1. Introduction
Zinc oxide (ZnO) is a widely used semiconducting material known for its ease of synthesis, cost-effectiveness, non-toxicity, transparency, and high electron mobility of 2000 cm²/(Vs) at 80 K. It typically crystallizes in the wurtzite phase and has a direct bandgap of 3.37 eV, with a high exciton binding energy of 60 meV, allowing for efficient excitonic emission at room temperature [1]. ZnO's various nanostructures, including films, nanowires, nanorods, and nanoparticles, are suitable for applications in sensors, detectors, and thin-film transistors, with nanostructured thin films particularly valuable for studying electrical, thermal, and optical properties [2]. The material's carrier transport behavior is influenced by light and other sensing materials, making it attractive for solar cells, luminescent devices, electrical and acoustic devices, and chemical sensors [3]. Al-doped ZnO thin films exhibit excellent optical and electrical properties, such as high electron mobility, uniformity, and transparency to visible light, positioning them as promising materials for next-generation flat panel displays [4]. These films also possess a broad sensing spectrum (200-300 nm), making them suitable for UV light applications, including solar UV radiation monitoring and ultra-high temperature flame detection, as well as potential use in transparent conducting oxide (TCO) electrodes and light-emitting diodes (LEDs) [5]. The material's optical absorption is linked to electron transitions from the valence band to the conduction band and defect levels, enhancing conductivity through the desorption of surface oxygen by photogenerated holes.
Additionally, Zhu et al. [6] developed a novel gas sensor with high response and selectivity using molecularly imprinted powders (MIPs). The sensor demonstrated excellent gas-sensing properties to methanol vapor, particularly with a methanol-to-methyl acrylic acid molar ratio of 1:4. At an optimal operating temperature of 130°C, the sensor showed a response of 41 to 1 ppm methanol, with response and recovery times of 40 seconds and 50 seconds, respectively. Mingzhi Jiao's research found that ZnO nanowires, synthesized at 90°C with low precursor concentration, show better nitrogen dioxide selectivity compared to other gases, with higher stability at 600°C [7]. Alaa's study on ZnO and Al-doped ZnO thin films revealed that increased Al doping reduced lattice parameters and bandgap energy [8]. Anandh discovered that Al doping alters ZnO thin films' structural and optical properties, increasing the bandgap up to 3% doping before it decreases [9] (Anandh et al., 2018). Aydın et al. [10] noted that Al doping in ZnO thin films enhances their suitability for ammonia gas detection. Kathwate's work demonstrated that Al doping decreases the bandgap of ZnO films and improves ammonia gas sensing [11]. Dubey found that higher Al doping in ZnO thin films enhances humidity sensor sensitivity [12]. Khojier showed that Al-doped ZnO thin films optimize formaldehyde sensitivity at 2 % Al [13]. Finally, Gulec reported that 20% Al-doped ZnO films exhibit superior photocatalytic performance post-annealing, despite their unique p-type characteristics [14].
The motivation for this research stems from the increasing demand for efficient, cost-effective, and environmentally friendly gas sensors, particularly for detecting hazardous gases like methanol vapor. Methanol is widely used in various industries but poses significant health and environmental risks due to its toxicity, making its detection critical. Al-doped ZnO thin films are promising materials for gas sensing applications due to their superior optical and electrical properties, including high transparency, tunable bandgap, and enhanced carrier mobility. Previous studies have demonstrated that Al doping improves ZnO’s sensitivity and selectivity for various gases, such as ammonia and formaldehyde, by altering its bandgap and structural properties. However, limited research exists on the optimization of Al-doped ZnO thin films specifically for methanol vapor detection. The purpose of this research is to investigate the optical properties of Al doped ZnO thin film and methanol sensing properties of Al-doped ZnO thin films with varying Al concentrations, focusing on their performance as methanol vapor sensors. This study's significance lies in its potential to contribute to the development of highly sensitive, low-cost, and reliable methanol sensors for industrial safety, environmental monitoring, and other applications requiring accurate detection of toxic gases.
