Study contribution
The development of antimicrobial drug resistance (AMR) is a global concern, and the incorrect administration of antimicrobial drugs is one of the main drives promoting AMR. The WHO has urged all Nations to improve their use in an attempt to slow down AMR. Of the β-lactam antibacterial drugs, the benzylpenicillin-G (BP-G) derivatives are among the most widely used antibacterial drugs in Latin America. In this paper, the main pharmacokinetic (PK) characteristics of benzylpenicillin-G derivatives are analyzed and a correlation with clinical applications (pharmacodynamics; PD) made (PK/PD analysis). A proposal on the use of BP-G based on PK/PD considerations is advanced to regulatory authorities and veterinarians. In Latin America, only non-combined BP-G should be marketed, unless evidence to confirm a potentiated effect with other drugs is presented. Comments on faulty design of some commercial preparations containing BP-G as well as notes on withdrawal times, are presented. Proposals on the dose ranges and dosing intervals, are also presented.
Background
The WHO has presented the world the problem of increase in serious and fatal infections in human medicine due to multi-resistant pathogens. All professionals involved in human, animal and even plant health have been urged to take the appropriate measures to optimize the use of antimicrobials. Benzylpenicillin-G (BP-G) is one of human and veterinary medicine’s most widely used antibacterial drugs. According to what has been proposed, it is pertinent to review how it is used in veterinary medicine in general and in production medicine in particular. Where appropriate, an analysis should be made to ensure the adequacy of its use based on rational fundamentals of pharmacokinetics/pharmacodynamics (PK/PD).1,2 It is recognized that bacterial resistance to penicillin occurs mainly though the inactivation of the so-called β-lactam nucleus of the drug (Figure 1). This occurs though the so-called β-lactamase enzymes produced by pathogens and many wild-type bacteria. However, other additional mechanisms include bacterial wall overproduction, bacterial efflux pumps, and structural modification of BP-G docking sites to the so-called penicillin-binding proteins (PFPs) embedded in the wall of pathogens.3
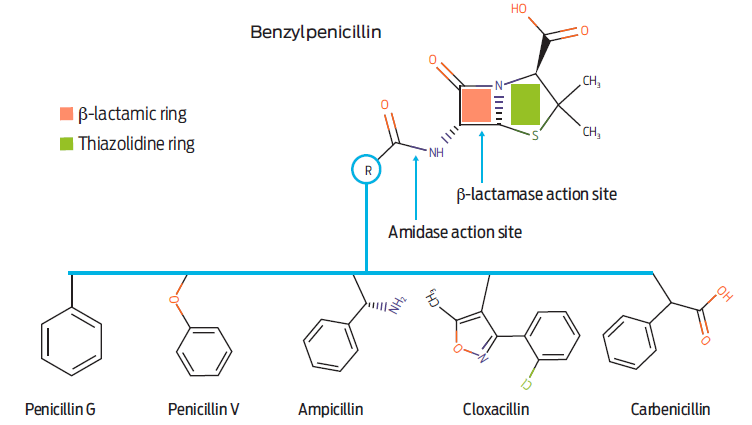
Figure 1 General structural formula of benzylpenicillin and some representative derivatives. The β-lactamase, and amidase action sites are illustrated.
The β-lactam ring is present in all antimicrobials related to this primary nucleus (β-lactam antimicrobials). It includes penicillins (the most used in veterinary medicine), monbactams, carbapenems, cephamycins, carbacephems and even cephalosporins.4 Together, β-lactam antimicrobials represent at least 200 valuable and active antimicrobials. From the preceding, it is clear that if there is an inappropriate use of one of the derivatives of the families mentioned above, the selection of bacteria that produce β-lactamases may exhibit cross-activity and facilitate resistance in other human and/or veterinary pathogens to the original drug and to many other β-lactam derivatives with predictable undesirable consequences for human and animal health. The antimicrobial drug that prompted this manuscript is benzylpenicillin-G (BP-G), the origin and precursor of the historical development of the β-lactam antimicrobial series. The following BP-G derivative salts are considered:5
Salt BP-G • Na and BP-G • K or sodium and potassium benzylpenicillin, also called crystalline or soluble penicillin derivatives. Each milligram of these salts should contain a potency expressed as 1 667 IU or 1 595 IU (IU = international units), respectively.
BP-G procaine salt, whose activity in terms of potency is 1 mg = 1000 IU
BP-G benzathine salt, whose activity in terms of potency fluctuates between 1 400 to 1 600 IU/mg of the salt.
