Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Medicina y ética
versión On-line ISSN 2594-2166versión impresa ISSN 0188-5022
Med. ética vol.36 no.3 Ciudad de México jul./sep. 2025 Epub 09-Sep-2025
https://doi.org/10.36105/mye.2025v36n3.04
Articles
Pain Without Consciousness? Rethinking pain-suffering in Disorders of Consciousness
* Professor of Bioethics at the Faculty of Medicine, Universidad Xochicalco, Ensenada Campus, Baja California, Mexico. Email: 000045092@ens.xochicalco.edu.mx
Disorders of consciousness (DoC) are a challenge for understanding pain and suffering, as they are complex subjective experiences involving multiple neural networks. Neurophysiological studies and neuroimaging suggest that some patients in a vegetative state may experience pain, and those with covert consciousness are more likely to perceive it. However, these patients are often ignored by medical staff because they cannot express it. This narrative review addresses recent neuroscientific research on pain in these patients, highlighting the need to reconsider it in clinical practice. In the context of diagnostic and prognostic uncertainty, it is crucial to deepen research and establish ethical frameworks to ensure respect for the autonomy and well-being of these patients, addressing the bioethical dilemmas derived from the use of neurotechnologies in patients who cannot express consent.
Keywords: vegetative state/unresponsive wakefulness syndrome; minimally conscious state; cognitive-motor dissociation; bioethics
Los desórdenes de la consciencia (DoC) son un desafío para la comprensión del dolor y del sufrimiento, ya que son experiencias subjetivas complejas que involucran múltiples redes neuronales. Estudios neurofisiológicos y de neuroimagen sugieren que algunos pacientes en estado vegetativo podrían experimentar dolor y que aquellos con consciencia encubierta tienen mayor probabilidad de percibirlo. Sin embargo, los pacientes al no poderlo expresar son ignorados por el personal médico. Esta revisión narrativa aborda las investigaciones neurocientíficas recientes sobre el dolor en estos pacientes, resaltando la necesidad de reconsiderarlo en la práctica clínica. En un contexto de incertidumbre diagnóstica y pronóstica, es fundamental profundizar en la investigación y establecer marcos éticos que garanticen el respeto a la autonomía y el bienestar de estos pacientes, por lo que se abordan los dilemas bioéticos derivados del uso de neurotecnologías en pacientes que no pueden expresar su consentimiento.
Palabras clave: estado vegetativo/síndrome de vigilia sin respuesta; estado de mínima consciencia; disociación cognitivo-motora; bioética
1. Introduction
Brain injury due to an external factor is known as acquired brain injury and can be mild, moderate, or severe. Disorders of consciousness (DoC), or altered states of consciousness, are several conditions characterized by severe brain damage with physical, cognitive, and emotional sequelae of varying severity. This complexity requires specialized and trained personnel for diagnosis and treatment, as seen in Figure 1.
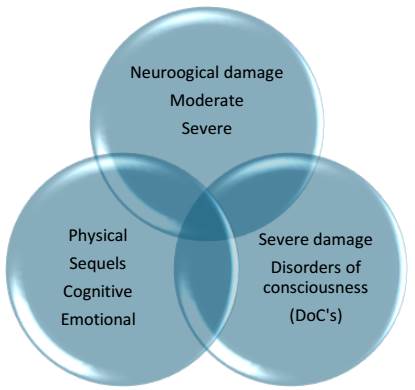
Source: prepared by the author. Schematic representation of neurological damage. DoC falls under severe neurological damage. Sequelae can be in different areas and intensities.
Figure 1 Acquired Brain Injury
The knowledge, terminology, definition, and treatment of DoC, the term that will be used in this review, have evolved over time, as seen in Figure 2. In 1940, the term “apalic syndrome” was used for the state of unconsciousness with preserved vegetative functions due to diffuse brain injury. In 1972, the term “vegetative state” (VS) was coined for patients with severe brain damage who were not in a coma but did not interact with their environment. In 2002, the minimally conscious state (MCS) was described for patients who were neither in a coma nor in a VS, and more recently, cognitive-motor dissociation (CMD) has been introduced.
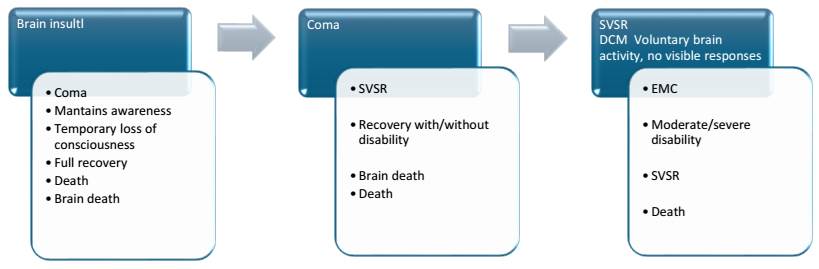
Source: prepared by the author. The diagram shows the evolution of DoC from brain insult, coma, unresponsive wakefulness syndrome (VS), MCS, and cognitive-motor dissociation (CMD). Recovery may occur at any point, potentially with some type of disability, or death.
Figure 2 Evolution of Disorders of Consciousness
The Multi-Society Task Force(1) defined VS as a condition of complete unconsciousness of both self and environment with the presence of sleep-wake cycles, autonomic functions preserved from the brainstem and hypothalamus, and no response to stimuli, or comprehension or expression of language. They introduced the term “permanent vegetative state” as synonymous with irreversibility (2).
In 1995, the Aspen Group (3) described a new condition in patients who were neither in a coma nor in VS, called the minimally conscious state (MCS). This condition is characterized by inconsistent and fluctuating consciousness, specific and intentional behavioral responses, such as following objects with the gaze and responding to external stimuli. Recognition of MCS is important because its prognosis is different from that of VS, and it contributed to the debate on end-of-life decisions in patients with permanent VS in the 1970s.
It is important to clarify that a vegetative state (VS) is not equivalent to brain death. A patient in a VS presents severe neurological damage with extreme disability, which requires total assistance not only at a medical and technological level but also at a human level, ensuring that the patient receives adequate nutrition, care, and stimulation.
Advances in neuroimaging (NI) and neurophysiology (NF) have radically transformed the understanding of Disorders of Consciousness (DoC). It is now recognized that a vegetative state is not a state of unconsciousness, but rather a dissociation between awakening and consciousness (4,5), as shown in Figure 3.
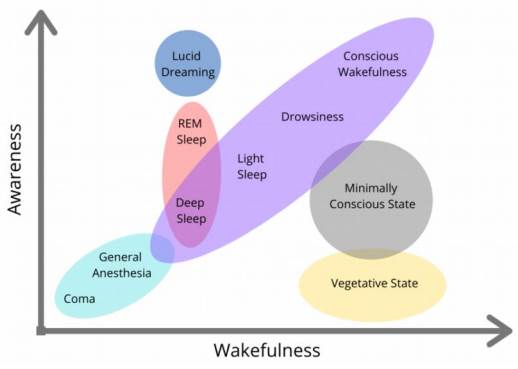
Source: Spielman R, Jenkins W, Lovett M. Introduction to Psychology & Neuroscience. Canada (NS): Dalhousie University Libraries Digital Editions Halifax: Stevens; 2022 (accessed 2024 March 24). Available at: https://digitaleditions.library.dal.ca/intropsychneuro/chapter/what-is-consciousness/ This textbook is an adaptation of Psychology 2e produced by OpenStax and licensed under a Creative Commons Attribution License 4.0. Access for free at https://openstax.org/books/ psychology-2e/pages/1-introduction
Figure 3 Diagram of the components of consciousness: awakening and consciousness
The following presents key research that shifted this paradigm and led to the recognition of cognitive-motor dissociation (DCM).