2. Materials and methods
2.1. Synthesis of aluminum doped zinc oxide films
Aluminum-doped zinc oxide (Al-doped ZnO) films are highly sought after due to their multifunctional properties, including piezoelectric, electrical, optical, and thermal characteristics. These films are particularly useful in applications such as gas sensors, ultrasonic oscillators, and transparent electrodes in solar cells. The effectiveness of Al-doped ZnO films largely depends on their microstructure and surface nanochemistry. Aluminum doping results in a reduced material density and smaller grain size, enhancing the electrical conductivity, magnetic performance, and optical transparency of ZnO. Extrinsic doping with elements like aluminum, indium, gallium, copper, or cadmium is a common method to improve these properties, as it can induce defects in the ZnO lattice and widen the bandgap. Al-doped ZnO is especially valuable in the fabrication of optoelectronic devices, heterojunctions, superlattices, and detectors. The sol-gel method, known for its low cost and ease of composition control, is frequently used to synthesize these films, offering benefits such as precise size control, low processing temperatures, and the production of cost-effective semiconducting materials.
2.2. Substrates Cleaning
Before depositing the films, the glass slides were cleaned with diluted HCl, boiled in acetone, and then dried completely using a dryer and cotton. Once fully dry, the slides were prepared for coating.
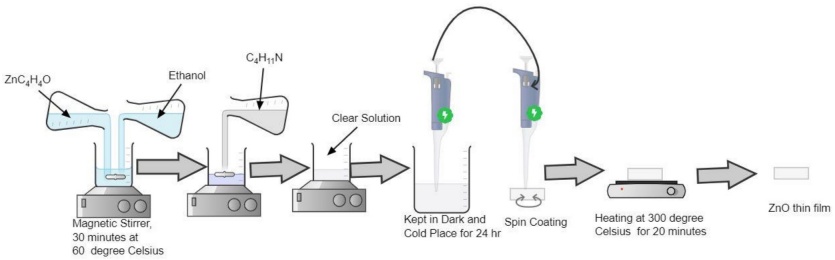
2.3. Preparation of ZnO thin films
To prepare the undoped ZnO precursor solution, 2.74 grams of zinc acetate (ZnC4H6O4) were dissolved in 25 ml of propanol. This mixture was then stirred at 60°C for 30 minutes using a magnetic stirrer, resulting in a curdy mixture. Diethylamine (C4H11N) was then added drop by drop while continuing to stir until a clear, water-like solution was formed. The prepared solution was stored in a cool, dark place for 24 hours before use. The spin coating technique was employed to deposit thin films, as thin as 10 nm, onto flat substrates. This method involves placing a liquid on a rotating substrate, with the material deposited at the center either manually or robotically. The uniformity of the film depends on the balance between centrifugal and viscous forces, which are influenced by the spin speed, solution viscosity, and spinning time. Typically used for transparent oxide thin films, this method ensures the production of uniform and repeatable films. To achieve consistent deposition, the spin speed and time (usually 30-45 seconds) were kept constant. For the preparation of Al-doped ZnO, 3.0178 grams of aluminum tetrachloride (AlCl3) were mixed with 25 ml of the previously prepared ZnO solution to achieve a 0.5M concentration. Five different Al-doped ZnO samples with varying concentrations (0.5%, 1%, 1.5%, 2%, and 2.5%) were prepared using the spin coating technique. During this process, the slides were placed in the spinner, and the solution of zinc acetate and propanol was added dropwise (2 drops per cycle). After each coating, the slides were heated to 300°C. This process was repeated for 10 to 15 coats which is equivalent to 0.001 mm to 0.009 mm thickness the film's resistance fell within the kΩ range.