It is worth highlighting that BP-G has very low toxicity. Cases of anaphylaxis, which have been observed mainly after the administration of very high doses and in long standing treatments, are rare.5 When adverse effects occur, they are considered not life-threatening. In horses, procaine toxicoses has been linked to supposedly allergic reactions to penicillin. However, the procaine moiety is considered the responsible chemical due to a 20-fold reduced ability of equine species to metabolize procaine by erithrocyte’s pseudocolinesterases.6 Also, pig susceptibility to severe trembling and a allergic-type rection has been described, particularly when concurrently affected with erysipelosis.7
Dosage of benzylpenicillin-G
Antimicrobials, including the BP-G, have an in vitro antibacterial activity critical concentration that is called the breakpoint. This value is established within the drug’s concentration range that can reasonably be achieved in tissues and blood, given the therapeutic dose range customary accepted for clinical purposes and based on the available pharmacokinetic data. If a higher concentration than the breakpoint is required in a given tissue or compartment, a certain degree or total bacterial resistance is considered to that antimicrobial drug. Hence, breakpoints may vary for each disease and each pathogen. For example: in human medicine breakpoints for BP-G in infections other than meningitis are graded as: susceptible = 2 µg/mL; intermediate = 4 µg/mL and resistant = 8 µg/mL, and for meningitis the breakpoints are: susceptible = 0.06 µg/mL and resistant = 0.12 µg/mL. This means high susceptibility to pathogens causing meningitis, but it also means that high concentrations of BP-G are not easily achieved as it may occur in other tissues.8 Obviously, as bacterial antibiotic resistance increases, breakpoints require revision and adjustment. Consequently, a higher dose is needed to overcome in situ antimicrobial resistance.9 Sometimes the breakpoint of BP-G would appear to be in the lower range in some publications i.e., ≥ 0.25 µg/mL for Staphylococcus aureus.10 This indicates that bacterial susceptibility is exhibited at very low concentrations, but such a breakpoint may not reflect the tendency of this microorganism to develop resistance.11
Streptococcus species tend to show a standard sensitivity and bacterial resistance towards BP-G worldwide. This is somehow predictable as, contrary to expectation, the frequency of antibiotic resistance in organic farms, in which the use of antibiotics must be very restrictive, has not been different from that found conventional farms.12-14 Formal studies of bacterial susceptibility towards BP-G carried out in Latin America are scares. Yet the few available information, allow the proposal that susceptibility of bacteria to BP-G is not different from the rest of the world.15-18
The minimum inhibitory concentration (MIC) needed to destroy 90 % of the strains causing disease is much higher as the resistance of Staphylococcus aureus to BP-G is common among isolates from bovine mastitis.19 Then, the breakpoint should be revised, which will mean that the dose of the antibacterial drug should be increased.20 The breakpoint of BP-G for Streptococcus spp. causing mastitis in cattle is approximately 0.5 µg/mL19 and 0.25 µg/mL for Streptococcus suis.21 Increasing the actual dose is not the only strategy recommended as rapid elimination would result in long periods of low concentrations of BP-G within a dose interval. Hence, adequate handling of BP-G dosage scheme i.e., increasing the dose and shortening the dosing interval, will surely increase clinical efficacy.22 Table 1 exemplifies how the MIC90 values and breakpoints vary among studies on bovine mastitis and depending on the pathogen theoretically susceptible to BP-G. Values presented include the MICs proposal of the Subcommittee on Veterinary Antimicrobial Susceptibility Testing of the European Community for BP-G for various pathogens cattle.21 In general, susceptibility of pathogens is being reduced, increasing the MIC90 value both in human and veterinary medicine. In the former case, clinical studies indicate MIC90 values between 0.25 and 1 µg/mL for Streptococcus spp. group B, and a similar trend has been appointed for Streptococcus suis.23