2. Key research in the revision of the Vegetative State (VS)
Laurey et al. (6) used positron emission tomography (PET) in 2000 to identify activation in the auditory cortex in response to sound stimuli in patients with post-anoxic vegetative state (VS). Years later, they recorded responses in somatosensory cortical areas in reaction to painful stimuli (7). Surprisingly, brain activation was similar to that of healthy controls, suggesting hidden cognitive functions, though with functional disconnection from higher cortical levels, which would explain the lack of integration of consciousness.
Owen et al. (8) studied a patient with a traumatic brain injury-induced vegetative state in 2006 using functional magnetic resonance imaging (fMRI), and recorded brain activity in response to auditory stimuli, similar to that of healthy controls. Furthermore, when asked to imagine activities like playing tennis or walking around her house, the same areas of the brain activated as in the controls. These findings demonstrated that, despite the diagnosis of VS, the patient understood verbal commands and responded with brain activity. Her intention to cooperate and respond showed that she was aware of herself and her environment, revealing a level of consciousness undetected by clinical evaluation.
Similarly, Monti et al. (9) documented intentional modulation of brain activity in a patient in VS in 2010. Using the same technique as Owen, the patient was asked to respond “YES/NO” using mental imagery (playing tennis = YES; walking around the house = NO), and the patient responded coherently. Despite this, the behavioral evaluation with the Coma Recovery Scale-Revised (CRS-R) did not show responses to visual, auditory, tactile, or painful stimuli. These findings confirm the existence of patients with hidden consciousness, which is undetectable through clinical exploration. The patient was conscious, responded to questions with brain activity, and was able to communicate.
The discrepancy between voluntary brain activity identified by neuroimaging techniques (PET, fMRI) and the absence of detectable behavioral responses in patients with Disorders of Consciousness (DoC) led to the concept of Cognitive-Motor Dissociation (DCM) (10). This means that the patient is conscious and responds to stimuli through brain activity, demonstrating hidden consciousness that remains unexpressed physically. As a result, this consciousness is undetected during clinical evaluation.
3. Change in Terminology: from VS to Unresponsive Wakefulness Syndrome (SVSR)
These discoveries transformed the understanding of DoC, replacing the term “permanent vegetative state” (VS), traditionally associated with irreversibility (2), with the term Unresponsive Wakefulness Syndrome (SVSR)(11), a descriptive term proposed by the International Task Force on The Vegetative State.
The term “consciousness” was replaced by “unresponsive” because the presence or absence of consciousness in these patients remains difficult to verify with certainty. From this point forward, the term SVSR replaces VS.
4. Neurotechnology and perspectives on DoC diagnosis
The growing interest in predicting and promoting potential neurological recovery has driven the development of integrated scientific and technological models (12). One example is the Brain Research Advancing Innovative Neurotechnologies (BRAIN 2025) project (13), aimed at understanding the dynamic interrelationships of neural circuits and their application in neurorehabilitation.
While neurotechnology has improved the diagnosis of DoC, there are still limitations in clinically assessing consciousness. To address this challenge, several institutions have developed clinical guidelines recommending the use of advanced technologies in evaluating patients with DoC.
The American Academy of Neurology, the American Congress of Rehabilitation Medicine, and the National Institute of Disability, Independent Living, and Rehabilitation Research (14) propose multimodal evaluations, such as functional neuroimaging or electrophysiological studies (EEG), to detect consciousness when clinical evaluation is uncertain.
The European Academy of Neurology and experts in coma and chronic DoC (15) developed the European Recommendations based on the GRADE methodology (Grading of Recommendations Assessment, Development, and Evaluation). They recommend using the Coma Recovery Scale-Revised (CRS-R) to assess consciousness. They propose using EEG to identify sleep patterns and evoked potentials (EPs). They suggest, when possible, functional neuroimaging (PET, fMRI) to improve diagnostic accuracy.
Additionally, the International Federation of Clinical Neurophysiology (16) conducted a thorough and critical review of the major electrophysiological studies used to assess diagnosis and prognosis in patients with DoC. Notable among them are conventional EEG, Event-Related Potentials (ERPs),1 and their potential clinical applications.
As a result of these advances, two neuroimaging (NI) methods have been developed to detect hidden consciousness in patients with Cognitive-Motor Dissociation (DCM). The first combines functional MRI (fMRI) with EEG and is based on imagined mental tasks.
The second is the brain-computer interface (BCI),2 which translates neural activation in response to verbal commands, facilitating communication in the absence of behavioral responses. Although these methods are not conclusive, they have demonstrated high sensitivity and specificity for identifying patients with a higher likelihood of regaining consciousness due to residual cognitive functions (17).
In this context, the Neurocritical Care Society launched the Curing Coma initiative in collaboration with institutions such as the National Institute of Neurological Disorders and Stroke and the Curing Coma Campaign’s DoC project. Its aim is to improve the management and prognosis of patients with DoC through the development of a shared data repository and the promotion of international collaboration. Furthermore, it seeks to standardize a common language for future research in this field (18).
5. Pain in patients with DoC: a multidisciplinary debate
One of the oldest and most controversial debates regarding patients with DoC is their ability to perceive and experience pain. While some argue that patients in SVSR do not consciously process painful stimuli due to disconnection from higher cortical centers, others contend that there is insufficient evidence to claim the absence of pain perception. Recently, neuroimaging (NI) has identified a subgroup of patients in SVSR with hidden consciousness, a phenomenon known as DCM. This finding has opened a bioethical debate with new questions:
What should be the intensity of nociceptive stimuli in a patient who cannot give consent?
How should neurological responses to these stimuli be interpreted?
What dose of analgesics is appropriate without further affecting the state of consciousness?
This paper is a narrative review focused on interdisciplinary studies that have transformed the understanding of DoC. Its primary goal is to present the most recent research in neuroscience, especially those employing neurotechnologies to study pain pathways. Furthermore, it mentions advances in the validation of different scales for pain assessment. The review also addresses the bioethical and neuroethical challenges arising from the use of neurotechnologies in individuals who cannot express consent. The topics covered include:
6. Pain, nociception, suffering, autonomic response, pain
In 2020, the International Association for the Study of Pain (IASP) redefined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.” While pain serves an adaptive function, it can also significantly affect a person’s well-being. In patients with Disorders of Consciousness (DoC), pain assessment is particularly challenging due to the absence of verbal communication, one of the primary ways to express pain. However, the inability to communicate does not necessarily mean these patients do not experience pain (19,20).
Pain is a personal experience conditioned by biological, psychological, and social factors. It differs from nociception because it is not limited to the activity of sensory pathways (19,20). Its perception varies depending on factors such as sex, individual sensitivity, pain inhibition or enhancement mechanisms, and response to treatment. Additionally, cultural and educational factors influence the way pain is expressed (21). Pain is also modulated by cognitive consciousness, subjective interpretation, and behavioral responses (22).