2.4. Fabrication of methanol gas sensor and gas sensing setup
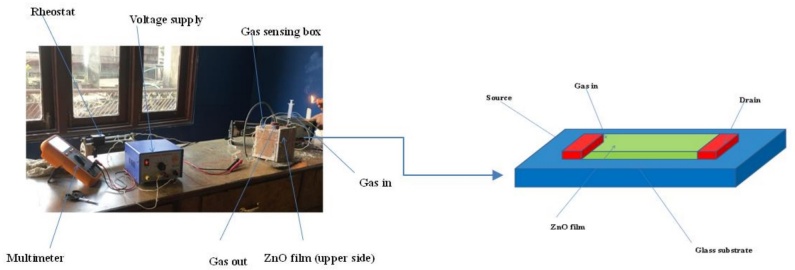
A cubical aluminum box with each side measuring 10 mm was constructed, featuring three air-tight holes sealed with rubber corks. These holes served different purposes: one for the methanol gas inlet, another for the outlet, and the third for the wire connections. The ZnO sample was placed on the upper side of the box, and a uniform heat source was applied consistently. The experiment involved varying the internal temperature of the box across three levels. Initially, the temperature was set to 60°C, and varying volumes of methanol (100µl, 200µl, 300µl, and 400µl) were introduced using a micropipette. The procedure was repeated at 80°C and 100°C, with the same methanol volumes being tested at each temperature. The experimental setup for gas sensing is depicted in Figure 2.
The heat inside the box facilitated the vaporization of the methanol. A rheostat was used to adjust the resistance, and the voltage supply was managed to maintain the desired temperature. Together, these components allowed precise control over the temperature within the box. The setup featured three holes on the ceiling of the box, all sealed with rubber corks. One cork served as the inlet for the gas, introduced via a syringe, while another cork was left open as the outlet. The third hole accommodated the connections for the multimeter, rheostat, and voltage supply. Additionally, a heater was positioned at the bottom of the box, with the ZnO film placed on the ceiling.
2.5. Mechanism of Conductivity
The mechanism of conductivity in Al-doped ZnO thin films is primarily influenced by the incorporation of aluminum ions into the ZnO crystal lattice, which alters its electrical properties. ZnO is an intrinsic n-type semiconductor, primarily due to oxygen vacancies and zinc interstitials that provide free electrons. When aluminum is doped into ZnO, the Al³⁺ ions, having a smaller ionic radius and higher valency than Zn²⁺ ions, replace some Zn²⁺ in the lattice. This substitution creates extra free electrons because Al³⁺ donates one more electron than Zn²⁺, thereby enhancing the electron carrier concentration, which leads to an increase in electrical conductivity. Al-doped ZnO composites exhibit improved charge storage behavior due to their enhanced carrier mobility and increased electron density. The higher concentration of free electrons in Al-doped ZnO enables greater polarization under an external electric field, which enhances the dielectric properties and thus the material's ability to store charge. This is especially relevant in dynamic electrical applications, such as capacitors and sensors, where the charge storage capacity plays a crucial role.
2.6. Characterization of ZnO films
UV-Visible spectroscopy is used to analyze materials by measuring their absorbance, reflectance, or transmittance of light in the UV to visible range, where electronic transitions occur in molecules. According to the Beer-Lambert law, absorbance is directly proportional to both the concentration of the absorbing species and the path length. This method is useful for determining concentration and calculating the optical band gap of thin films [15].
A represents the measured absorbance, I0 is the intensity of the incident light, I is the transmitted intensity, L denotes the path length through the sample, and c is the concentration of the absorbing species. Additionally, the band gap of a thin film can be calculated using UV-Visible spectroscopy:
The optical band gap (Egap) of a semiconductor can be determined using UV-Visible spectroscopy. Here, hν represents the incident photon energy, B is a constant, and m is 0.5 for direct and 1 for indirect band gaps. The absorption coefficient (α(ν)) is calculated using Beer-Lambert's law: α(ν)= (2.303×Abs(λ))/d, where Abs(λ) is the absorbance and d is the film thickness. When light interacts with a semiconductor, it can be absorbed, transmitted, or reflected. The energy required for electron transitions between the valence and conduction bands varies with different materials. In direct optical absorption, the conduction and valence band edges align at the same wave vector (𝑘k), allowing electrons to transition directly from the valence band to the conduction band without a change in momentum when hν>Eg [16-17].