Table 1 Range of values of benzylpenicillin-G for the main pathogens of mastitis and potentially sensitive to this antimicrobial
| Pathogen | MIC50 | MIC90 | MIC * | Reference |
| Streptococcus uberis | < 0.015 - 0.25 | (19) | ||
| Streptococcus dysgalactiae | < 0.015 | (19) | ||
| Streptococcus agalactiae | 0.03 - 0.06 | (19) | ||
| Staphylococcus aureus | 0.1** | (24) | ||
| Staphylococcus aureus | 0.4 and 24 | (25) | ||
| Staphylococcus aureus | 0.5 | (26) | ||
| Escherichia coli | 64 | > 128 | (27) | |
| Klebsiella pneumoniae | 128 | 128 | (28) | |
| Staphylococcus aureus | 4 | 128 | (28) | |
| Staphylococcus epidermidis | 8 | 32 | (28) | |
| Staphylococcus hyicus | 0.125 | 8 | (28) | |
| Staphylococcus intermedius | 0.125 | (28) | ||
| Staphylococcus xylosus | 0.125 | 0.125 | (28) | |
| Streptococcus agalactiae | 0.125 | 0.125 | (28) | |
| Streptococcus dysgalactiae | 0.125 | 0.125 | (28) | |
| Streptococcus uberis | 0.125 | 0.25 | (28) | |
| Enterococcus faecalis | 2 | 4 | (28) |
* Unspecified susceptibility
** IU/mL
It is clear that breakpoint values are linked to the pharmacokinetics of a given antimicrobial drug. Hence, it is necessary to measure blood and tissue concentrations that can reasonably be achieved and determine for how long such concentrations are maintained between dosing intervals. In this sense, BP-G is considered a time-dependent antimicrobial. That is, β-lactam antibiotics such as BP-G, potentiated aminopenicillins, and cephalosporins are bactericidal drugs. However, their action is slower than other bactericides and generally do not have a post-antibiotic effect. Therefore, its concentration must be maintained above MIC90 (90 % of pathogens in each clinical case) for as long as possible between dosage intervals (T ≥ MIC90). If this is accomplished, optimal bactericidal action will occur, and the clinical effect will be close to ideal.1 For BP-G it is more important to remain at or slightly above the MIC90 value or the breakpoint value in plasma and target tissues throughout the dosing interval (DI), than to achieve a large peak concentration.29 In other words, the idea that a loading dose of BP-G is necessary for clinical efficacy is a misconception. A sufficiently large dose must be injected to achieve the MIC90 as mentioned above or breakpoint values. However, the dosage interval must be adjusted to comply with T ≥ MIC90.1 Thus, the known plasma concentration profiles and pharmacokinetics of the different BP-G derivatives is summarized here:
BP-G • Na and BP-G • K derivatives are administered at high doses of 50 000 IU and up to 100 000 IU/kg body weight by the IV route. These derivatives are recommended in exceptional cases and only in horses. Its duration in plasma is not greater than 4-6 h, even when administered IM or SC. Clearly, this pharmacokinetic behavior does not comply with what is required from the PK/PD point of view when considering the doses and intervals of the existing preparations in Mexico and Latin America.30
BP-G benzathine is another derivative that is slowly absorbed from the site of administration, whether SC or IM and gives rise to what is called FlipFlop pharmacokinetics. That is, the absorption phase is more relevant in describing the drug’s pharmacokinetics than the elimination phase, which occurs in customary pharmacokinetics. However, the sustained and delayed absorption of BP-G from the benzathine derivative fails to achieve the necessary blood and tissue concentrations, as the renal elimination of BP-G is far more efficient. Once in the bloodstream BP-G, has an elimination halflife of less than an hour.3 So regardless of the dose to be evaluated, benzathine BP-G cannot reach useful concentrations, i.e., near the lowest MIC considered. This is why this BP-G derivative has been banned in many countries.