6. Pain assessment in non-communicative patients
There are several scales for assessing pain in patients with communication difficulties, such as those with cognitive deficits, dementia, or advanced age. Some examples include the Pain Assessment in Advanced Dementia Scale (PAINAD), Non-Communicative Patient’s Pain Assessment Instrument (NOPPAIN), and Faces Pain Scale (NRS). These tools rely on physiological indicators (heart rate, respiratory rate), behavioral indicators (facial expressions), and vocalizations (sighs, moans), as well as body movements, changes in interpersonal reactions, activity, and mental state (23 - 27).
In patients with DoC, pain is primarily assessed using the Nociceptive Coma Scale (NCS)(28) and its revised version, the Revised Nociceptive Coma Scale (NCS-R), which is the most commonly used. This scale measures three types of responses: motor, verbal, and facial. Motor Response: none, abnormal posture, flexor withdrawal, localization of the painful stimulus. Verbal Response: none, grunting, vocalization, intelligible verbalization. Facial Response: none, oral reflex movements/Moro reflex, gestures, crying.
The scale assigns a score from 0 to 9, where higher values indicate a greater response to pain. In the revised version, the visual response was removed due to its potential influence by non-painful stimuli, such as noise (29 - 32). NI studies have shown a correlation between the NCS-R and activation of nociceptive cortical processes, particularly in the anterior cingulate cortex (ACC), a key region involved in pain perception (30,33,34).
6.1. Nociception
While pain and nociception are closely related, they are distinct concepts. Nociception is the physiological process by which nociceptors detect harmful stimuli and transmit signals to the central nervous system. However, the presence of pain is not always linked to a nociceptive stimulus or the severity of an injury.
Nociception is the neural mechanism that encodes and processes harmful stimuli, activating an extensive cortical network, either consciously or unconsciously (21). Pain is a complex phenomenon that encompasses nociception and the subjective interpretation of the sensation. While nociception is a universal phenomenon, as certain noxious stimuli trigger similar sensory responses across all cultures, pain is an individual experience that varies depending on the meaning each person attributes to it, influenced by cultural and personal factors (22).
6.2. Suffering
Suffering is the emotional response to pain; it is an unpleasant experience that manifests through various cognitive, emotional, and autonomic reactions (35). Its relationship with pain is complex, as pain is not always associated with suffering, nor is suffering always related to physical pain (21,22,35).
In general, suffering is linked to emotions or situations perceived as a threat to a person’s integrity. However, it is not always easy to identify or assess in clinical practice (21). When treating the patient, the doctor must gather evidence of their suffering, which is not an easy task, as this experience is subjective and difficult to express verbally (22).
There is an ongoing debate about the boundaries between pain and suffering, as well as their neural correlation. Addressing this relationship without resorting to reductionism is essential for a comprehensive understanding of the pain experience and its impact on patients with DoC.
7. Neural Correlation of Sensation, Pain, Perception, and Suffering
According to De Ridder et al.(35), pain consists of three main elements: pain sensation, suffering, and duration over time, which correspond to different pathways involved in their processing. Upon a painful stimulus, sensory receptors and the somatosensory cortex are activated, generating the initial sensation. Subsequently, the Salience Network, Default Mode Network, and Frontoparietal Control Network intervene in the perception of pain. Perception is the process by which the brain organizes and interprets sensory stimuli, creating a meaningful experience of the world and oneself.
The stimulation of nociceptors activates the spinothalamic pathway, which informs the thalamus and the cerebral cortex, responsible for the reflex responses to pain. The nociceptive cortical network involves the secondary somatosensory cortex and the posterior part of the insula. However, the conscious experience of pain requires the activation of a more complex network called the pain matrix, which is divided into two subsystems: the lateral network, which encodes the sensory discriminative information of pain, and the medial network, which processes the affective-cognitive aspects of pain (36).
8. Dynamics of connectivity in the pain matrix
Pain studies have not only identified the brain areas involved in pain perception but also the activation dynamics and functional connectivity within the pain matrix.
The ascending pain pathways are divided into two main tracks: the first, medial pathway, involves areas such as the anterior insular cortex and the rostral-dorsal cingulate cortex (rdACC). This pathway is involved in integrating the cognitive, emotional, somatosensory, and autonomic aspects of pain, as well as in generating negative emotions of discomfort. The second, lateral pathway, includes the somatosensory cortex and the parietal area, responsible for the sensory discrimination of pain, determining its location and characteristics (35).
Studies of cortical potentials suggest that pain processing begins in the posterior insula, responsible for the initial detection of the stimulus, and then involves the anterior insula, where the emotional reaction to pain is processed (36).
9. Inhibitory Mechanisms of Pain
The ascending pain pathways, responsible for transmitting harmful stimuli to the brain, are modulated by a descending pain control pathway. This pathway involves serotonergic and noradrenergic systems that help regulate the intensity of the pain sensation. Specifically, the amygdala plays a key role in pain inhibition by releasing substances such as endogenous opioids, somatostatin, and corticotropin. These compounds inhibit the transmission of pain signals, contributing to its regulation. An efficient inhibitory system can effectively suppress pain, while a dysfunctional system can lead to chronic and constant pain, or even variable and unpredictable pain if the system is deficient (35).
In addition to these brain areas, other regions also participate in the pain experience. The cerebellum, for example, is involved in the sensory discriminative processing of pain, while motor areas contribute to both the perception and processing of pain (36).
10. Pain in DOC
As mentioned above, pain and suffering are often subjective expressions of the individual, which means that the person must be conscious in order to communicate them. This poses a challenge for clinicians when identifying pain in patients with DoC, whether nociceptive or non-nociceptive in origin. In addition to their complexity, both the pain matrix and its phenomenology have not been fully understood to date.
There are various methods for assessing pain in these patients. Some are based on comparing physiological variables such as eye opening, changes in breathing, heart rate, and blood pressure. However, these indicators have a subcortical origin and do not necessarily reflect a conscious perception of pain. Other studies analyze stereotypical responses and the location of the painful stimulus, which allows for the differentiation of different levels of activity in the brainstem, subcortical structures, and cerebral cortex, including the nociceptive network or pain matrix. (36)
Neuroimaging (NI) and Neurophysiological Studies on Pain in DoC Neuroimaging (NI) and neurophysiological (NF) studies confirm that both suffering and the cognitive evaluation of pain are mediated by specific brain areas such as: Insula Anterior cingulate cortex Prefrontal cortex Subcortical limbic structures Evidence of Pain Perception: Despite the lack of communication in DoC patients, some studies document that these patients are capable of perceiving pain. In particular, it has been found that some patients in Unresponsive Wakefulness Syndrome (SVSR) perceive the affective components of pain through the activation of the limbic circuit (21).
Additionally, retrospective studies have shown that coma survivors, even without visible responses, remember experiences of pain, noise, sleep deprivation, thirst, hunger, heat, fear, anxiety, isolation, and lack of sunlight. These findings support the theory that, although many patients do not show an obvious response to painful stimuli, some can still perceive them. Thus, although it has traditionally been considered that these patients do not experience pain in a conscious manner, neuroimaging and neurophysiological studies show activation in brain areas associated with pain experience. Moreover, the presence of physiological responses to noxious stimuli suggests that some patients can perceive pain, even if they are unable to express it in a conventional way (36).
