Study contribution
This scoping review aims to map the evidence on treatments used during Bovine in vitro embryo production. The majority of related publications have focused on the oocyte maturation process, where hormones, growth factors, and FBS administered at the proper concentrations improved such process. Likewise, hormones, gonadotropins, growth factors, sera, and reproductive fluids applied at the right measured during embryo culture improved the yield and quality of embryos produced in vitro. The information presented here can be used to classify the knowledge on the topic and direct future research.
Introduction
In cattle, assisted reproductive techniques rely heavily on embryo production. In vivo embryo production has been a well-established technique for several decades, allowing for up to 80 % of commercialized embryos to be obtained worldwide.1 However, in recent years, in vitro embryo production has become an alternative system for producing Bovine embryos from immature oocytes due to its advantages and flexibility.2 Although in vivo embryo production is considered the gold standard due to the higher quality and yield of embryos produced, in recent years, the number of in vivo-produced embryos has stabilized worldwide. Meanwhile, the transfer of in vitro-produced embryos has continued to increase at a rate of 12 % per year. According to the International Embryo Transfer Society, in 2016, the number of transferable in vitro-produced embryos surpassed the number of in vivo-produced embryos (http://www.iets.org/comm_data.asp). This trend demonstrates a shift toward in vitro embryo production, which may also be combined with the tendency toward more efficient in vitro procedures.3
In vitro production of embryos is replacing traditional multiple ovulation embryo transfer.4 However, one of the main challenges of in vitro techniques is the reduced rate of embryo development to the blastocyst stage.5 Embryos produced through in vitro culture systems often have lower quality than those produced through in vivo culture systems, resulting in lower pregnancy rates for in vitro-produced blastocysts compared to in vivo-produced blastocysts.6 The primary objective for veterinarians and embryologists is to identify and select Bovine embryos with the highest developmental potential for transfer.7
To achieve this, the International Embryo Transfer Society recommends assessing embryo quality using metrics such as blastomere symmetry, fragmentation, thickness of the zona pellucida, and the rate of cleavage.8 Embryo quality is classified using a numerical code based on the morphological integrity of the embryo: Code 1 represents excellent or good quality, with symmetrical embryos having a spherical mass with uniform blastomeres; Code 2 represents fair quality, with embryos having moderate irregularities in shape; Code 3 represents poor quality, with embryos having major irregularities; and Code 4 represents dead or degenerating embryos.9
The production of embryos involves crucial steps such as oocyte maturation (meiotic, cytoplasmic, and molecular), fertilization, and embryo development, which significantly affect embryo survival.10 Therefore, the culture system used during the oocyte maturation and embryo culture stages has a significant impact on the development and quality of the blastocysts produced.11 Despite the importance of culture conditions during the different stages of embryo production, there is still no consensus on the optimal medium for Bovine oocyte maturation or the optimal culture conditions and characteristics after fertilization for embryo development.12
The quality of the oocyte is influenced by the ovarian follicle, which in turn determines the quality of the embryo during in vitro production. Therefore, to comprehend and assess the various factors that influence the developmental potential of in vitro-produced Bovine embryos, it is essential to have a detailed understanding of the three main processes that significantly impact oocyte quality: oocyte developmental competence (including meiosis resumption, cleavage after fertilization, blastocyst development, pregnancy to term, and production of healthy offspring), oocyte maturation, and follicular differentiation.13 However, in vitro conditions for oocyte development have not yet been improved to produce consistent blastocyst rates above 40 %. Therefore, one of the main challenges in oocyte maturation is to mimic the ovarian follicle environment to increase the success of Bovine blastocysts production.11
Similarly, in vitro culture conditions for embryo development are inferior compared to those in vivo. In consequence, both developmental competence and embryo quality are often compromised.12 As a result, there has been a substantial increase in primary research over the last two decades focused on obtaining additional knowledge regarding culture conditions during oocyte maturation and embryo development. An extensive and diverse body of scientific literature has been produced, making it challenging to acquire current knowledge to aid evidence-based decision making. Therefore, we conducted a scoping review to systematically map and summarize published primary studies that investigate the impact of treatments on the embryo production process, specifically during oocyte maturation and embryo development.
The purpose of this research was to conduct a scoping review to answer two questions related to Bovine in vitro embryo production: 1) What is the body of evidence on the characteristics and types of treatments used during Bovine in vitro embryo production?, and 2) What are the effects of hormones, growth factors, serum, and reproductive fluids on in vitro oocyte maturation and embryo culture in bovines? To answer the first question, we did not provide a summary of the publication’s results. Instead, we mapped the research to classify it into defined categories, which allowed us to understand its nature and extent.14
To address the second question, we qualitatively described and summarized the findings of the selected sources of evidence to disseminate the main results to technical experts and decision-makers. Scoping reviews are a contemporary and innovative method for synthesizing evidence on various veterinary topics.15,16) They utilize rigorous and explicit methodologies to address research questions that aim to identify key concepts and types of evidence.17 Scoping reviews employ a systematic approach to identify, select and synthesize the most relevant sources of evidence,14 which allows summarizing findings from a body of evidence of a heterogeneous nature, both in methods and approaches.18
The evidence presented in this study may serve as a guide for mapping and categorizing the diversity and extension of scientific research assessing Bovine in vitro embryo production and for understanding, in a broad sense, the main effects of some of the treatments used during such process.
Methods
Protocol
The research followed a protocol developed a priori in accordance with the PRISMA-P statement (Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocol).19 The protocol is available upon reasonable request to the corresponding author. Additionally, the study was reported in compliance with the PRISMA-ScR extension for scoping reviews.18
Eligibility criteria
The Population, Intervention, Outcomes, Study (PIOS) approach of the PRISMA-ScR statement was chosen to define the inclusion criteria due to the nature of the research,18 and Table 1 provides a summary of the definitions of each criterion. To find the most recent literature on Bovine in vitro embryo production, we considered primary research published in English, Portuguese, or Spanish from 2000 to 2021. The research focused on factors affecting and regulating culture conditions during oocyte maturation and embryo development. Only primary research available in full text was considered. To ensure a sufficient level of methodological rigor, we excluded gray literature such as theses, association or governmental reports, unpublished studies, or abstracts presented at congresses.20
Table 1 The eligibility criteria according to the PIOS approach of the PRISMA statement
Information sources and search strategy
A three-step approach was utilized to search for potential studies related to the topic. Firstly, two reviewers defined a broad and varied collection of search terms (thesaurus). Secondly, the reviewers conducted pilot searches using combinations of these terms. From these searches, they chose the final search terms based on their yield of records related to the topic. Finally, a single reviewer conducted comprehensive searches in the following electronic databases using the final search terms: PubMed, Scopus, Virtual Health Library (VHL), Redalyc, ScienceDirect, Web of Science, and CAB abstracts.
To conduct the final searches, a reviewer utilized a search command that incorporated the following term groups in combination with Boolean operators (AND and OR): 1) Bovine OR cow OR cattle OR calf, 2) embryo OR superovulation OR blastocyst OR oocyte OR cumulus oophorus complex OR in vitro, and 3) quality OR quantity OR transferable OR number OR fertility OR fecundation OR conception OR gestation. Additionally, methodological filters were applied to each electronic database, and searches were restricted to titles and abstracts. Table S-1 displays the search commands utilized in each database. The searches were conducted between October 3rd and 10th, 2021. Once the search process was completed, the study records were downloaded and added to an EndNote X9 library (Clarivate, USA).