Only procaine BP-G has been considered capable of achieving adequate concentrations in plasma and target tissues in production animals. Absorption from injection sites is faster than with the former derivative but slower than with BP-G Na o K. Then, therapeutically useful concentrations are achieved at doses equivalent to or greater than 20 000 IU/kg of body weight. As an example, Figure 2 shows three dose levels of procaine BP-G, and three levels of MIC values are laid out for pharmacodynamic reference. It is noteworthy that small doses of BP-G do not achieve therapeutic concentrations, sometimes throughout the entire dosing interval. Also, based on projected population pharmacokinetics, two dosage intervals are depicted in Figure 3, every 12 and every 24 hours.31
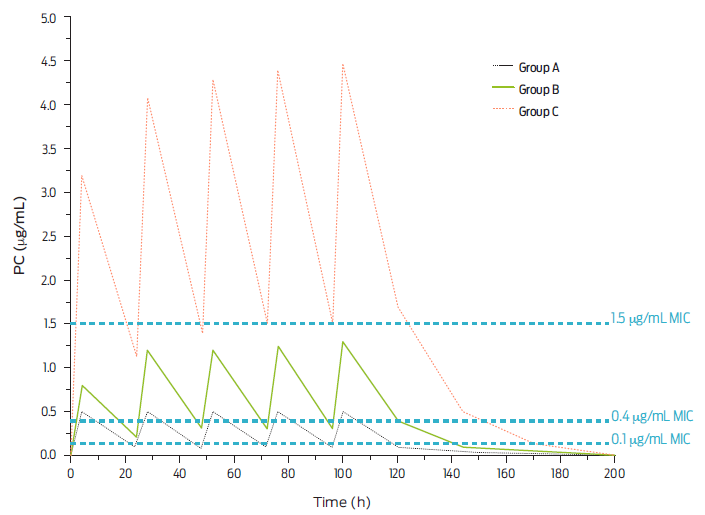
Figure 2 Plasma concentrations of procaine BP-G after daily IM injections of these dose levels of procaine BP-G. Group A: 6 600 IU/kg of body-weight; Group B: 20 000 IU/kg of body-weight and Group C: 66 000 IU/kg of body-weight. Three levels of bacterial sensitivity (minimal inhibitory concentration [MIC]) are noted i.e., 0.1 µg/mL (hypersensitive); 0.4 µg/ mL (proposed breakpoint) and 1.5 µg/mL (slightly sensitive) (adapted from DeDonder et al., 2013).31
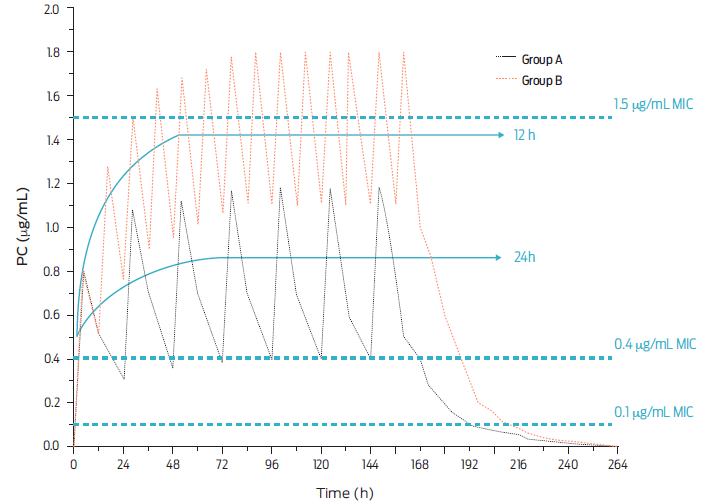
Figure 3 Plasma concentrations of procaine BP-G after a population projection, and given the IM injection of the β-lactam drug at a dose of 20 000 IU/kg body weight either every 12 (Group A) or 24 h (Group B). Steady state is indicated and thee levels of bacterial sensitivity (minimal inhibitory concentration [MIC]) are noted i.e., 0.1 µg/mL (hypersensitive); 0.4 µg/mL (proposed breakpoint) and 1.5 µg/mL (slightly sensitive) (adapted from DeDonder et al., 2013).31
The veterinary pharmaceutical industry has insisted on adding benzathine BP-G to products intended for production animals. However, it is well established as a basic pharmacokinetic principle that a rapidly eliminated active ingredient such as BP-G cannot be formulated for prolonged action unless very low plasma and tissue concentrations of the drug are required. This is the case of benzathine BP-G authorized in human medicine only to treat some diseases caused by hypersensitive pathogens.32 This assumption does not exist in food-producing animals or equines. For this reason, many countries have prohibited the commercialization of preparations with this penicillin derivative for food-producing animals.33 Table 2 presents some different regulatory agencies deployments and comments on this issue. No comments from the rest of the Latina American countries were deemed necessary in this table, as they all approve the use of benzathine BP-G and combinations with other derivatives and many other active principles.