3. Result and discussion
3.1. Optical properties
Transmittance, which measures the fraction of incident light passing through a substance, was analyzed for ZnO films using a UV-visible spectrophotometer. This analysis compared the optical transmittance of undoped ZnO films with those doped with varying concentrations of aluminum. The thickness of the films was kept constant (0.001 mm) to accurately determine the absorption coefficient and evaluate the optical properties. The transmittance of 1% Al-doped ZnO films was found to be 91%, indicating a high level of optical clarity on figure 3a. This high transmittance suggests that the 1% Al doping enhances the crystalline quality of the films, making them suitable for applications such as solar cell electrodes. The transmittance spectra for these films displayed interference fringes, which are indicative of high surface quality. These fringes result from reflections at the film surface with minimal absorption and scattering in the bulk, further confirming the excellent quality of the 1% Al-doped ZnO films [18].
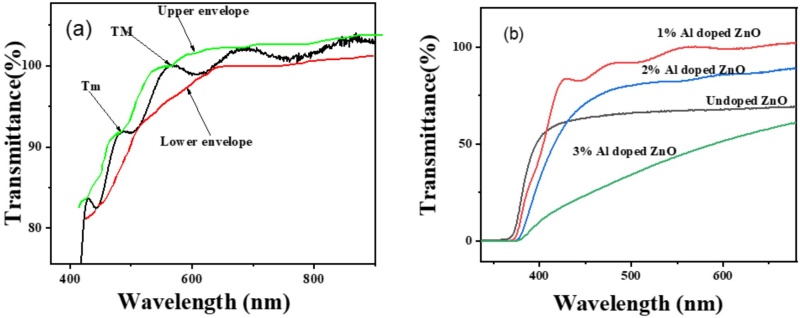
Figure 3 (a) Transmittance with 1% Al doped for TM(Tmax) and Tm (Tmin), and (b) transmittance of undoped, 1%, 2%, 3% Al doped ZnO.
In contrast, the 3% Al-doped ZnO films exhibited a much lower transmittance of 11%, which increased to 60% with varying methanol volumes. This significant reduction in transmittance is attributed to the increased doping concentration, which introduces higher charge density and consequently enhances photon absorption. The presence of defect sites from higher doping levels leads to increased light scattering, which degrades the optical performance of the films as shown in figure 3b. This scattering effect is evident from the lower transmittance values observed for the 3% Al-doped ZnO films [18]. The transmittance of 2% Al-doped ZnO films was recorded at 74%. This value is intermediate between the 1% and 3% doping levels, reflecting a balance between improved crystalline quality and the introduction of defect sites. The decrease in transmittance compared to the 1% doping level indicates that while some benefits of doping are retained, further increasing the concentration introduces more defects, leading to increased light scattering and reduced transmittance [19]. Undoped ZnO films exhibited a transmittance of 62%, which is lower than that of 1% Al-doped ZnO but higher than that of 3% Al-doped ZnO shown in figure 3. This suggests that while undoped ZnO films have relatively lower optical clarity compared to the 1% Al-doped films, they perform better than the higher doping concentrations in terms of light transmission.
Figure 4a illustrates the variation in the direct bandgap of ZnO films with increasing Al doping concentration. The bandgap of undoped ZnO is measured at 3.3 eV. For 1% Al-doped ZnO, the bandgap decreases to 3.25 eV. Further increasing the Al concentration to 2% and 3% results in bandgaps of 3.2 eV and 3.15 eV, respectively. This decrease in bandgap with higher doping concentrations can be attributed to the interactions between the dopant and the host material. The reduction in bandgap with increased Al doping is explained by the incorporation of Al3+ ions, which introduce additional electronic states within the bandgap. Specifically, the 3d levels of the Al3+ ions interact with the sp-electrons of the ZnO lattice, resulting in strong sp-d exchange interactions. This interaction modifies the electronic structure of ZnO, leading to a narrowing of the bandgap [20]. As shown in Figure 4b, the bandgap reduction is linearly related to the Al concentration, indicating a consistent impact of doping on the electronic properties of the ZnO films.