Selection of the evidence sources
The process of selecting studies for the narrative synthesis involved the following steps: duplicate records from the library were removed both automatically and manually by one reviewer; the same reviewer then applied the inclusion criteria to the titles and abstracts to eliminate any records that were not relevant to the topic; full-text articles of the selected records were retrieved for the eligibility process; finally, a single reviewer performed the final eligibility process using a pilot-tested standardized format based on the inclusion criteria. The main reason for exclusion was defined in each of the excluded studies.
Process for creating data tables and graphs
Two reviewers collaborated to identify the variables to be extracted and created an Excel database to generate the data tables and graphs. The format was evaluated independently by both reviewers, who standardized the extraction format in 5% of randomly selected articles to ensure that all relevant information was extracted. Once the questionnaire was refined, it was applied to the remaining studies to complete the process. The information from each individual study was extracted by a single reviewer and organized into data tables and graphs. A second reviewer then verified all extracted information, and any discrepancies were discussed and resolved. The process of creating the data tables was iterative.
Extraction of individual data
To achieve the initial objective of the research, which is to map the sources of evidence, we extracted the following information from each of the included articles: a description of the general characteristics of the study, including the author, year of publication, and country where the study was performed; a description of the source of the biological material, including the breed and age of the females, the origin of the specimen, and the method for collecting the biological material; and a description of the type of intervention, including the category of intervention (culture conditions, medium composition, bioactive supplements, or other additives), the treatment regimen (dose, concentration, group, comparison, and so on), the time of treatment (oocyte maturation or embryo development), and the main outcomes evaluated.
To cover the effects of hormones, growth factors, sera, and reproductive fluids during oocyte maturation and embryo culture, we extracted the following data from each article: 1) the study’s objective, including a brief description of its main goal; 2) a methodological description of the study, including a brief overview of the research methods and techniques used; and 3) the main findings, including a qualitative summary of the principal results.
Synthesis of results and visualization of the body of evidence
To map the research, the studies were grouped into four treatment categories: A) culture conditions, B) medium composition, C) bioactive supplements, and D) other additives. A general summary of the body of evidence is presented based on the characteristics of all the studies. Then, each category and its studies are described individually. Additionally, visual aids such as descriptive graphs, maps, and Sankey diagrams were created to accompany the narrative synthesis of the individual studies. To summarize the effects of hormones, growth factors, and sera or reproductive fluids during maturation, summary tables were constructed. Except for the Sankey diagrams (https://sankeymatic.com) and the geographic map (Excel), all the graphs were constructed with Prism 10 (GraphPad, Inc., USA).
Results and discussion
Selection process of the sources of evidence
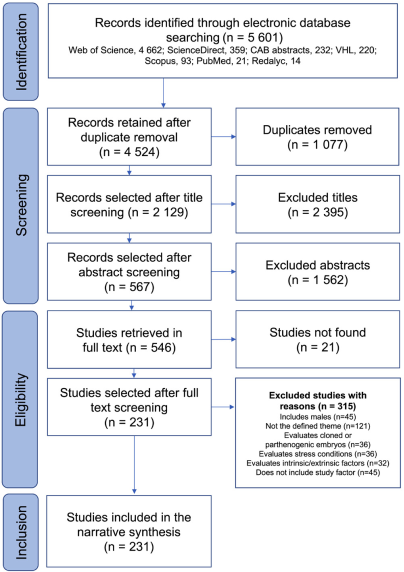
Figure 1 depicts the process of selecting studies for the narrative synthesis. A total of 5 601 study records were identified by searching electronic databases. The Web of Science contributed the most (83.2 %, 4 662 studies), while PubMed and Redalyc contributed the least (21 and 14, respectively). After automatic and manual removal of duplicates, 4 524 records remained for selection. During the title review, 2 129 unrelated titles were excluded. After reviewing the abstracts, 567 studies were selected for full-text retrieval, of which only 546 were retrieved for the full-text eligibility process. Finally, after applying the eligibility criteria, 315 studies were excluded because they did not meet the inclusion criteria. The reasons for exclusion are summarized in Figure 1. A total of 231 studies (4.1 %) were ultimately identified and included. The complete list of studies included in the review grouped by treatment category and subcategory is presented in Table S-2.
General characteristics of the sources of evidence
Among the 231 studies identified, 133 were accumulated in the last decade between 2012 and 2021. According to Figure 2A the number of publications per year increased gradually, with the peak in 2017 (21 studies). These results indicate a growing interest in this topic in recent years. Based on the origin of the studies, the continents that contributed the highest number of publications were the Americas, Asia, and Europe with 85, 71, and 71 publications, respectively. Australia contributed the least with only four studies (Figure 2B). Brazil, the United States of America, and Argentina had the highest number of contributions in the American continent with 34, 20, and 14 studies, respectively.
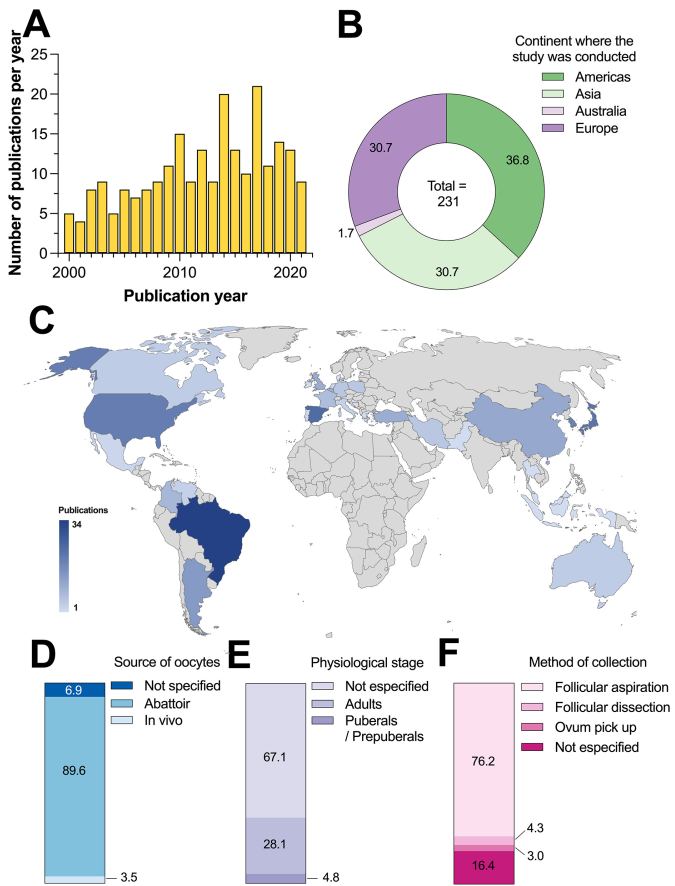
Figure 2 A) Number of studies per year of publication, B) percentage of publications according to the continent of origin, C) distribution of publications per country, D) source of the oocytes, E physiological state, and F) method of collection.