Table 2 Examples of banning benzathine benzylpenicillin-G according to various regulatory agencies
| Country | Comment | Source |
| Canada | Benzathine penicillin is not available for marketing in veterinary medicine | Canadian Food inspection agency https://inspection.canada.ca/animal-health/livestockfeeds/medicating-ingredients/name/eng/1331229958978/1331230120881 |
| Australia | The marketing of natural penicillin derivatives in combination with streptomycin and/or dihydrostreptomycin has been banned since 1999. | Australian Pesticides and Veterinary Medicines Authority https://apvma.gov.au/node/12721 |
| United States | Benzathine BP-G is not marketed either alone or in combination with other antibiotics, and the marketing of natural penicillin derivatives in combination with streptomycin and/or dihydrostreptomycin has been banned. | FDA https://www.fda.gov/media/71374/download |
| Costa Rica | The marketing of veterinary products with benzathine penicillin is banned since 2014 | SENASA https://www.senasa.go.cr/resultados-de-busqueda?q=penicilina |
| European Union | Marketing of veterinary products containing benzathine BP-G has been banned since 2003 | EMEA https://www.ema.europa.eu/en/medicines/veterinary/referrals/benzathine-benzylpenicillinintended-administration-food-producing-species |
A further example of dose ranges is presented in Figure 4. The serum concentrations of BP-G in pigs are presented after the injection of benzathine BP-G or procaine BP-G at doses of 100 000 IU/kg and 33 000 IU/kg of body weight, respectively. Note that serum concentrations of BP-G rapidly fall after achieving their peak plasma concentration (Cmax) and are remarkably low after 24 h in both cases, requiring a second administration at 12 or 24 hours.34
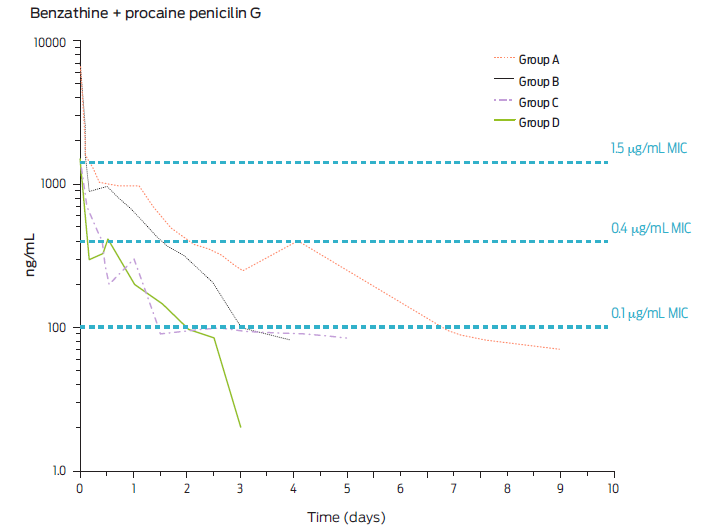
Figure 4 Mean plasma concentration of BP-G in pigs after repeated intramuscular injection of procaine BP-G at a dose of 20 000 every 24 (Group A) or 12 h (Group B) or 30 000 IU/kg bodyweight every 24 (Group C) or 12 h (Group D) and for several days (adapted from Sjölund et al., 2020).30 Steady state is indicated and thee levels of bacterial sensitivity. Minimal inhibitory concentrations (MIC) are noted i.e., 0.1 µg/mL (hypersensitive); 0.4 µg/mL (proposed breakpoint) and 1.5 µg/mL (slightly sensitive). Note that serum concentrations are very low after 24 h (adapted from Ranheim et al., 2002).34
Again, the MICs of very sensitive and breakpoint values are depicted for pharmacodynamic reference.34 It can be highlighted that even with doses much higher than those recommended for any existing preparation in Mexico and Latin America for procaine BP-G, therapeutic concentrations at the breakpoint level are not achieved for much longer than 24 hours. Hence, the existing long-acting pharmaceutical preparations available are misleading clinicians. These studies comply well with the plasma profiles achieved after dosing 20 000 or 30 000 IU/kg of body weight every 12 or 24 h, utilizing only procaine BP-G injected IM into pigs (Figure 5).29, 35
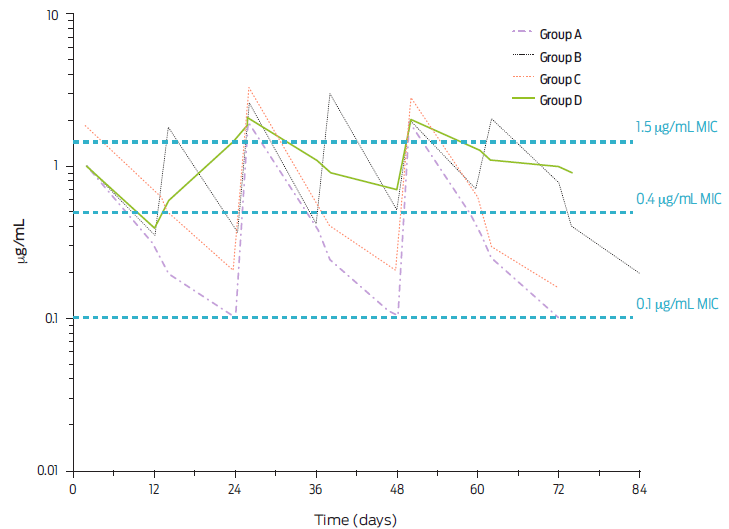
Figure 5 Mean plasma concentration of BP-G in pigs after repeated intramuscular injection of procaine BP-G at a dose of 20 000 every 24 (Group A) or 12 h (Group B) or 30 000 IU/kg body-weight every 24 (Group C) or 12 h (Group D) and for several days (adapted from Sjölund et al., 2020).35 Steady state is indicated and thee levels of bacterial sensitivity (Minimal Inhibitory Concentration) are noted i.e., 0.1 µg/mL (hypersensitive); 0.4 µg/mL (proposed breakpoint) and 1.5 µg/mL (slightly sensitive).