The inability to communicate pain in DoC patients may not only be due to alterations in consciousness but also to other neurological conditions, such as: Aphasia (difficulty with language) Fluctuations in alertness Spasticity and rigidity Motor and cognitive changes resulting from multiple focal brain injuries or diffuse brain damage Additionally, sub-syndromes may coexist that affect pyramidal and extrapyramidal tracts, brainstem pathways, and cortical areas, making pain assessment even more complex(37).
Although it has traditionally been considered that these patients do not experience pain consciously, neuroimaging (NI) and neurophysiological (NF) studies show activation in brain areas associated with the painful experience. Additionally, the presence of physiological responses to noxious stimuli suggests that some patients may perceive pain, even if they cannot express it, as will be discussed further below.
11. Pain and consciousness assessment
The assessment of pain and nociception in patients with Disorders of Consciousness (DoC) is undoubtedly essential. However, one of the primary challenges lies in the limited knowledge about the neural correlation of consciousness, which makes it difficult to fully understand DoC. Moreover, the variability in the type and extent of neurological lesions in each patient prevents establishing a linear relationship between the location of brain damage and the resulting alteration of consciousness. Consciousness, far from being the product of a single brain structure, emerges from extensive processes within a generalized neural network that goes beyond the cortical area (37).
The same applies to pain. From a neurophysiological perspective, pain is not the result of a specific brain region, but rather of a salience network or pain matrix that involves multiple areas of the brain. As mentioned, the absence of obvious signs of consciousness during clinical examination does not rule out the presence of cortical activity, meaning that a patient could be conscious and process pain without displaying observable manifestations. Similarly, the lack of visible signs of pain does not necessarily mean that the patient does not consciously experience it or that their body does not generate physiological responses to noxious stimuli (32).
From a clinical perspective, it could be stated that patients in Unresponsive Wakefulness Syndrome (SVSR) do not consciously experience pain, and their responses to painful stimuli are reflexive. In contrast, patients in a minimally conscious state (EMC) could perceive pain, as their responses are specific and intentional.
However, both neuroimaging (NI) and neurophysiological studies have shown that, following a nociceptive stimulus, a network of brain structures involved in affective-cognitive processing is activated, including the anterior insula, the anterior cingulate cortex (ACC), and the prefrontal cortex, even in patients with SVSR. These activations are more extensive than those of areas responsible for sensory-discriminative processing, such as the primary and secondary cortices, the lateral thalamus, and the posterior insula (38).
These findings have paved the way for the development of new clinical scales and studies in neurofeedback (NF) and neuroimaging (NI) aimed at assessing and monitoring pain in patients with SVSR and EMC. The following presents the results of these investigations.
12. Clinical scales to assess pain
12.1. NCS-R and its correlation with NI
One of the most relevant aspects of the NCS-R scale is its sensitivity in differentiating levels of consciousness, showing higher scores in EMC patients compared to those with SVSR. Additionally, it helps distinguish between harmful and non-harmful stimuli (39).
Studies with positron emission tomography (PET) have demonstrated a positive correlation between brain activity in the anterior cingulate cortex and the total score on the scale, suggesting that the score reflects cortical processes related to pain. Therefore, it is proposed as a useful tool for evaluating, monitoring, and treating pain in these patients (30).
Cortese et al.(40) compared the NCS and NCS-R scales, finding that scores equal to or greater than 5 on the NCS and 3 on the NCS-R are associated with a better prognosis. They also consider that the scale can act as a probability indicator for the recovery of consciousness. A score of 3 or higher in response to nociceptive stimuli, measured one week before improvement, predicts the transition from Unresponsive Wakefulness Syndrome (SVSR) to a Minimally Conscious State (EMC).
On the other hand, Chatelle et al.(41) recommend using the NCS-R scale to regularly monitor patients, as an increase in the score could indicate complications or the presence of pain, especially during mobilization or medical procedures.
Additionally, studies on brain metabolism with FDG-PET have shown that patients with a score of 5 or higher on the scale activate the cortical pain pathways, suggesting the presence of covert consciousness or minimally conscious state (DCM). However, a lower score does not completely rule out the possibility that the patient experiences pain (32).
This indicates that the NCS-R scale is a sensitive tool for differentiating levels of consciousness and assessing pain perception in patients with Disorders of Consciousness (DoC). Its correlation with brain activity suggests that its scores reflect cortical processes related to pain, making it useful for monitoring and treatment. Moreover, its ability to predict the recovery of consciousness and detect potential complications or pain reinforces its clinical value. However, its use should be complemented with other evaluations, as low scores do not entirely rule out the experience of pain in these patients.
12.2. New scales
Currently, new scales are being developed, such as the personalized version of the scale, NCS-R-PS (personalized painful stimulation), which uses stimuli adapted to each patient. This has demonstrated a 42.8% increase in response detection compared to standard stimuli. Although the results are preliminary, they suggest that a greater proportion of patients with DoC could be experiencing pain (42).
The Pain Assessment Scale (PAS) evaluates multiple dimensions of the pain response, including autonomic responses, body language, verbal communication, and behavior during painful procedures (32).
As mentioned, the evaluation of pain using these scales is based on the presence or absence of observable behaviors, which may be absent or less evident in patients with Unresponsive Wakefulness Syndrome (SVSR) and Minimally Conscious State (DCM). More studies are still required to validate their reliability and applicability in various clinical contexts. On the other hand, in patients who appear unconscious, the use of physiological markers during painful stimulation provides additional information about the possible presence of covert consciousness.
13. Autonomic response to pain and biomarkers
Although the perception of painful stimuli may or may not be conscious, the autonomic response to pain is undeniable. For example, an increase in heart rate (HR), sweating, changes in pupil diameter, among others. Behavioral responses, such as the reflex withdrawal of a limb in flexion, may also be observed (32).
In healthy individuals, the autonomic response to pain is characterized by an acute and transient increase in HR, blood pressure, pupil dilation, and skin conductance (43). For a more detailed analysis, see (32,38,44).
DeValle et al.(45) evaluated the autonomic response to pain in patients with DoC using a multimodal approach: ECG, fingertip blood pressure, and total peripheral resistance (TPR). In patients with DCM, they found a faster and more significant response, with increases in HR and TPR both in the short and long term. In contrast, in patients with SVSR, only a short-term increase in HR was observed, possibly due to greater brain impairment.
It is known that patients with DCM score higher on the NCS scale, suggesting a greater perception of pain. Moreover, the correlation between physiological markers and behavioral responses indicates that the long-term response involves the same brain centers responsible for pain perception. These findings highlight the importance of higher cortical centers in the autonomic modulation of pain and suggest that this evaluation could also be useful for monitoring the evolution of consciousness in patients with DoC.