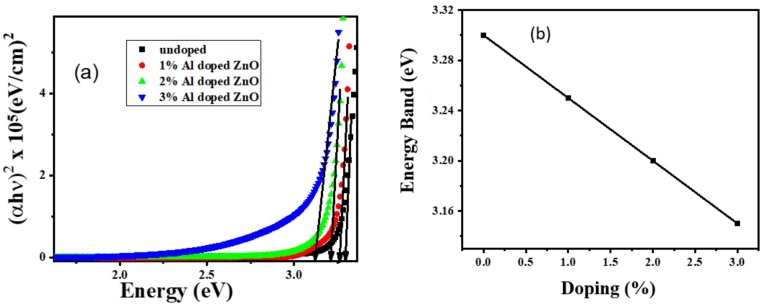
Figure 4 (a) Comparison bandgaps of undoped, 1%, 2%, and 3% Al-doped ZnO and (b) Band gap vs doping concentration
A narrower bandgap generally enhances electrical conductivity, as it reduces the energy required for electron excitation from the valence band to the conduction band. Therefore, the observed decrease in bandgap with increased Al doping suggests that the electrical conductivity of the ZnO films is likely to improve with higher doping concentrations. The improved conductivity is beneficial for applications requiring efficient charge transport, such as in transparent electrodes and optoelectronic devices.
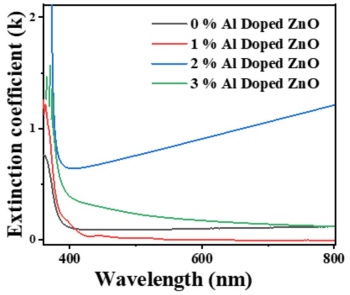
Figure 5 presents the extinction coefficient for ZnO films with varying Al doping concentrations. Notably, the 2% Al-doped ZnO sample exhibits a higher extinction coefficient compared to the 1% Al-doped ZnO sample. The extinction coefficient is a measure of how much light is absorbed and scattered by a material, influencing its dielectric loss. A higher extinction coefficient indicates greater light absorption and scattering. The extinction coefficient trends observed in the samples correlate with their doping levels. For the 2% Al-doped ZnO, the extinction coefficient initially decreases and then increases with wavelength. This behavior suggests that the optical properties of the film are significantly influenced by the doping concentration. Conversely, undoped, 1%, and 3% Al-doped ZnO films show a similar extinction coefficient pattern, indicating a more stable relationship between wavelength and extinction coefficient within these samples.
The general trend for all samples shows that the extinction coefficient decreases with increasing wavelength. This decrease is attributed to the scattering of light, which reduces absorbance. As the wavelength of light increases, the absorption decreases, leading to a lower extinction coefficient. This trend is consistent across the undoped, 1%, and 3% Al-doped ZnO films. The increase in the extinction coefficient for higher Al doping levels, specifically for the 2% Al-doped ZnO, is due to reduced light scattering and increased absorbance. With more Al doping, the ZnO film's microstructure becomes more conducive to absorbing light, leading to higher absorbance and, consequently, a higher extinction coefficient. This improvement in absorbance is linked to the enhanced electronic interactions introduced by the Al dopants, which modify the optical properties of the ZnO film.