A detailed analysis of the recommended doses of procaine BP-G of existing commercial preparations thoughout Latin America shows an enormous disparity in dose recommendations. However, none present the dose of procaine BP-G of 20 000 IU/kg body weight every 12 or 24 h, advanced here as the minimum dose to be recommended. Commercially available penicillin preparations in Mexico and Latin America recommend dose ranges that can be described as inadequate if care is taken to only quantify procaine BP-G, as the other derivatives are either eliminated very quickly (Na BP-G and K BP-G) or do not achieve adequate serum nor tissue concentrations (benzathine BP-G). To highlight the relationship between the procaine BP-G dose and the concentrations achieved at the target site, the mean concentrations of BP-G in milk from dairy cattle after the daily intramuscular or subcutaneous injection of procaine BP-G for 5 consecutive days and employing four dose levels is presented in Figure 6.36 Also, two bacterial sensitivities are highlighted. This figure shows that if the recommended dose of a commercial product does not approach 20 000 IU of procaine BP-G/kg of body weight, the therapeutic efficacy can be set as borderline. Additionally, concentrations achieved are compatible with the emergence of bacterial resistance, mainly because the dosing interval, often recommended, is every 24 h and sometimes longer if the chosen drug preparation contains benzathine BP-G. Likewise, the intramammary dosages of procaine BP-G that are recommended for clinicians in commercial preparations marketed in Mexico and Latin America range from low to very low. Studies have determined that the intramammary dose should be 600 000 IU/mammary quarter in approximately 10 mL volume.37 Commercially available options for treating clinical mastitis in these countries do not reach this level of inclusion. Instead, formulations often combine procaine BP-G with other antibacterial drugs who’s chemical and pharmacodynamics rationale is questionable.38 It has been established that the efficacy of parenteral procaine BP-G treatment at a dose of 20 000 IU/kg of weight every 24 h has an efficacy indistinguishable from treatment with an intramammary preparation at a dose of 600 000 IU/quarter one or twice daily. Furthermore, there are extreme cases of the irrationality of some pharmaceutical preparations commercially available. For example, preparations intended for intramammary use combine BP-G with sulfonamides, which has been shown to be a pharmacological antagonism described since 1949.39 A preparation that includes procaine BP-G in the list of active vitamin ingredients is not presented as an antibacterial preparation according to manufacturer’s instructions. It is recommended to be administered orally to prevent diseases and as an appetite stimulant in almost all species of veterinary interest.