14. Neurophysiological studies
Electrophysiological studies are valuable tools for assessing pain in patients with DoC, especially in those with covert consciousness. Event-Related Potentials (PREs) evaluate the integrity of central and peripheral sensory pathways. Among them, somatosensory evoked potentials (SEPs), brainstem auditory evoked potentials (BAEPs), and visual evoked potentials (VEPs) are useful for the prognosis of patients in a coma. In particular, SEPs evaluate the Aδ fiber pathways, which encode thermal nociceptive and non-nociceptive stimuli, and Aβ fibers, which are responsible for pressure and vibration sensitivity (32,38).
On the other hand, laser-evoked potentials (LEPs) are specific for studying nociceptive processes, as they reflect the integrity of the spinothalamic pathway and the residual cortical capacity to perceive pain. Additionally, LEPs record the activity of Aδ and C fibers, which respond to both nociceptive stimuli and non-painful thermal stimuli, such as heat and cold (32).
In patients with SVSR, cortical reactivity to nociceptive stimuli is diminished, possibly due to impaired functional connectivity (21,32,38). However, Naro et al.(33) identified cortical activity in some of these patients following the stimulation of C fibers (nociceptive), even without Aδ-LEP responses. This suggests that, in the absence of Aδ-LEPs, exploring C-LEPs might help detect a potential perception of pain and guide treatment.
EEG and LEPs are quick and adaptable tools for evaluating pain in these patients. However, their interpretation remains challenging due to the lack of a universal standard for measuring conscious pain perception. The use of neurophysiological markers could improve this differentiation, especially between EMC and SVSR in terms of pain perception (38,46).
Neurophysiological studies such as PESS and LEPs are valuable tools for assessing pain perception in patients with DoC, particularly those with covert consciousness and SVSR. While PESS allows for analysis of sensory pathway integrity, LEPs are more specific for studying nociception, reflecting the activity of Aδ and C fibers. Although cortical connectivity may be reduced in SVSR, the detection of responses to nociceptive stimuli suggests the need for more precise strategies to evaluate pain. However, the lack of a universal standard for interpreting these findings remains a challenge, underscoring the importance of developing more accurate neurophysiological markers.
15. Neuroimaging studies (NI)
Although no study has conclusively confirmed whether patients with SVSR perceive pain consciously, some neuroimaging studies have provided valuable information about pain pathways in patients with DoC.
Boly et al.(47) used PET to compare brain activity in 15 healthy controls, 15 patients with SVSR, and 5 with EMC. They found a reduction in functional connectivity in SVSR compared to EMC, although none showed behavioral responses to pain. In contrast, in patients with EMC, pain matrix activation was similar to that of the controls, but with a smaller spatial extension.
On the other hand, Markl et al. (48) used fMRI in 30 patients with SVSR and 15 healthy controls who were subjected to alternating electrical nociceptive stimuli and rest periods. The controls showed activation in S1, S2, CCA, inferior frontal gyrus, insula, thalamus, and cerebellum. In contrast, the activation in SVSR patients was less homogeneous but significant: 50% in the sensory part of the pain matrix and cerebellum, 30% in the affective part (including CCA and/or anterior insula), and 26.7% in both. Only 4 patients activated higher-order structures, suggesting that some may experience pain.
Neuroimaging studies have provided key information about pain perception in patients with DoC, although it is still unclear whether patients with SVSR experience pain consciously. While in EMC, activation of the pain matrix is more similar to that of healthy subjects, in SVSR, functional connectivity is reduced, and brain activation is less consistent. However, some patients with SVSR have shown responses in structures related to nociceptive processing, suggesting the possibility of some pain perception in specific cases. These findings emphasize the need for continued research to better understand the subjective experience of pain in these patients.
These contributions have a significant impact on clinical management and therapeutic decisions, as relying solely on the NCS-R scale might underestimate pain perception. Moreover, the findings suggest that pain perception increases with the level of consciousness, even in the absence of consistent pain responses.
16. Bioethical and neuroethical considerations
The increase in clinical trials involving patients with DoC presents significant bioethical and neuroethical challenges. As of March, this year, 625 clinical trials have been registered on ClinicalTrials.Gov(49).
These encompass observational studies to interventions with devices, drugs, stimulation techniques, and diagnostic procedures. Since these patients are unable to make decisions for themselves, questions arise regarding their inclusion in research, the use of neurotechnologies, and their potential participation in medical decisions.
Neuroethics addresses the boundaries of evaluating and manipulating the central nervous system (CNS), as well as the impact of neurotechnology on human identity and mind (13,50).
One of the main challenges is informed consent (IC), as most of these patients require substitute consent (SC) (51).
Advances in neurotechnology, such as fMRI and electrophysiological evaluations, have allowed the detection of covert consciousness, providing crucial information that traditional assessments may not identify. Additionally, ICC offers a direct communication pathway with these patients (52). However, the technical limitations of fMRI and ICC restrict their participation in medical decisions, as responses are often reduced to binary options, questioning the validity of their participation (53).
Finally, these dilemmas must be addressed in conditions of vulnerability in light of fundamental bioethical principles: autonomy, challenged by the patient’s inability to express it; beneficence and non-maleficence, in the face of high diagnostic and prognostic error margins; and justice, which requires ensuring equitable access to treatment and resources (52).
17. Key bioethical aspects in research and challenges in inclusion in clinical trials
Young et al.(51) identify four fundamental bioethical aspects in research with DoC: autonomy and informed consent, the balance between benefits and risks, justice in access to clinical trials, and transparency in the disclosure of results. These principles face various challenges in practice, particularly concerning IC.
Since these patients cannot directly give their consent, substitute consent (SC) is used. However, this raises questions about the fidelity of the substitute to the patient’s values (51,52). Moreover, IC should be an ongoing process, where the patient’s participation is reevaluated. If the patient recovers the capacity to communicate, researchers should provide them with information about the study they are enrolled in and offer the opportunity to confirm their consent, express their agreement or disagreement, a decision that must be respected. Tools like ICC could, in the future, improve the protection of their autonomy and strengthen the ethical soundness of the research (51).
Since IC is a demanding process, it is essential to ensure that both participants and their representatives fully understand it.
Regarding beneficence and non-maleficence, it is crucial to ensure that the benefits outweigh the risks, avoiding unnecessary interventions or erroneous diagnoses that could harm the patient. SC must be carefully evaluated to maintain an ethical balance in selecting patients (51).
Justice demands that studies be inclusive and equitable, avoiding the exploitation of vulnerable populations. While SC facilitates the inclusion of these patients, it may also justify their participation without fully ensuring the defense of their interests. It is crucial that researchers implement safeguards to ensure that the interests of patients with DoC are prioritized and protected (51).
Transparency and accountability in the SC process are essential to strengthen trust between researchers, patients, and their families (52). This involves clear communication about the risks, benefits, and roles of substitutes, as well as the disclosure of methods and results without conflict of interest (51).
Given the dilemma of preserving the autonomy of these patients and the need to develop new therapies to prevent disability and mortality, alternative models of IC have been proposed (51):
Substitute or surrogate consent, granted by a legal representative.
Exemption from IC, in emergency cases.
Deferred consent, with retrospective information.
Community consultation, based on the values and preferences of recovered patients.
Consensus consent for patients with DoC, involving the legal representative, treating physician, clinical researcher, and an independent advocate (51).
Despite these options, the debate continues regarding the most ethical approach, with the Declaration of Helsinki and Research Ethics Committees still applicable.