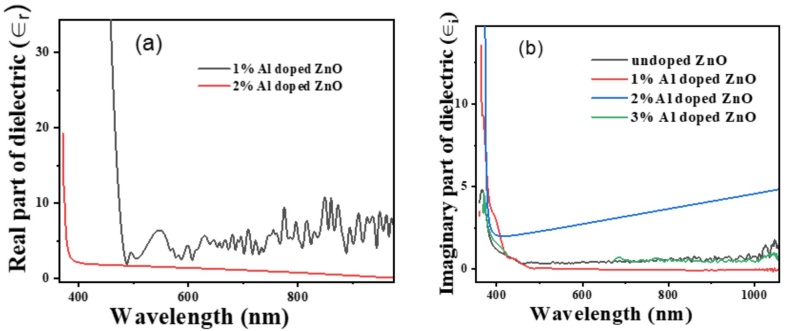
Figures 6a and 6b illustrate the real and
imaginary parts of the dielectric functions for ZnO films with varying Al doping
concentrations. The dielectric functions provide crucial insights into the
optical properties of the films, with the real part
3.2. Electrical properties
The gas sensing mechanism in Al-doped ZnO thin films is primarily based on surface sensing, where the interaction between the target gas (methanol vapor) and the film's surface leads to changes in electrical properties. When exposed to air, oxygen molecules adsorb onto the ZnO surface, trapping free electrons from the conduction band and forming negatively charged oxygen species (O2⁻, O⁻). This creates a depletion layer near the surface, reducing the film's conductivity.
When methanol vapor is introduced, it reacts with these oxygen species, releasing the trapped electrons back into the conduction band, thereby reducing the depletion layer and increasing conductivity. The extent of this change in conductivity depends on the concentration of methanol vapor and the efficiency of electron transfer. Al-doping enhances this surface interaction by increasing the electron density, which boosts the sensor's sensitivity, allowing it to detect lower concentrations of methanol with faster response and recovery times. This surface reaction mechanism is central to the gas sensing properties of Al-doped ZnO thin films.
The I-V curve illustrates the relationship between the applied voltage and the resulting current in ZnO films. As depicted in Figure 7a, there is a clear linear relationship where an increase in voltage corresponds to an increase in current. This behavior is typical of conductive materials, where the current is directly proportional to the applied voltage, following Ohm's Law. However, the magnitude of the current varies depending on the doping concentration. The undoped ZnO film exhibits a high current of 431 µA at elevated voltages. This high current is attributed to the specific dimensions of the sample, which can affect the current measurement. Undoped ZnO generally has lower resistivity due to the absence of doping-induced defects that would otherwise impede charge carrier movement. In contrast, the 2.5% Al-doped ZnO film shows a significantly lower current of 17 µA. This reduction is likely due to the increased defect density and potential scattering of charge carriers at higher doping concentrations, which impedes carrier mobility.
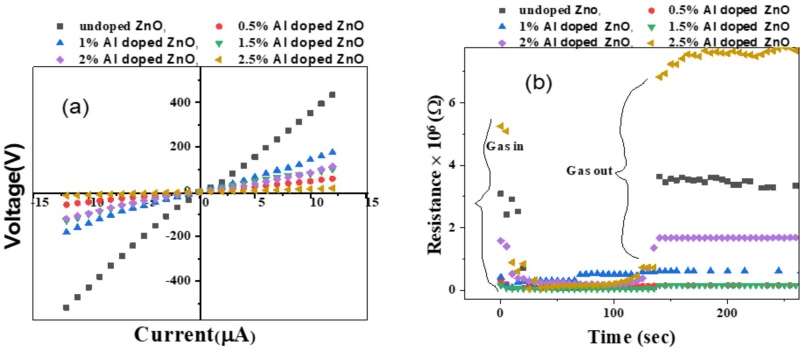
Figure 7 (a) Current vs Voltage and (b) Comparison of resistance of undoped, 0.5%Al, 1%Al, 1.5%Al, 2%Al, and 2.5%Al doped ZnO vs time at 100μ l of serum at 60 oC
The current increases from 69 µA at 0.5% Al doping to 121 µA at 1% Al doping. This increase is due to the enhanced carrier concentration resulting from Al doping. Aluminum introduces extra electrons into the ZnO lattice, which increases electrical conductivity by providing additional charge carriers. Carrier Concentration: The rise in current with moderate Al doping (0.5% to 1%) indicates improved carrier concentration and enhanced electrical conductivity. As Al concentration increases, more electrons are introduced into the ZnO lattice, enhancing carrier mobility and conductivity. At higher doping levels (e.g., 2.5% Al), the observed decrease in current can be attributed to increased defect density. Excessive doping creates a higher number of scattering centers and traps, which can hinder carrier mobility and reduce the overall current. This phenomenon reflects a common trade-off where initial increases in carrier concentration improve conductivity, but excessive doping can lead to diminished performance due to defect-related scattering and carrier recombination [22].