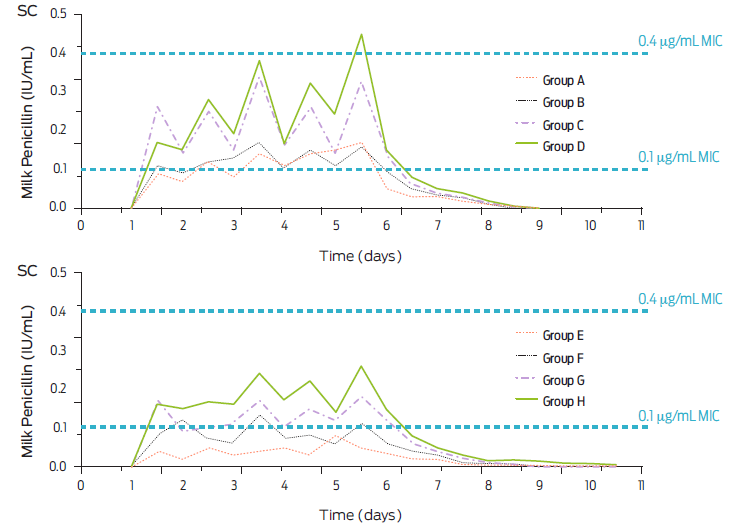
Figure 6 Mean concentrations of BP-G in milk from dairy cows after the daily intramuscular (IM) injection at the following doses: 7 000 IU/kg (Group A), 14 000 IU/kg (Group B), 24 000 IU/kg (Group C) and 28 000 IU/kg (Group D). Similarly, the milk concentrations of BP-G are presented after the subcutaneous (SC) injection of procaine BP-G for 5 consecutive days i.e., 7 000 IU/kg (Group E), 14 000 IU/kg (Group F), 24 000 IU/kg (Group G) and 28 000 IU/kg (Group H). Two minimal inhibitory concentrations (MIC) are highlighted (adapted from Dubreuil et al., 2001).36
Old habits die hard
It is challenging to establish historically when the use of combinations of aminoglycosides with BP-G preparations began, under the perception that their joint action generates an additive, enhancing, or synergistic antibacterial effect. One of the earliest suggestions that aminoglycosides may be synergistic with BP-G may have derived from a comment in a review of a thesis work in human medicine at Boston University in 1949 entitled: “The uses of penicillin and streptomycin”.40 This work advanced the hypothesis that perhaps a synergistic activity of these antimicrobials could be obtained vs. Streptococcus spp. However, no laboratory or clinical trials were presented in the text. Evidently, the comment adjusted to a very different reality from the current one. Despite the absence of evidence and based only on the perception of increased antibacterial effect, many preparations contain the association of an aminoglycoside with BP-G, and these preparations have generated significant sales for many years. They have imprinted the belief that a synergistic antibacterial association is being administered to a veterinary patient. Cure rates for procaine BP-G treatments with and without neomycin have been shown to be virtually identical,41 the same has been documented for streptomycin plus BP-G.42 These conclusions were reached despite the fact that it was attempted that these aminoglycoside-antibacterial drugs somehow became present at the site of infection by experimental methods since normally, the concentrations they achieve in milk and/or mammary epithelium, are marginal and not useful to treat mammary infections, even those caused by gram-negative bacteria.43 This and other arguments strongly suggest that the combined use of BP-G with an aminoglycoside does not increase the treatment efficacy over that achieved with penicillin G alone in bovine clinical mastitis caused by gram-positive bacteria sensitive to penicillin. Worldwide evidence explains why there is a worldwide tendency to revoke marketing permits for these combinations. Furthermore, when this supposed potentiation of actions is administered parenterally, it is incompatible from the pharmacokinetic point of view i.e. BP-G blood:tissue distribution ratio is accepted to approach 1:1, while diffusion of aminoglycosides to mammary tissue or milk is negligible.43,44
Added to the above is the fact that there are indications that the use of aminoglycosides in human and veterinary medicine is associated with a higher prevalence of resistance in both areas, and from the perspective of “One health”, it has been postulated that it is of great importance to safeguarding the clinical efficacy of aminoglycosides by rationalizing their use in human and veterinary medicine,44 mainly because aminoglycosides have an “ɣ” phase of clearance, called residue elimination, with a half-life of 30 h or more 43,44 and therefore the potential to incur in illegal residues in products of animal origin is considerable.45 As discussed, arguments that penicillin-streptomycin combination formulations and other combinations of β-lactams with aminoglycosides, provide synergistic efficacy in the treatment of infections in farm animals, are not well supported to date.46 In addition, the plasma concentration profiles achieved by aminoglycosides and procaine PBG are incompatible with providing a combined effect at the tissue level and plasma levels, i.e., if there is a synergy, it would last only 4-5 h given the rapid elimination of streptomycin and aminoglycoside derivatives in general (T½β = approximately 1 h).43 Based on the pharmacokinetics of a dose of 66 000 IU/kg of body weight of procaine BP-G,46 Figure 7 shows the plasma profiles of the β-lactam derivative and that of streptomycin concurrently injected at a dose of 10 mg/kg. The MIC of 0.4 µg/mL is laid out to show no congruency in the plasmatic concentrations of both active principles at the plasmatic level.