The Declaration of Helsinki (1964, updated 2024) (54) establishes strict criteria for the inclusion of people unable to consent, requiring that the research benefits the group they represent cannot be conducted on people capable of consenting, and presents minimal risk.
Moreover, ethics committees may also require safeguards to protect patients without hindering research (51).
18. Proposals to promote inclusion
Fins et al., as cited by Young (51), warn that these patients have been excluded from research in the name of protection, which contradicts the principle of justice. To correct this situation, four primary strategies are proposed:
1. Inclusion of subjects with DoC
It is suggested to incorporate them into studies whenever ethically justified, and the foreseeable risks and benefits are considered. The importance of evaluating their potential participation in consent as they evolve or respond to the research is emphasized. It is recommended to seek their assent when possible and consider their preferences and values to ensure their voice is heard.
2. Individualized assessment of cognitive abilities
Since some patients with DoC may have non-evident levels of consciousness, it is essential to use appropriate tools to detect covert cognitive abilities. Recognizing this diversity not only fosters their inclusion in research but also respects their dignity and rights.
3. Alternative consent models
It is suggested to complement substitute consent with approaches like deferred consent and community consultation, thus ensuring respect for the patient’s autonomy, even if they cannot communicate conventionally. There is also a proposal to investigate strategies for obtaining deferred consent in patients with covert consciousness, considering the attitudes of both the patients and their families.
4. Collaboration with neuroeticists
It is imperative that researchers work alongside neuroeticists and ethics committees to design clinical trials that respect the autonomy of participants. This involves developing protocols that consider individual differences in patient abilities and conducting empirical neuroethics research to better understand the perceptions of patients and their families.
In conclusion, there is no single approach to resolve the ethical and regulatory complexities of research in patients with DoC. Collaboration between researchers, neuroeticists, and ethics committees is essential to ensure ethical clinical trials, adapted to each case, that fully protect participants.
Conclusions
In light of the above, it is concluded that pain in these patients is particularly complex due to their inability to communicate it. Neuroimaging research has revealed that some patients with SVSR may experience pain and that those with DCM are more likely to perceive it. In this regard, the NCS-R scale could underestimate pain perception in certain patients, particularly those with covert consciousness.
While technological advances have enabled the detection of neurophysiological responses to pain, crucial questions remain about how these stimuli are processed at sensory, cognitive, and affective levels and whether they generate conscious suffering. In this uncertainty, clinical management requires integrating scientific, ethical, and bioethical criteria for informed and responsible decision-making.
In research, the protection of the autonomy and dignity of these patients must be balanced with their inclusion in clinical trials. For this, it is necessary to develop alternative consent models and personalized cognitive assessment strategies that allow for more ethical and representative participation. Collaboration between researchers and neuroeticists is essential to ensure an equitable and respectful approach in research with patients with DoC.
Finally, rethinking pain and suffering in this population requires profound interdisciplinary reflection. In a context where clinical practice faces diagnostic and prognostic uncertainties, a scientific training that dialogues with other disciplines and a medicine that, in addition to seeking the patient’s overall well-being, is sensitive to the pain of those who cannot express it clinically and have been historically marginalized is essential.
REFERENCES
1. Multi-Society Task Force on PVS. Medical aspects of the persistent vegetative state. New England Journal of Medicine. 1994; 330(21):1499-508. [ Links ]
2. Multi-Society Task Force on PVS. Medical Aspects of the Persistent Vegetative State. Prognosis for Recovery. New England Journal of Medicine [Internet]. 1994 [citado 1 de mayo de 2023]; 330(22):1572-9. Disponible en: https://www.nejm.org/doi/full/10.1056/NEJM199406023302206 [ Links ]
3. Giacino JT, Ashwal ; S, Childs ; N, Cranford ; R, Jennett ; B, Katz ; D I. The Minimally Conscious State. Definition and diagnostic criteria. American Academy of Neurology. 2002; 58:349-53. [ Links ]
4. Laureys S, Berré J, Goldman S. Cerebral Function in Coma, Vegetative State, Minimally Conscious State, Locked-in Syndrome, and Brain Death. Yearbook of Intensive Care and Emergency Medicine 2001 [Internet]. 2001 [citado 26 de abril de 2023]; 386-96. Disponible en: https://doi.10.1007/978-3-642-59467-0_33 [ Links ]
5. Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol [Internet]. 2013 [citado 26 de abril de 2023]; 23(2):239-44. Disponible en: https://doi.10.1016/J.CONB.2012.12.003 [ Links ]
6. Laureys S, Faymonville ME, Degueldre C, Fiore G Del, Damas P, Lambermont B. Auditory processing in the vegetative state. Brain. 2000; 123:1589-601. [ Links ]
7. Laureys S, Faymonville ME, Peigneux P, Damas P, Lambermont B, Del Fiore G. Cortical Processing of Noxious Somatosensory Stimuli in the Persistent Vegetative State. Neuroimage. 2002; 17(2):732-41. [ Links ]
8. Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science (1979) [Internet]. 2006 [citado 28 de marzo de 2024]; 313(5792):1402. Disponible en: https://doi.10.1126/science.1130197 [ Links ]
9. Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L. Willful Modulation of Brain Activity in Disorders of Consciousness. New England Journal of Medicine [Internet]. 2010 [citado 28 de marzo de 2024]; 362(7):579-89. Disponible en: https://doi.10.1056/nejmoa0905370 [ Links ]
10. Peterson A, Owen AM, Karlawish J. Alive inside. Bioethics [Internet]. 2020 [citado 28 de marzo de 2024]; 34(3):295-305. Disponible en: https://doi.10.1111/bioe.12678 [ Links ]
11. Laureys S, Celesia GG, Cohadon F, Lavrijsen J, León-Carrión J, Sannita WG. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010 ;8. [ Links ]
12. Luppi AI, Cain J, Spindler LRB, Górska UJ, Toker D, Hudson AE. Mechanisms Underlying Disorders of Consciousness: Bridging Gaps to Move Toward an Integrated Translational Science. Neurocrit Care [Internet]. 2021 [citado 28 de marzo de 2024]; 35:37-54. Disponible en: https://doi.10.1007/s12028-021-01281-6 [ Links ]