Spain had the highest number of studies in Europe with 24, followed by the United Kingdom with 10 studies, and Belgium with nine studies. In Asia, Japan, South Korea, and China had 22, 19, and 11 studies, respectively (Figure 2C). In 2020, the commercial production of in vitro embryos increased by 12 %, resulting in 1.5 million declared embryos. This technique is the preferred method for beef cattle breeding in the main countries of the international meat market, including Argentina, Australia, Brazil, and the USA. This increase in production may be attributed to the economic significance of this industry in these countries.21
In total, 89.6 % (207/231) of the studies used biological material from the abattoir. Sixteen studies did not specify the source, and eight publications reported the use of cows in vivo as the source of the oocytes (Figure 2D). The use of ovaries, mainly from slaughtered females at the abattoir, indicates that this last source is preferred for experimental studies because it allows for a greater number of experiments to be performed without affecting animals that are productively active. For this reason, using ovaries obtained from slaughtered animals ensures a continuous supply of oocytes while avoiding the use of animals with high genetic value. Additionally, these oocytes are obtained at a low cost, come from animals in different stages of the estrous cycle, and can be matured, fertilized, and cultivated in vitro for study or commercial purposes. However, oocytes obtained from a slaughterhouse may come from ovaries of mixed quality due to low reproductive status or illness in cattle.8
Out of the 231 studies, 65 reported the use of oocytes obtained from adult females, while only 11 out of 231 studies used pubescent/prepubertal animals. The type of female from which the biological material was obtained was not specified in most studies (155/231) (Figure 2E). However, it is possible that these studies used adult females, as culled cows are the most common type of female that enters the abattoir for slaughtering. Out of the reviewed studies, 176 reported using follicular aspiration with needle and syringe as the primary technique for oocyte collection. Meanwhile, 38 studies did not specify the collection method, while follicular dissection (ovary slicing) and ovum pick-up were the least common methods (Figure 2F).
According to the Sankey diagram depicted in Figure 3, 47.2 % (109/231) of the studies assessed oocyte maturation, 38.5 % (89/231) reported results during embryo culture, and 14.3 % (33/231) included both processes. Of the four treatment categories, bioactive supplements were evaluated in 34.2 % of the studies (79/231), medium composition in 19.5 % (45/231), and culture conditions in 12.1 % (28/231). The remaining 34.2 % of the studies (79/231) focused on the effect of other additives on embryo production (Figure 3).
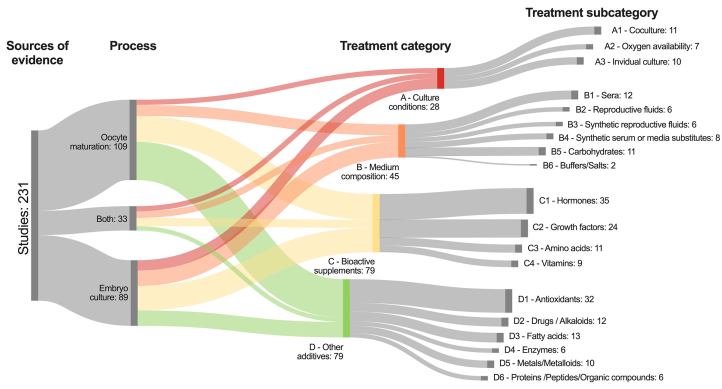
Figure 3 Sankey diagram showing the distribution of thee studies reviewed based on their process and treatment category and subcategory.
The 231 studies reviewed were classified mutually exclusive and collectively exhaustive into one of 19 subcategories based on the main type of treatment category that the study reported. This allowed for grouping of articles with similar characteristics based on the treatment, though with heterogenous methodology. The general characteristics of the studies in each treatment subcategory are described below. Characteristics and types of treatments used during Bovine in vitro embryo production: A review of the evidence
A - Culture conditions: sources of evidence
In this review, we identified 28 studies that evaluated culture conditions for in vitro embryo production. These studies were classified into three subcategories (Table S-3).
A1 - Coculture (11 studies). Five studies reported coculturing with oviductal cells as the most common treatment. Two studies evaluated the effect of coculturing with cumulus or luteal cells, and four other studies evaluated the addition of other kind of somatic cells (amniotic membrane cells, mesenchymal stem cells, or Vero cells). These treatments were used both during in vitro oocyte maturation and embryo culture. Interestingly, there were contrasting results arising from these treatments, because coculture with amniotic membrane cells enhanced oocyte maturation and developmental competence.22
Meanwhile, another study showed that the developmental competence of Bovine embryos depended more on the type of the culture media than on the presence or absence of coculture with cumulus cells.23 Additionally, researchers have investigated coculture with all the above types of somatic cells to create an environment that closely resembles the maternal environment of the embryo. This environment secretes growth and embryotrophic factors.24 Coculture has in this way been found to increase the efficiency of Bovine in vitro embryo culture.25
A2 - Oxygen availability (7 studies). Six studies evaluated the effect of oxygen (O2) availability, and one additional study analyzed the effect of nitric oxide (NO) concentration on embryo production. During in vitro embryo production, high concentrations of reactive oxygen species and reactive nitrogen species are often produced, especially at high O2 tension (20 %).26 Research has shown that reducing O2 tension from 20 % to 5 % can increase embryo development in vitro because high levels of O2 can be harmful due to the accumulation of reactive oxygen species, which causes oxidative stress and reduces embryo quality.27 Previous studies have reported that inhibiting NO synthesis has a negative effect on oocyte maturation, cleavage, and blastocyst development.28 Furthermore, higher concentrations of NO apparently have a beneficial effect during in vitro culture at lower O2 tensions.26 Although O2 tension and NO are important during embryo production, there are few studies that assess their effects on embryo production.
A3 - Individual/Small group culture (10 studies). Five studies assessed the effect of individual embryo culture, whereas five other studies analyzed the effect of small group embryo culture. Previous research has demonstrated that culturing embryos in small groups enhanced the rate of development and the quality of the embryos, mainly due to the production of embryokines as a result of paracrine and autocrine stimulation from the neighboring embryos.29) In contrast, individual culture does not stimulate the production of such factors. However, single embryo culture allows tracking and monitoring of individual oocytes and embryos, which may be used to identify biomarkers for assessing embryo quality and development.30
Nevertheless, there exist contrasting evidence on the effect of small group culture. Ahumada et al.31 showed that when cultured in large groups, a greater proportion of embryos reached the blastocyst stage, whereas in small groups, the addition of growth factors could help to partially prevent the deleterious effect of small group culture and increase the percentage of blastocysts. Similarly, Cebrian-Serrano et al.32 reported that embryos cultured in large groups showed a greater ability to develop than those cultured in smaller groups and that adding some growth factors improved embryo quality when small groups of embryos were cultured. Further research is needed to provide more evidence on this pivotal topic.
B - Medium composition: sources of evidence
We identified a total of 45 studies that reported the effect of medium composition on Bovine embryo production. The studies were classified into six subcategories (Table S-4).