Figure 7b shows the resistance of ZnO films with varying Al concentrations over time. The resistance initially decreases when gas is introduced, stabilizes for approximately 130 seconds, and then gradually returns to its original value within 100 seconds. Upon gas introduction, resistance drops, indicating a quick reaction to the gas exposure. This decrease is likely due to the interaction between the gas and the ZnO film, which alters the carrier concentration or surface properties temporarily. After the initial drop, the resistance stabilizes and eventually increases back to the original value. This behavior suggests that the gas exposure causes a transient change in carrier dynamics or surface chemistry, which later returns to equilibrium. The resistance of ZnO films decreases with higher Al doping concentrations. This is because high doping concentrations lead to increased defect density by trapping or scattering electrons which lead to higher resistance over time [23].
Figure 8 shows that sensitivity to methanol vapor increases with Al doping concentration in ZnO films, with 2.5% Al-doped ZnO exhibiting the highest sensitivity and 0.5% Al-doped ZnO the lowest. Higher doping concentrations introduce more free electrons into the ZnO lattice, which enhances the film's response to methanol vapor. The presence of methanol, a reducing agent, decreases the resistance of the film by reducing oxygen species at the surface and grain boundaries, leading to increased sensitivity. Specifically, the 2.5% Al-doped ZnO film shows a significant reduction in resistance due to a higher concentration of free electrons and more active reaction sites, whereas the 0.5% Al-doped ZnO film, with fewer free electrons and reaction sites, demonstrates lower sensitivity. Thus, optimizing Al doping levels improves the sensitivity of ZnO films for gas detection applications. Also, Al-doped ZnO thin films offer high sensitivity, fast response, and recovery times, making them ideal for developing efficient, low-cost sensors for real-time methanol vapor monitoring. This aligns with the need for accurate, portable sensors in industrial and environmental applications, ensuring safe handling and compliance with regulations.
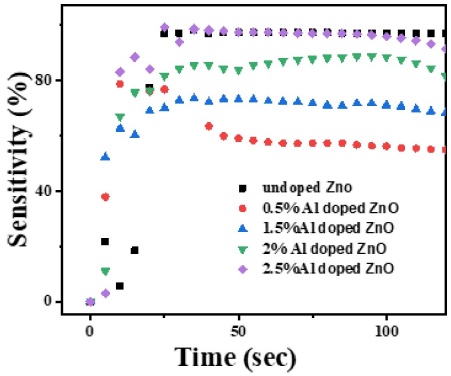
Figure 8 Comparisons of sensitivity of undoped, 0.5% Al, 1.5% Al, 2% Al, 2.5% Al-doped ZnO vs Time at 100 μ l and 60 oC.
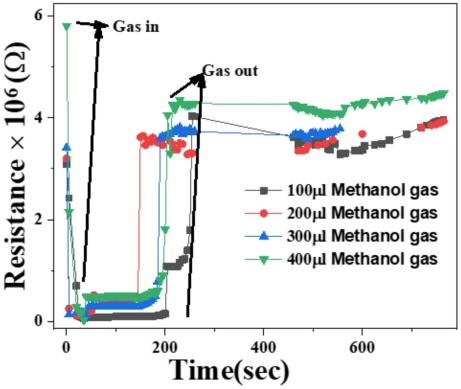
Figure 9 illustrates the resistance response of ZnO films to various volumes of methanol vapor. The graph shows that resistance decreases rapidly upon introducing methanol vapor, with the greatest reduction occurring in the first few seconds. This decrease in resistance stabilizes around 180 seconds before starting to rise as the methanol vapor is removed. Notably, the 200 µL methanol volume exhibits a more pronounced early increase in resistance compared to other volumes. Specifically, the resistance drops from 5.8 Ω to 1.4 Ω for 400 µL, from 3.4 Ω to 1.31 Ω for 300 µL, from 3.1 Ω to 1.0 Ω for 200 µL, and from 3.5 Ω to 4.0 Ω for 100 µL. The observed trend across all volumes reflects a similar pattern of resistance change. This resistance decrease is attributed to the chemisorption of methanol molecules on the ZnO surface, leading to the formation of O₂⁻ ions, which act as electron acceptors. This process increases the electron carrier concentration near the surface, enhancing conduction and reducing resistance [23].