13. Ramos KM, Grady C, Greely HT, Chiong W, Eberwine J, Farahany NA. The NIH BRAIN Initiative: Integrating Neuroethics and Neuroscience [Internet]. Neuron. Cell Press; 2019 [citado 28 de marzo de 2024]; 101:394-8. Disponible en: https://doi.10.1016/j.neuron.2019.01.024 [ Links ]
14. Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S. Practice Guideline Update Recommendations Summary: Disorders of Consciousness. Arch Phys Med Rehabil [Internet]. 2018 [citado 28 de marzo de 2024]; 99(9):1699-709. Disponible en: https://doi.10.1016/j.apmr.2018.07.001 [ Links ]
15. Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol [Internet]. 2020 [citado 28 de marzo de 2024]; 27(5):741-56. Disponible en: https://doi.10.1111/ene.14151 [ Links ]
16. Comanducci A, Boly M, Claassen J, De Lucia M, Gibson RM, Juan E. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group [Internet]. Clinical Neurophysiology. Elsevier Ireland Ltd; 2020 [citado 28 de marzo de 2024]; 131:2736-65. Disponible en: https://doi.10.1016/j.clinph.2020.07.015 [ Links ]
17. Pan J, Xie Q, Qin P, Chen Y, He Y, Huang H. Prognosis for patients with cognitive motor dissociation identified by brain-computer interface. Brain [Internet]. 2020 [citado 28 de marzo de 2024]; 143(3):1177-89. Disponible en: https://doi.10.1093/brain/awaa056 [ Links ]
18. Edlow BL, Claassen J, Suarez JI. Common data elements for disorders of consciousness. Neurocrit Care [Internet]. 2024 [citado 28 de marzo de 2024]. Disponible en: https://doi.10.1007/s12028-023-01931-x [ Links ]
19. IASP Revises Its Definition of Pain for the First Time Since 1979 [Internet]. [citado 26 de abril de 2024]. Disponible en: https://www.iasp-pain.org/wp-content/uploads/2022/04/revised-definition-flysheet_R2-1-1-1.pdf [ Links ]
20. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S. The Revised IASP definition of pain: concepts, challenges, and compromises HHS Public Access. Pain [Internet]. 2020 [citado 26 de abril de 2024]; 161(9):1976-82. Disponible en: https://www.iasp-pain.org/wp-content/uploads/2022/04/revised-definition-flysheet_R2-1-1-1.pdf [ Links ]
21. Zasler ND, Formisano R, Aloisi M. Pain in Persons with Disorders of Consciousness [Internet]. Brain Sciences. MDPI; 2022 [citado 23 de mayo de 2024]. Disponible en: http://10.3390/brainsci12030300 [ Links ]
22. Bueno-Gómez N. Conceptualizing suffering and pain. Philosophy, Ethics, and Humanities in Medicine [Internet]. 2017 [citado 10 de mayo de 2024]; 12(7):2-11. Disponible en: 10.1186/s13010-017-0049-5 [ Links ]
23. Scarponi F, Tinelli G. Disturbi di coscienza: approccio riabilitativo al dolore. Disorders of consciousness: Rehabilitative perspective in management of pain. Il Fisioterapista. 2018; 2:11-5. [ Links ]
24. Lukas A, Barber JB, Johnson P, Gibson SJ. Observer-rated pain assessment instruments improve both the detection of pain and the evaluation of pain intensity in people with dementia. European Journal of Pain [Internet]. 2013 [citado 9 de mayo de 2024]; 17(10):1558-68. Disponible en: https://onlinelibrary.wiley.com/doi/full/10.1002/j.1532-2149.2013.00336.x [ Links ]
25. Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: A state-of-the-science review. J Pain Symptom Manage [Internet]. 2006 [citado 9 de mayo de 2024]; 31(2):170-92. Disponible en: 10.1016/J.JPAINSYMMAN.2005.07.001 [ Links ]
26. Herr K. Pain assessment strategies in older patients. Journal of Pain [Internet]. 2011 [citado 9 de mayo de 2024]; 12(3 SUPPL.):S3. Disponible en: 10.1016/J.JPAIN.2010.11.011 [ Links ]
27. Bartolo M, Chiò A, Ferrari S, Tassorelli C, Tamburin S, Avenali M. Assessing and treating pain in movement disorders, amyotrophic lateral sclerosis, severe acquired brain injury, disorders of consciousness, dementia, oncology and neuroinfectivology: Evidence and recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. European Journal of Physical and Rehabilitation Medicine; 2016. [ Links ]
28. Schnakers C, Chatelle C, Vanhaudenhuyse A, Majerus S, Ledoux D, Boly M. The Nociception Coma Scale: A new tool to assess nociception in disorders of consciousness. Pain [Internet]. 2010; 148(2). Disponible en: https://journals.lww.com/pain/fulltext/2010/02000/the_nociception_coma_scale__a_new_tool_to_assess.9.aspx [ Links ]
29. Guldenmund P, Stender J, Heine L, Laureys S. Mindsight: Diagnostics in disorders of consciousness [Internet]. Vol. 2012, Critical Care Research and Practice. 2012 [citado 31 de mayo de 2024]. Disponible en: 10.1155/2012/624724 [ Links ]
30. Chatelle C, Thibaut A, Bruno MA, Boly M, Bernard C, Hustinx R. Nociception coma scale-revised scores correlate with metabolism in the anterior cingulate cortex. Neurorehabil Neural Repair [Internet]. 2014 [citado 30 de mayo de 2024]; 28(2):149-52. Disponible en: 10.1177/1545968313503220 [ Links ]
31. Sattin D, Schnakers C, Pagani M, Arenare F, Devalle G, Giunco F. Evidence of altered pressure pain thresholds in persons with disorders of consciousness as measured by the Nociception Coma Scale-Italian version. Neuropsychol Rehabil [Internet]. 2018 [citado 31 de mayo de 2024]; 28(8):1295-310. Disponible en: 10.1080/09602011.2017.1290532 [ Links ]
32. Bonin EAC, Lejeune N, Szymkowicz E, Bonhomme V, Martial C, Gosseries O. Assessment and management of pain/nociception in patients with disorders of consciousness or locked-in syndrome: A narrative review [Internet]. Frontiers in Systems Neuroscience. Frontiers Media. 2023 [citado 31 de mayo de 2024]. Disponible en: 10.3389/fnsys.2023.1112206 [ Links ]
33. Naro A, Russo M, Leo A, Rifici C, Pollicino P, Bramanti P. Cortical responsiveness to nociceptive stimuli in patients with chronic disorders of consciousness: Do c-fiber laser evoked potentials have a role? PLoS One [Internet]. 2015 [citado 1 de junio de 2024]; 10(12). Disponible en: 10.1371/journal.pone.0144713 [ Links ]
34. Calabrò RS, Pignolo L, Müller-Eising C, Naro A. Pain perception in disorder of consciousness: A scoping review on current knowledge, clinical applications, and future perspective [Internet]. Brain Sciences. MDPI AG; 2021 [citado 1 de junio de 2024]. Disponible en: 10.3390/brainsci11050665 [ Links ]
35. De Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev [Internet]. 2021 [citado 10 de mayo de 2024]; 130:125-46. Disponible en: 10.1016/j.neubiorev.2021.08.013 [ Links ]
36. Riganello F, Macrì S, Alleva E, Petrini C, Soddu A, Leòn-Carriòn J. Pain perception in unresponsive wakefulness syndrome may challenge the interruption of artificial nutrition and hydration: Neuroethics in action [Internet]. Vol. 7, Frontiers in Neurology. Frontiers Media. 2016 [citado 8 de junio de 2024]. Disponible en: 10.3389/fneur.2016.00202 [ Links ]