B1 - Sera (12 studies). Nine studies used fetal Bovine serum (FBS), while other three studies used estrus cow serum (ECS) or dairy cow serum. FBS is often included in culture medium to enhance oocyte competence and maturation, leading to the formation of a blastocyst and subsequent offspring generation.33 However, it has been shown to have negative effects on in vitro development (inhibition of first cleavage division), embryo morphology (altered ultrastructure, abnormal blastulation, or impaired compactation), cryotolerance (excessive accumulation of lipid droplets), and fetal and postnatal development in certain conditions (large calf syndrome); therefore, it is important to consider these potential drawbacks when using FBS in culture medium.(34, 35) Furthermore, FBS may serve as protein supplement to protect embryos from toxic substances, provide growth factors, and reduce the surface tension of the medium, preventing embryos from adhering to the instruments. However, some aspects of its function are not yet fully understood.36
B2 - Reproductive fluids (6 studies). Three studies evaluated the effect of Bovine oviductal fluid, while three studies reported the use of follicular fluid. Reproductive fluids play a crucial role in providing an optimal microenvironment for oocyte growth and development. This is due to their complex composition of growth factors, hormones, electrolytes, proteins, steroids, cytokines, metabolites, and antioxidants, which have been shown to have a positive impact on embryo production.30 For example, oviductal fluid contains carbohydrates, ions, lipids, phospholipids, proteins, and other unknown components that enhance both the quality and quantity of embryos.36
B3 - Synthetic reproductive fluids (6 studies). Six studies included synthetic oviductal fluid (SOF) to assess its effect on oocyte maturation and embryo culture. SOF is a commonly used medium for Bovine embryo culture,37 and previous studies have demonstrated that serum-free media based on SOF can be used for Bovine embryo production.38,39 George et al.40 reported that the addition of insulin, transferrin, and selenium to SOF medium improved embryonic development rate and quality. Palasz et al.41 cultured zygotes in SOF with or without BSA and hyaluronic acid (HA) and found that the addition of these substances to the SOF increased the number of blastocyst developed and enhanced the mRNA expression of hyaluronan synthases.
B4 - Synthetic serum/Media substitutes (8 studies). Four studies assessed the effect of serum-free media and other four studies included serum substitutes or media substitutes. Given that serum is produced as a pathological fluid during blood clotting, it has been suggested to cause some detrimental effects on embryo culture.42 Therefore, several studies have reported the use of serum substitutes, though with variable response. Sung et al.43 supplemented zygotes with sodium citrate and two brands of BSA (Sigma™ vs. ICPbio™). The authors found that both sodium citrate and Sigma-BSA increased total blastocyst development.
Another study reported that both BSA-free medium and FBS supplemented with other compounds (growth factors, cytokines, albumin, and hyaluronan) increased the blastocyst rate and improved embryo development and survival.44 Del Collado et al.45 matured oocytes in different concentrations of fatty acid-free BSA and commercial embryonic fluid to compare them against FBS-supplemented media (10 %). The authors concluded that, even at low concentration, FBS is necessary during oocyte maturation. Another study revealed that the percentages of blastocysts and hatched blastocysts in undefined media (FBS or BSA) were significantly greater than those in defined media containing polyvinyl alcohol.46
Sagirkaya et al.47 reported that the cleavage rate after the addition of 10 % FBS was significantly greater than that after the addition of 10 % synthetic serum substitute. According to the authors, the lack of effect of the serum substitute relies in its differential composition in comparison to FBS, which contains growth factors that may cause a synergistic effect.47 Nevertheless, in a further experiment Sagirkaya et al.48 showed that the replacement of FBS with a synthetic substitute favors oocyte maturation and embryo development in vitro. Therefore, such a contrasting result requires further research to provide more insights on the role of synthetic serum substitute. Even though these types of compounds may offer more constant results due to its known composition, some studies have suggested that serum supplementation, even at low concentration, may still be necessary during embryo production.47
B5 - Carbohydrates (11 studies). Four studies were conducted to evaluate the effect of glucose or fructose on embryo production, six studies assessed the effect of hyaluronic acid, while another study determined the effect of nutrient depletion. Glucose utilization significantly increases embryonic development in advanced preimplantation stages; however, during earlier preimplantation stages, pyruvate, lactate, and glutamine are preferentially used as energy sources.34 Regarding the effect of hyaluronic acid, some authors have demonstrated that the production of hyaluronic acid is essential for cumulus cell expansion and embryo development,49 and reported that adding hyaluronic acid to the medium favored the rate of oocyte maturation in BSA-enriched medium50 as well as improved blastocyst hatching and embryo quality.51 Therefore, since hyaluronic acid has shown to improve the developmental capacity of cultured embryos it is recommended as a supplement for in vitro embryo production.52
B6 - Buffers/Salts (2 studies.: One study determined the effect of EDTA during culture, while the other analyzed the effect of various buffer solutions (HEPES, TES, MOPS, and PBS).
C - Bioactive supplements: sources of evidence
The bioactive supplements category included a total of 79 studies, which were classified into four subcategories (Table S-5):
C1 - Hormones (35 studies). Twelve studies reported the effect of metabolic hormones such as ghrelin or insulin, growth hormone (GH), or leptin as their main treatment, followed by eight studies that reported the effect of melatonin. In addition, eight studies utilized steroid hormones, such as estradiol and progesterone, while four studies evaluated the effects of gonadotropins, and three studies included prostaglandins, activin A, and follistatin as the primary treatments on embryo production. To improve maturation conditions, experimental research aims to replicate the in vivo environment in which oocyte maturation occurs physiologically. Hormones have been found to be helpful in stimulating maturation.53
A recent review reported that steroid hormones, including progesterone, estradiol, and cortisol, had little effect on embryo development during in vitro embryo production. Indeed, some of the studies reviewed showed a negative effect when excessive amounts of hormones were used.4 This contrasts with the positive effect exerted by melatonin, which acts as an embryokine during in vitro embryo production.4 Melatonin has been shown to increase blastocyst cell number, blastocyst production, hatching, and quality.54,55
C2 - Growth factors (24 studies). Nineteen studies evaluated several growth factors, with epidermal growth factor (EGF, 6 studies), insulin-like growth factor-1 (IGF-1, 6 studies), fibroblast growth factor (FGF, 4 studies), and bone morphogenetic protein (BMP, 3 studies) as the most frequently studied. Additionally, five studies examined a variety of factors, including erythropoietin, IMD/ADM21-47, embryotrophic factors, leukemia inhibitory factor (LIF), or Interleukin 6 (IL6). FGF is particularly interesting due to its involvement in various cellular processes, such as chemotaxis, cell migration, differentiation, cell survival, apoptosis, embryo development, and angiogenesis. FGF10 has been found in Bovine theca cells, luteal cells, and oocytes in conjunction with the expression of its two main receptors (FGFR1B and FGFR2B), which are regulated by FSH, suggesting that they play an important role in oocyte maturation.56 However, there is conflicting evidence regarding the effect of FGF on blastocyst development. Further studies are needed to provide more conclusive evidence concerning the impact of FGF on in vitro embryo production.