Figure 10a presents the sensitivity of undoped ZnO films to methanol vapor at 60 °C across different methanol volumes (100, 200, 300, and 400 µL). Initially, sensitivity increases rapidly within the first 50 seconds, remains stable for approximately 100 seconds, and then begins to decrease. This behavior is attributed to the high initial concentration of methanol vapor, which increases the number of reducing molecules, thereby enhancing the reduction of oxygen at the grain boundaries of the ZnO film. This reduction leads to a significant drop in resistance, resulting in higher sensitivity. As methanol molecules evaporate over time, their concentration decreases, leading to fewer oxygen molecules being reduced and causing the resistance to rise. Consequently, the sensitivity decreases as the methanol concentration diminishes. Among the different volumes tested, 100 µL methanol exhibits the highest sensitivity at 97%, indicating that lower methanol concentrations yield better sensitivity in this setup.
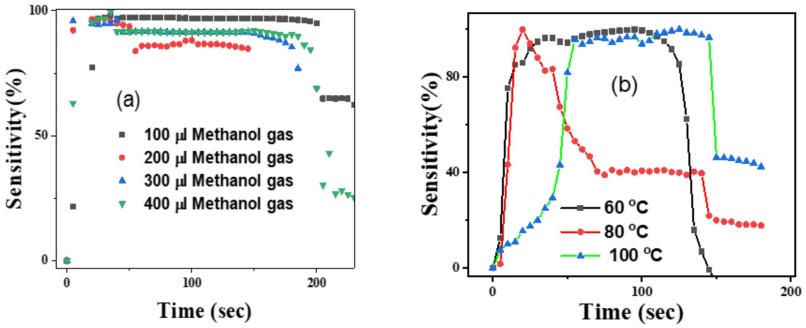
Figure 10 (a) Sensitivity of undoped ZnO with 100, 200, 300, 400 µl methanol gas at 600°C temperature vs time and (b)Sensitivity of 2% Al-doped ZnO of 100 µl methanol gas at 60°C, 80°C and 100°C temperature vs time.
Figure 10b explores the sensitivity of 2% Al-doped ZnO films to 100 µL methanol vapor at different temperatures: 60 °C, 80°C, and 100 °C. At both 60 °C and 100 °C, sensitivity initially increases rapidly, remains stable for a brief period, and then starts to decline. This pattern suggests that the sensor responds well to methanol vapor at these temperatures, with a consistent initial response followed by a gradual decrease as the methanol vapor disperses. Conversely, at 80 °C, sensitivity peaks abruptly and then drop sharply without maintaining a stable phase. This sudden decline may be due to the higher temperature accelerating the evaporation rate of methanol and possibly affecting the adsorption dynamics on the ZnO surface, leading to a less stable sensitivity response.
4. Conclusion
The results demonstrate that doping ZnO with aluminum significantly impacts its optical and electrical properties. The optimal doping concentration for maintaining high optical transmittance and improving electrical conductivity is 1%. Higher doping concentrations, while enhancing sensitivity to methanol vapor, introduce more defect sites and reduce optical clarity. Specifically, 2% Al-doped ZnO films offer a good balance between high sensitivity and reasonable optical properties. Sensitivity to methanol vapor is maximized with lower methanol concentrations (100 µL) and at specific temperatures (60 °C and 100 °C), indicating that fine-tuning doping levels and operational conditions can optimize ZnO-based sensors for various applications.











 nueva página del texto (beta)
nueva página del texto (beta)