37. Pistoia F, Sacco S, Stewart J, Sarà M, Carolei A. Disorders of consciousness: Painless or painful conditions?-evidence from neuroimaging studies [Internet]. Brain Sciences. MDPI AG; 2016 [citado 5 de junio de 2024]. Disponible en: 10.3390/brainsci6040047 [ Links ]
38. Calabrò RS, Pignolo L, Müller-Eising C, Naro A. Pain perception in disorder of consciousness: A scoping review on current knowledge, clinical applications, and future perspective [Internet]. Brain Sciences. MDPI AG; 2021 [citado 31 de mayo de 2024]. Disponible en: 10.3390/brainsci11050665 [ Links ]
39. Chatelle C, Majerus S, Whyte J, Laureys S, Schnakers C. A sensitive scale to assess nociceptive pain in patients with disorders of consciousness. Journal of Neurology, Neurosurgery & Psychiatry [Internet]. 2012; 83(12):1233-7. Disponible en: https://dx.doi.org/10.1136/jnnp-2012-302987 [ Links ]
40. Cortese MD, Arcuri F, Nemirovsky IE, Lucca LF, Tonin P, Soddu A. Nociceptive Response Is a Possible Marker of Evolution in the Level of Consciousness in Unresponsive Wakefulness Syndrome Patients. Front Neurosci [Internet]. 2021 [citao 11 de junio de 2024]; 15. Disponible en: 10.3389/fnins.2021.771505 [ Links ]
41. Chatelle C, Hauger SL, Martial C, Becker F, Eifert B, Boering D. Assessment of Nociception and Pain in Participants in an Unresponsive or Minimally Conscious State After Acquired Brain Injury: The Relation Between the Coma Recovery Scale-Revised and the Nociception Coma Scale-Revised. Arch Phys Med Rehabil [Internet]. 2018 [citado 23 de mayo de 2024]; 99(9):1755-62. Disponible en: 10.1016/j.apmr.2018.03.009 [ Links ]
42. Formisano R, Contrada M, Aloisi M, Ferri G, Schiattone S, Iosa M. Neuropsychological Rehabilitation. 2020 [citado 11 de junio de 2024]: 1893-2904 Nociception Coma Scale with personalized painful stimulation versus standard stimulus in non-communicative patients with disorders of consciousness. Disponible en: 10.1080/09602011.2019.1614464 [ Links ]
43. Lee IS, Necka EA, Atlas LY. Distinguishing pain from nociception, salience, and arousal: How autonomic nervous system activity can improve neuroimaging tests of specificity. Neuroimage [Internet]. 2020 [citado 17 de junio de 2024]; 204:116254. Disponible en: 10.1016/J.NEUROIMAGE.2019.116254 [ Links ]
44. Riganello F, Tonin P, Soddu A. I Feel! Therefore, I Am from Pain to Consciousness in DOC Patients. Int J Mol Sci [Internet]. 2023 [citado 17 de junio de 2024]; 24(11825). Disponible en: 10.3390/ijms241411825 [ Links ]
45. Devalle G, Castiglioni P, Arienti C, Abbate C, Mazzucchi A, Agnello L. Cardio-respiratory autonomic responses to nociceptive stimuli in patients with disorders of consciousness. PLoS One [Internet]. 2018 [citado 3 de junio de 2024]; 13(9). Disponible en: 10.1371/journal.pone.0201921 [ Links ]
46. Calabrò RS, Naro A, Manuli A, Leo A, De Luca R, Lo Buono V. Pain perception in patients with chronic disorders of consciousness: What can limbic system tell us? Clinical Neurophysiology [Internet]. 2017 [citado 10 de junio de 2024]; 128(3):454-62. Disponible en: 10.1016/J.CLINPH.2016.12.011 [ Links ]
47. Boly M, Faymonville ME, Schnakers C, Peigneux P, Lambermont B, Phillips C. Perception of pain in the minimally conscious state with PET activation: an observational study. www.thelancet.com/neurology [Internet]. 2008 [citado 22 de junio de 2024]; 7:1013-20. Disponible en: http://www.thelancet.com/neurology [ Links ]
48. Markl A, Yu T, Vogel D, M€ Uller F, Kotchoubey B, Lang S. Brain processing of pain in patients with unresponsive wakefulness syndrome. Brain Behav [Internet]. 2013 [citado 22 de junio de 2024]; 3(2):95-103. Disponible en: 10.1016/S1474 [ Links ]
49. National Library of Medicine [Internet]. 2025 [citado 26 de marzo de 2025]. Disorder of Consciousness | Card Results | ClinicalTrials.gov. Disponible en: https://clinicaltrials.gov/search?cond=Disorder%20of%20Consciousness [ Links ]
50. Amadio J, Bi GQ, Boshears PF, Carter A, Devor A, Doya K. Neuroethics Questions to Guide Ethical Research in the International Brain Initiatives [Internet]. Vol. 100, Neuron. Cell Press; 2018 [citado 28 de marzo de 2024]:19-36. Disponible en: https://doi.10.1016/j.neuron.2018.09.021 [ Links ]
51. Young MJ, Bodien YG, Edlow BL. Ethical Considerations in Clinical Trials for Disorders of Consciousness. Brain Sci [Internet]. 2022 [citado 24 de marzo de 2024]; 12(2). Disponible en: 10.3390/brainsci12020211 [ Links ]
52. Young MJ, Bodien YG, Giacino JT, Fins JJ, Truog RD, Hochberg LR. The neuroethics of disorders of consciousness: A brief history of evolving ideas. Brain [Internet]. 2021 [citado 28 de marzo de 2024]; 144(11):3291-310. Disponible en: https://doi.10.1093/brain/awab290 [ Links ]
53. Peterson A, Mintz K, Owen AM. Unlocking the Voices of Patients with Severe Brain Injury. Neuroethics [Internet]. 2022 [citado 6 de abril de 2025]; 15(1). Disponible en: https:/doi.10.1007/s12152-022-09492-0 [ Links ]
54. Declaración de Helsinki de la AMM - Principios éticos para las investigaciones médicas con participantes humanos -WMA- The World Medical Association [Internet]. [citado 27 de marzo de 2025]. Disponible en: https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/?utm_source=chatgpt.com [ Links ]
55. Huerta-Chávez V, Rivera-Tello S, Ramos-Loyo J. Los Potenciales Relacionados a Eventos (PREs): una técnica para estudiar el funcionamiento del cerebro durante el procesamiento de información: Event-Related Potentials (ERPs): a technique to study brain functioning during information processing. e-CUCBA [Internet]. 2022 [citado 3 de abril de 2025]; (19):183-94. Disponible en: http://e-cucba.cucba.udg.mx/index.php/e-Cucba/article/view/278 [ Links ]
56. Alonso-Valerdi LM, Arreola-Villarruel MA, Argüello-García J. eBrain computer-interfaces: Conceptualization, redesign challenges and social impact. Revista Mexicana de Ingeniería Biomédica [Internet]. 2019 [citado 28 de marzo de 2024]; 40(3). Disponible en: https://doi.10.17488/RMIB.40.3.8 [ Links ]
1Neurophysiological study that analyzes sensory, motor, and cognitive processes. It captures the signal of brain electrical activity after an auditory, written, or visual stimulus to follow the course and dynamics of the neural processes involved in information processing.
2System that measures the activity of the central nervous system (CNS) and converts it into outputs that replace, restore, improve, and supplement natural outputs from the CNS.
CÓMO CITAR: Montiel Boehringer, Z. V. (2025). Pain Without Consciousness? Rethinking pain-suffering in Disorders of Consciousness. Medicina y ética, vol. 36, núm. 3. DOI: https://doi.org/10.36105/mye.2025v36n3.04
Received: February 17, 2025; Accepted: April 07, 2025











 texto en
texto en 