C3 - Amino acids (11 studies). Four studies reported the effect of L-carnitine, three more studies included L-arginine, while four other studies reported results with L-cysteine, glycine, L-ergothioneine, or taurine. While there is limited evidence about the impact of amino acids on Bovine embryo production, a previous study has shown that amino acids, such as L-carnitine, can aid in the transportation of fatty acids to the mitochondria for beta-oxidation, enhancement of cumulus cell expansion in bovines, and improvement of mitochondrial activity in embryos by reducing lipid content.57
C4 - Vitamins (9 studies). Three studies analyzed the addition of vitamin A metabolites such as retinoic acid or retinol, three studies included vitamin B (B6 or dimethylglycine), two more studies assessed choline or vitamin E analogue, and one study reported the effect of vitamin K on oocyte maturation and embryo production.
D - Other additives: sources of evidence
We identified 79 articles within the category of other additives, which were divided into six subcategories (Table S-6):
D1 - Antioxidants (32 studies). Resveratrol was the most used compound in five studies, followed by cysteamine and glutathione in four and three studies, respectively, and other four studies included astaxanthin, anethole, or pterostilbene. Alpha-tocopherol, lycopene, nobiletin, and native plant extracts were included in two studies each, whereas the other eight studies evaluated a variety of antioxidants. The quality of embryos produced in vitro is influenced by the culture environment, in which different types of natural or synthetic antioxidants are added to the culture medium. Recently, it was demonstrated that nobiletin enhances embryo quality by increasing blastocyst formation rates and decreasing the levels of reactive oxygen species and apoptotic cells in embryos.12
D2 - Drugs/alkaloids (12 studies). Three studies reported the effect of nicotine or caffeine, three studies assessed urolithin A, butaphosphan, or trichostin A, and one study included lupeol. The other five studies evaluated a wide variety of compounds including flunixin meglumin, dexamethasone, metformin, steroidal glycoalkaloids, or iloprost.
D3 - Fatty acids (13 studies). Four studies evaluated the effect of Omega-3 (alpha-linolenic acid or eicosapentaenoic acid), two studies included Omega 6 (linoleic acid), and two more studies used conjugated linoleic acid. The five additional studies examined lysophosphatidic acid, arachidonic acid, or docosahexaenoic acid, among other compounds.
D4 - Enzymes (6 studies). Two studies reported the use of plasminogen activator, whereas cathepsin B, protein kinase kinase inhibitor (U0126), peroxiredoxin, and phosphodiesterase isoenzyme inhibitor were included in one study, each.
D5 - Metals/Metalloids (10 studies). Ten studies evaluated the effect of zinc, manganese, copper, selenium, and strontium as the main metals or metalloids assessed.
D6 - Proteins/Peptides/Organic compounds (6 studies). Two studies examined the natriuretic C-type peptide, whereas sericin, coagulansin-A, plant protein hydrolysates, and osteopontin were evaluated in one study each.
Effects of hormones, growth factors, sera, and reproductive fluids used in Bovine in vitro oocyte maturation and embryo culture
Figure 4 summarizes the frequency of negative/no effect or positive effect results on Bovine embryo production reported by 77 studies that used hormones, growth factors, sera, or reproductive fluids during oocyte maturation and embryo culture. Out of the 77 studies, 53 (68.8 %) reported a positive effect of the intervention. Hormone-based interventions accounted for 49.0 % of the positive results (26/53), followed by growth factors at 30.2 % (16/53). Among the 24 studies that reported negative/no effects after an intervention, 33.3 % (8/24) of these reports were obtained using growth factors, followed by 37.5 % (9/24) from treatments that used hormones (Figure 4A). In the following sections, we provide a study-specific summary of the results for each of the 77 studies, divided according to each of the four treatment categories.
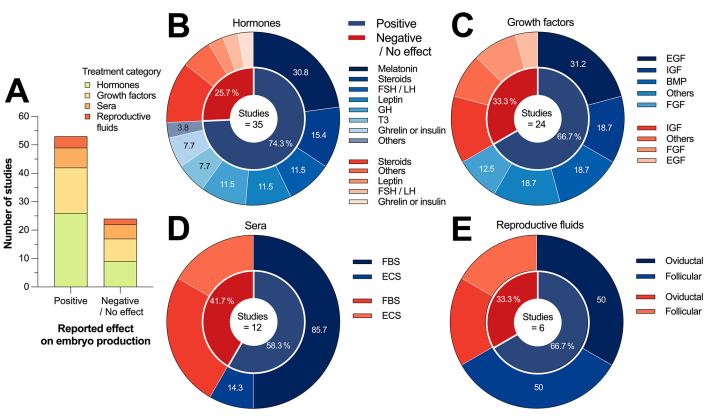
Figure 4 A) Number of studies that reported positive or negative effects on Bovine embryo production of the four selected subcategories and B-E) donut charts summarizing intervention-specific effects of the four selected subcategories. In donut charts, the percentages shown sum up to 100 %, corresponding to the number of studies that reported a positive effect.
Evidence on the effect of hormones
Based on the data presented in Figure 4B, 26 out of 35 studies (74.3 %) that utilized hormone-based interventions reported a positive effect during oocyte maturation and embryo culture. The main interventions that yielded positive results were melatonin, gonadotropins (FSH/LH), steroids (P4/E2), leptin, and GH, which accounted for 80.8 % (21/26) of the positive outcomes reported. Among the nine studies that reported negative or no effects, interventions that used steroids were the most common. Next. We present a summary of 35 studies that evaluated the impact of hormones on the in vitro production of Bovine embryos.
Gonadotropins. Ali et al.58 cultured oocytes in 0.5µg/mL r-hFSH for 2 or 6 hours, and the results showed that the treatment enhanced cytoplasmic maturation. Choi et al.59 found that the increase in cumulus expansion in proportion to the gonadotropin concentration and the rates of cleavage and blastocyst development were not different between two treatment groups when oocytes were cultured in combinations of 1 or 10 ng/mL FSH and 1 or 10 μg/mL LH . Liu et al.60 after culturing oocytes in media supplemented with 0.075, 0.75, 7.5, and 75 IU/mL FSH and LH, reported that low to moderate concentrations (0.75-7.5 IU/mL) of FSH enhanced nuclear maturation and development and that the highest LH concentrations induced detrimental effects on oocyte nuclear maturation and embryo development. Finally, Salgado et al.61) found that a greater proportion of LH than FSH in the maturation medium induced a greater maturation rate after culturing oocytes in the following groups (concentrations in μg/mL): 50 FSH + 150 LH; 100 FSH + 100 LH; and 150 FSH + 50 LH.
Leptin. Kaya et al.62 reported a positive effect on the 8-16-cell stage with 5 μg/mL leptin and 5 μg/mL leptin + 100 μg/mL IGF-1. The authors concluded that the addition of IGF-1 improved the cleavage rate, although the percentage of blastocysts did not differ between groups. In three studies, oocytes were cultured in media supplemented with 1, 10, or 100 ng/mL leptin; Boelhauve et al.63 and Jia et al.64 reported that treatment during oocyte maturation improved development to the blastocyst stage and enhanced meiotic maturation and embryo development and quality. In contrast, Arias-Alvarez et al.65 found that leptin did not influence day-7 blastocysts and that high concentrations of leptin negatively affected embryo survival.
