediating neuroprotective actions.27 Therefore, the actions of lodoxamide goes beyond GPR35 receptors.
It has been widely reported that analgesic and anti-inflammatory agents may produce conditioning place preference and other manifestations of reinforcing actions.28 Hence, as lodoxamide may produce neuroprotective and anti-inflammatory actions, a logical speculative idea is that its reinforcing effects may be linked to both effects. Nevertheless, our animals remained 7-10 days in recovery time after surgery, so inflammatory post-surgery processes are unlikely. As lodoxamide include multiple pathways and modulation in the secretion of different mediators (histamine, serotonine, adenosine, etc.),29,30 the indirect participation of other compounds which might alter the dopaminergic system should be also considered and studied to determine lodoxamide mechanisms of action.
Admittedly, this study failed to find the action mechanisms for the reinforcing effects induced by lodoxamide. Nevertheless, our data may motivate further research protocols directed to understand these mechanisms and its possible pharmacological effects on the brain reward system.
Conclusion
Our data showed that intracerebral injections of lodoxamide at a concentration of 100 pmol into the ventral striatum elicit an increase in place preference switching through mechanisms that have yet to be identified, but that are most likely independent of GPR35. Likewise, a role for GPR35 in the brain reward systems (at least in ventral striatum) seems improbable.
Authors’ contributions
AD-B carried out the experimental protocols, and contributed to data curation, writing, review and editing the manuscript; RG-A and JLQ contributed to conceptualization, writing, review and editing; BAM-C wrote the original draft, and contributed to formal analysis, funding acquisition and project administration.











 nueva página del texto (beta)
nueva página del texto (beta)