Cortisol. Da Costa et al.66 reported that supplementation with 0.1 μg/mL cortisol during oocyte maturation increased blastocyst formation rates and that cortisol positively affected oocyte competence. In contrast, another study reported that hormones did not affect the rate of embryo development or the number of cells per blastocyst after culturing zygotes in either control, 55 ng/mL P4, 120 pg/mL E2, 40 ng/mL cortisol medium, or their combination for 8 days.67
Triiodothyronine. One study revealed that medium supplemented with 50 or 100 nM triiodothyronine (T3) applied during oocyte in vitro maturation increased the number of blastocysts after 8 days of culture. The authors concluded that T3 may positively affects embryo development kinetics.68 Another study showed that after culturing oocytes in 50 ng/mL T3 and thyroxine, the thyroid hormones had a positive effect on blastocyst formation and hatching rates, and it was concluded that embryo quality improved significantly.69
GH. Three studies reported the effect of culturing oocytes or morulae on 0, 50, 100, or 200 ng/mL GH. Dong et al.70 found that the proportion of metaphase II oocytes was significantly greater at 200 than at 0ng/mL GH, and the authors concluded that the treatment favored oocyte maturation and embryo development. Moreira et al.71 reported that 100 ng/mL GH increased the cleavage rate and thus enhanced embryo development, while Iwata et al.72 reported that GH at 100 ng/mL improved the rate of development and cell number starting from the morula.
Ghrelin or insulin. After culturing oocytes in 0, 200, 800, and 2 000 pg/mL ghrelin during in vitro maturation of oocytes, Dovolou et al.73 found that ghrelin groups significantly suppressed blastocyst formation rates, whereas Dovolou et al.74 found no differences between the control, 200, and 2 000 pg/mL ghrelin groups, and fewer blastocysts were produced with 800 pg/mL than in the control group when the hormone was administered daily during the culture of zygotes. Dashtizad et al.75 reported that insulin at 10 µg/mL exerted beneficial effects on oocyte and embryo development up to the morula stage.
E2 and P4. Beker et al.76 cultured oocytes in control medium or with 1 μg/mL E2 with or without 0.05 IU/mL recombinant hFSH. The authors found that E2 alone decreased the percentage of metaphase II oocytes compared to that in the control group and increased the percentage of nuclear aberrations. Additionally, the authors found that E2 negatively affected the maturation of oocytes and subsequent embryo development, although this effect was attenuated in the presence of FSH. Larson et al.77 after culturing zygotes in 1 or 100 ng/mL P4 for 1-3 or 4−7 days, reported that the development rate from the morula to the hatched blastocyst stage was similar among the treatments. The authors concluded that supplementation with P4 during culture does not improve cleavage of presumptive zygotes, embryo development, and blastocyst rate.
Matsuo et al.78 used a 3-step oocyte culture system that mimicked temporal physiological changes in E2 and P4, and reported that the treatment enhanced in vitro embryo development reflected as a greater blastocyst formation rate. Reis et al.79 cultured zygotes in the following groups (concentrations in µg/mL): control, 1 E2+ 100 P4, 1 E2, and 100 P4. The authors reported that E2 and P4 together and E2 alone resulted in greater hatching rates and improved embryo quality.
Testosterone. Silva et al.80 reported that 100 nM testosterone and 100 nM dihydrotestosterone increased the rate of oocyte cleavage without affecting embryo development. Finally, Soares et al.81 cultured oocytes in the following groups: 1) control; 2) 100 nM natriuretic C-type peptide; 3) 500 ng/mL E2+ 50 ng/mL P4+ 50 ng/mL androstenedione + 10-4IU/mL FSH, and 4) 500 ng/mL E2+ 50 ng/mL P4+ 50 ng/mL androstenedione + 10-4IU/mL FSH + 100 nM natriuretic C-type peptide. The authors reported that steroids and the peptide did not alter blastocyst formation rates and that steroids interacted with the peptide to delay meiotic resumption and prolong oocyte and cumulus cell communication.
Melatonin. Marques et al.82 cultured zygotes in groups supplemented with 10-9M melatonin or 10-9M melatonin + blastocoel fluid on day 7 and found that treatment with melatonin and blastocoel fluid increased the hatching rate. Another six studies reported the effect of culturing oocytes in 0, 10-7, 10-9, and 10-11M melatonin. A concentration between 10-7 and 10-9M melatonin improved the cytoplasmic maturation of Bovine oocytes,83 and enhanced blastocyst yield and quality.84,85
Marques et al.86 found that 10-9M melatonin group presented a greater percentage of Grade I blastocysts than did the control group, and Pang et al.87) reported that oocyte maturation was enhanced by 10-9M melatonin, although this may affect subsequent embryo development. Tian et al.88 found that melatonin increased the expression of genes associated with oocyte maturation such as GDF9, MARF1, and DNMT1a and PTX3 and HAS1/2 which are genes related to expansion in cumulus cells. Finally, Gutierrez-Añez et al.89 found that 0.01 nM melatonin during oocyte maturation enhanced oocyte competence, embryo development, and embryo quality.
Other hormones. Algarra et al.90 cultured zygotes in groups treated with 10 or 50 µg/mL recombinant porcine oviductin (pOVGP1) and found that the treatments induced no differences in embryo development. Rodrigues et al.53 showed that 1-10µM PGE2 and PGF2a alone or in combination had no effect on embryo development. Another study revealed that, in comparison to 10 ng/mL of follistatin, 10 ng/mL of activin A may enhance the development of Bovine embryos by shortening the duration of the lag-phase which may result in an enhancement of the developmental kinetics at early stages of cleavage.91
Evidence on the effect of growth factors
According to Figure 4C, 16/24 studies (66.7 %) that used growth factors as the intervention reported a positive effect during Bovine embryo production. Interventions using EGF, FGF, IGF-1, and BMP contributed 81.2 % (13/16) of the positive results reported. Among the eight studies that showed a negative effect on oocyte maturation or no effect on embryo culture, three used IGF-1 as the primary intervention, followed by two studies that included FGF, and two other studies from the “Others” group. Next, we present a study-specific summary of the 24 studies that assessed the effect of growth factors on the in vitro production of Bovine embryos.
EGF. Sakagami et al.92 reported that EGF (100 or 200 ng/mL) or IGF-1 (50 or 100 ng/mL) increased the percentage of embryos that developed into blastocysts. Additionally, Sirisathien et al.93 found that 50 ng/mL 5 ng/mL EGF and IGF-1 improved embryo performance and increased embryo yield. Another study revealed that 5 ng/mL EGF improved the proportion of 4-cell stage embryos that reached the blastocyst stage in comparison to 0, 1, or 25 EGF groups, though no differences were documented in the number of 4-cell stage embryos among these four groups.94
Mesalam et al.95 showed that the number of embryos that cleaved, formed a blastocyst, and hatched was greater in the medium containing insulin-transferrin-sodium selenite + EGF + BSA than in the medium containing FBS. Mtango et al.96 found that 10 ng/mL EGF had favorable effects on oocyte maturation and embryo production because it increased the number of metaphase II oocytes and blastocysts. Only one study showed no differences between groups, although the authors concluded that 100 ng/mL EGF is the optimal concentration.97
IGF-1. Wang et al.98 reported that supplementation with polyphenols at 15 μM, 100 ng/mL IGF-1, and 5.6 mM glucose produced higher cleavage and blastocyst rates than did polyphenols at other concentrations. The authors concluded that these compounds could improve oocyte maturation, embryo development, and blastocyst quality in cattle. Neira et al.99 concluded that a combination of cytokines and growth factors that included IGF-1 improved blastocyst yield. Kocygit et al.100 reported that BSA alone or in combination with IGF-1 + EGF resulted in increased embryo yield and embryo cell mass. Three other studies did not find a significant effect of IGF-1.
Hernandez-Fonseca et al.101 concluded that 5 ng/mL IGF-1 supplementation during in vitro culture did not improve the cleavage rate or blastocyst production. Velázquez et al.102 showed that 1 000 ng/mL IGF-1 did not enhance blastocyst formation. Indeed, high concentrations of IGF-1 had detrimental effects on Bovine embryos. Finally, Dhali et al.103 assessed the single or combined effect of 100 ng/mL IGF-1 and 50 ng/mL stem cell factor and found that the developmental competence of embryos was similar among groups. The authors conclude that serum-free media supplemented with these growth factors, alone or combined, may result satisfactory for producing Bovine embryos.
FGF. Zhang et al.104 concluded that FGF2 treatment during oocyte maturation enhances blastocyst development, whereas Fields et al.105 showed that the addition of FGF2 increased the percentage of oocytes that developed into blastocysts. In contrast, two studies did not find a significant effect of FGF. Diogenes et al.56 found that 0.5 ng/mL FGF10 administered during oocyte maturation did not affect cleavage and blastocyst rates. Additionally, the authors reported that the growth factor showed no effect on the developmental kinetics of the embryo. Another study reported that 0.5 ng/mL FGF10 during oocyte prematuration did not improved the quality of the embryo and changed the expression of genes associated with embryo quality.106
BMP. Machado et al.107 reported that BMP15 alone or in combination with FGF in maturation medium enhanced cumulus expansion, meiosis progression, and embryo development. Sudiman et al.108 cultured oocytes in 100 ng/mL pro-mature BMP15, 100 ng/mL mature BMP15, or 100 ng/mL mature GDF9 alone or combined with FSH. The authors assessed the developmental competence of the oocytes and found that pro-mature BMP15 improved blastocyst development, whereas the mature form induced only intermediate levels of blastocyst development that only were higher than those of GDF9 group. In another study, the authors concluded that the preservation of gap junctional communication using 100 ng/mL BMP15 may facilitate the efficient transfer of metabolites from cumulus cells to oocytes, thereby enhancing oocyte developmental competence.109
Other factors. Conde et al.110 reported that the presence of hematopoietic erythropoietin and kit ligand during oocyte maturation had a positive effect on oocyte cytoplasmic maturation and developmental competence without impacting nuclear maturation. Garcia-Hernandez et al.111 cultured oocytes in 153 µg/mL IMD/ADM21-47 peptide and reported that the presence of this factor was correlated with the production of healthy metaphase II oocytes. Salgado et al.112 cultured zygotes in control media or >1, >5, or >10 kDa fractions of embryotrophic factors in conditioned media from BRL cells and found that the fractions increased the number of embryos that reached the 2-8 cell stage. The highest proportion of cleaved embryos was achieved at >10 kDa. However, embryo development to the morula/blastocyst stage was similar among the groups.
Seekford et al.113 found no significant difference in blastocyst formation after treating embryos with IL6, although this factor may normalize the developmental trajectory of embryos produced in vitro. Finally, Vejlsted et al.114) cultured zygotes in 0 or 1 000 U/mL recombinant LIF. Their results showed that in the presence of this factor, less embryos reached the hatched blastocyst stage, and the quality of blastocysts was reduced. The authors concluded that LIF may have adverse effects on embryo development.
Evidence of the effect of sera
According to Figure 4D, 7/12 studies (58.3 %) that used serum-based interventions reported a positive effect during oocyte maturation and embryo culture. Interventions using FBS contributed 6/7 (85.7 %) and ECS 1/7 (14.3 %) of the positive results reported. Among the five studies in which negative/no effects were reported, FBS contributed with three studies, whereas two other studies used ECS. Next, we present a study-specific summary of 12 studies that assessed the effect of sera during the in vitro production of Bovine embryos.
ECS or dairy cow serum. Collares et al.115 supplemented cumulus-oocyte complexes with dairy cow serum at the beginning and end of lactation and reported no differences between treatments. Mucci et al.34 showed that the hatching rate was lower in embryos cultured in media supplemented with 5 % ECS in comparison to serum-free media. Finally, Pereira et al.116 matured cumulus-oocyte complexes in 10 ECS or 0.1 % polyvinyl alcohol under two O2 tension conditions (5 % or 20 %) for each compound. The authors found that, under 20 % O2 tension, total cell number and blastocyst rate were higher in ECS than in polyvinyl alcohol, though at 5 % O2 tension the results did not differ between treatments. Nevertheless, polyvinyl alcohol + 5 % O2 produced a lower apoptosis index.
FBS. Fernandes-Franca et al.117 concluded that 5 % or 10 % FBS and 0.8 % BSA can be used alone or in combination during in vitro maturation. Mesalam et al.118 cultured zygotes in heat-inactivated FBS or FBS devoid of charcoal and dextran. The authors found that developmental competence and embryo mitochondrial activity were both enhanced in media supplemented with FBS devoid of charcoal and dextran. Sudano et al.119 concluded that the use of 2.5 % FBS and the addition of phenazine ethosulfate increased embryo survival and quality. Leivas et al.120 reported that zygote culture in SOF + BSA + FBS improved the yield of blastocyst and Quality I blastocyst and that the addition of serum increased the efficiency of Bovine embryo production.
Reis et al.121 cultured zygotes in either one of three groups: SOF + BSA, SOF + FBS, or SOF + FBS + vitamin E. The authors found that supplementing the culture medium with both FBS and vitamin E improved blastocyst yield and quality. Murillo et al.122 cultured oocytes in SOF + BSA with or without 0.1 % FBS and reported that early blastocyst rate was greater for embryos cultured with FBS. Other studies reported a negative effect of FBS. For instance, Restrepo et al.35 found that the presence of 10 % FBS during oocyte maturation decreased the mitochondrial activity of the oocytes and reduced the rate of morulae production in comparison to oocytes cultured in serum-free media.
The authors concluded that FBS may have a deleterious effect on the rate of embryo development. Alm et al.123 reported that medium supplemented with 20 % FBS achieved a greater rate of blastocyst development in comparison to medium supplemented with 5 % ECS + FSH, HCG, and E2. Finally, Caínzos et al.124 found no significant difference after the culture of oocytes supplemented with FBS, BSA, or polyvinylpyrrolidone.
Evidence on the effect of reproductive fluids
According to Figure 4E, 4/6 studies (66.7 %) that used reproductive fluids as the main intervention reported a positive effect during Bovine embryo production. Bovine oviductal fluid and follicular fluid were the two treatments reported by the studies; two studies reported a positive effect of oviductal fluid, whereas two studies reported that follicular fluid improved embryo production. In contrast, negative/no effect results were reported in one study that used oviductal fluid and other that included follicular fluid. Next, we present a study-specific summary of such six studies.
Follicular fluid. Ali et al.125 cultured oocytes for 24 hours in medium supplemented with 1, 5, or 10 % follicular fluid, and reported that more oocytes reach the blastocyst stage when cultured with a concentration of 5 %. Momozawa et al.126 cultured oocytes in basic maturation medium supplemented with either one of three fractions of follicular fluid or one of three fractions of FBS. The authors reported that the second fraction of follicular fluid and the third fraction of FBS improved the rate of development to the blastocyst stage. In contrast, Cruz et al.127 showed that a higher concentration (75-100 %) of follicular fluid added to the maturation medium reduced cleavage and blastocyst rates, whereas a lower concentration (50 %) of follicular fluid increased the inner cell mass number.
Bovine oviductal fluid. Lopera-Vasquez et al.128 cultured zygotes in medium supplemented with oviducal fluid (5 %, 10 %, or 25 %) or FBS (5 %) and found that oviductal fluid at low concentrations had a positive effect on the development rate and quality of Bovine embryos. Hamdi et al.36 cultured zygotes with 1.25 % oviductal fluid from days 1 to 4 after insemination, followed by 1.25 % uterine fluid from days 4 to 9. The authors showed that supplementation with both reproductive fluids favored embryo development. In contrast, Cebrian-Serrano et al.129 matured oocytes treated or not with Bovine oviductal fluid and reported no effect on embryo development rate. Implications of all the available evidence and perspectives for future studies
The scoping review results enabled us to answer our research questions. Firstly, we identified the body of evidence on the characteristics and types of treatments used during Bovine in vitro embryo production. We successfully mapped the nature and extent of studies reporting different treatments during the in vitro production of Bovine embryos. We identified 231 studies published between 2000 and 2021, with 133 published in the last decade, indicating a growing interest in the topic. The majority of publications came from the Americas, Europe, and Asia.
The studies primarily evaluated ovaries from abattoirs and did not specify the female population used. The 231 studies were categorized into four groups: culture conditions (28 studies), medium composition (45 studies), bioactive supplements (79 studies), and other additives (79 studies). These were further divided into 19 subcategories, with interventions using hormones, antioxidants, and growth factors having the largest number of reports (35, 32, and 24 respectively, Figure 3).
Secondly, the scoping review was conducted to qualitatively summarize the effects of 77 studies that reported the effects of hormones, growth factors, sera, and reproductive fluids during oocyte maturation and embryo development. Overall, among the 77 studies summarized, positive results were reported in 68.8 % of cases (53 studies, Figure 4), among which hormone-based interventions were the most effective, because 26/35 studies that used hormones reported a positive effect. Interventions based on melatonin, gonadotropins, and steroids were the most effective in promoting Bovine embryo production. The second most effective interventions were those that added growth factors to culture medium (16/24 positive effect). In particular, interventions that utilized EGF, FGF, BMP, and IGF-1 were the most effective. The evidence suggests that hormones, growth factors, sera, and reproductive fluids improves oocyte competence, cytoplasmic and nuclear maturation of Bovine oocytes, oocyte quality, embryonic development, cleavage and blastocyst development rates, and the number of grade I blastocysts. In some cases, it may be possible to substitute serum with artificial or alternative substances during embryo production. While some studies propose reducing the percentage of serum used in culture to achieve optimal performance, others have concluded that sera or serum proteins, such as BSA, are necessary during oocyte maturation and embryo development, even at low concentrations.
Out of the 77 studies summarized, 24 reported a negative/no effect after the intervention. Sera-based interventions provided the highest number of negative/no effect results (41.6 %, 5/12), followed by hormone-based treatments (37.5 %, 9/35). Several studies have shown that high concentrations of sera or hormones can have detrimental effects on oocyte nuclear maturation, which impairs embryo development and results in decreased embryo survival, as well as during embryo culture resulting in altered ultrastructure, abnormal blastulation, or impaired compactation. Furthermore, the presence of certain serum molecules can delay meiotic resumption and decrease the percentage of zygotes that develop morulae and their further structures.
Further research should be conducted on hormone-, serum-, and growth factor-based interventions and their potential combinations, as they have shown relatively greater efficacy. In certain instances, studies may be necessary to determine the exact mechanisms that lead to an elevated response, such as in the case of melatonin and certain growth factors. It is important to further investigate the limitations of reducing BSA concentrations in maturation and culture media, as well as the use of synthetic substances with more defined compositions. Future research should concentrate on quantitatively summarizing the effects of various treatments through meta-analysis to determine the optimal culture conditions and substance concentration and doses for oocyte maturation and embryo culture.
Besides, it is recommended to analyze the minimum serum concentration required or the conditions for serum substitution without compromising maturation and embryo development rates. When interventions have both positive and negative effects, it is important to identify the concentrations, combinations, and conditions (including intrinsic and extrinsic factors) that promote a positive response. Although the productive demand for Bovine embryos has encouraged the use of in vitro embryo production, as well as the exploration of different culture conditions through various interventions, some research is insufficient or presents contrasting results, preventing the generation of solid conclusions. Therefore, future research should prioritize based on the most effective interventions to generate a larger and solid body of evidence on their potential impact and practical application.
Limitations of the study
Our study has several limitations. Firstly, we did not include gray literature, which may have caused selection bias in the published evidence. Secondly, our search for published studies was limited to seven databases. Third, we limited our mapping to studies in 4 treatment categories and 19 subcategories due to the disparate methods and approaches reported in the literature. This may oversimplify the diversity of treatments and approaches used to experimentally explore Bovine in vitro embryo production. Fourth, to provide a summary of evidence, we included only 4 treatment subcategories, which may introduce bias in the results produced by these groups. Fifth, the scoping review approach prevented an overall estimation of the effect and assessment of the methodological quality of these studies, despite the positive effects found on oocyte maturation and embryo development.
Conclusions
In our scoping review, the inclusion of the 231 selected studies allowed us to map body of evidence and thus assess the extent and nature of scientific publications reporting the use of various treatments during in vitro Bovine embryo production. In addition, our summary of the evidence on the effects of hormones, growth factors, sera, and reproductive fluids during oocyte maturation and embryo culture allowed us to synthesize and disseminate the main characteristics and findings of these studies. The evidence presented in our study demonstrates a growing interest in the study of medium composition, since the medium provides the ideal conditions to ensure nutritional and energetic support during oocyte maturation in vitro.
On the one hand, we found a wide variety of treatments used during the oocyte maturation process, among which studies reporting the use of hormones (E2, GH, FSH, and LH) and growth factors (FGF, EGF, and IGF-1) showed positive effects. In addition, the reduction of FBS from 10 % to 3.5 % in the maturation medium was sufficient for oocyte maturation. On the other hand, during embryo culture, the response to growth factors such as EGF, IGF-1, and BMP15 is reflected in greater quantity and quality of embryos. Similarly, under some conditions, FBS and ECS improved the yield and quality of embryos produced in vitro. The information presented here may be useful in identifying hot topics for future investigation, especially for research topics that are inconclusive or have conflicting results. Our categorization of the body of evidence highlights the need for further investigation of those treatments that have shown the best performance in terms of oocyte maturation and embryo culture. In addition, meta-analyses are warranted to quantitatively assess the pooled effect of some of these treatments to enable evidence-based decision making.











 nueva página del texto (beta)
nueva página del texto (beta)