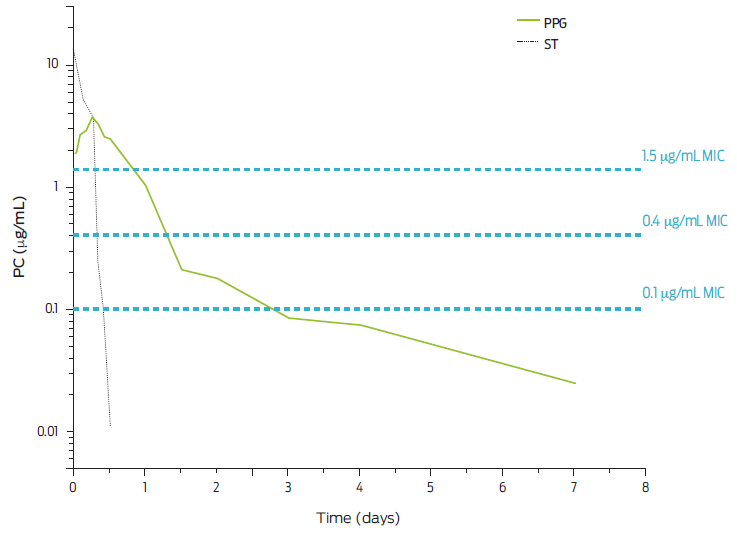
Figure 7 Plasma profiles for five consecutive days of a single IM dose of 66,000 IU of procaine-penicillin G (PPG)/kg bw and 10 mg/kg bw of streptomycin (ST). Three bacterial sensitivities (MIC) for BP-G are highlighted. Adapted from Whittern et al., 1997.35
β-lactam antibacterial drugs are extremely unstable, and in many countries, they are handled in a cold chain. Also, it is preferred that they be suspended immediately before use to preserve the greatest possible antibacterial activity.47 Additionally, it has been reported in reputable monograph that aminoglycosides can be inactivated by many β-lactam antibacterial drugs.48 It is known that in acid or alkaline conditions, BP-G degrades rapidly, with a half-life of approximately 2 h, giving rise to the metabolites: penitic acid, penicilloic acid and peniloic acid mainly, which are highly allergenic.47 Furthermore, it has been described in Mexico that powdered BP-G pharmaceutical preparations have a significantly higher potency than preconstituted ones.2 Therefore, it is not easy to understand how commercial preparations have successfully achieved the stablility of BP-G with other antibacterial drugs and/or some steroidal and non-steroidal anti-inflammatory drugs. Some pharmaceutical preparations include worldwide banned drugs i.e, diclofenac, which caused the decrease of more than 95 % of the vultures of the genus Gyps spp. in South Asia until its ban in 2006.49,50 Furthermore, it is essential to review the safety of the vehicles that allow the stabilization or semi-stabilization of commercial penicillin preparations. For example, the hypersensitivity of cattle to carboxymethylcellulose, a component of many preconstituted penicillin preparations, has been documented.51
Milking and meat withdrawal times
Given the danger of having penicillin residues and its metabolites in milk or meat, it is essential to ensure that these products comply with internationally accepted MRLs. However, the milking and slaughter withdrawal times present significant variations between pharmaceutical preparations. For example, one of the best-selling brands of a transnational company indicates a slaughter withdrawal of 30 days and a milking withdrawal of only 72 h. After suspending the powder with a diclofenac-containing vehicle containing benzathine BP-G 200 000 IU; procaine BP-G 100 000 IU; potassium BP-G 100 000 IU; streptomycin sulfate 166 mg and 15 mg of sodium diclofenac. The manufacturer recommends a range of 1 to 4 mL/40 kg of body weight, equivalent to 10 000 to 40 000 IU/kg of the combined penicillin salts, but only from 5 000 to 10 000 IU/kg of body weight, considering only the procaine BP-G.
In the formal literature, it has been highlighted that the sole elimination of procaine penicillin G (not counting benzathine BP-G, which, due to its FlipFlop pharmacokinetics, has a much longer stay in the body) requires a milk withdrawal time of 5-6 days, considering two milking per day and an MRL value of 4 ppb (4 µg/L of milk).52 According to probabilistic Monte Carlo simulations, if the doses of procaine BP-G administered are equal to or greater than 33 000 IU/kg and up to 66 000 IU/kg, 7 to 8 and up to 10 days of milk withdrawal will be required.3 As far as slaughter withdrawal time is concerned, and based on FARAD data (Food Animal Residue & Avoidance Databank; University of Florida), a withdrawal time of 21 days is required.53 Other authors have also postulated this withdrawal time.3, 54
Proposal for the use of BP-G in veterinary medicine in food-producing species
Driven by the need to rationalize the use of benzylpenicillin-G in foodproducing species, and in order to help mitigate the growth of the rate of bacterial resistance in man and production species, the following actions are proposed:
Only procaine benzylpenicillin-G should be used in food-producing species (sheep, goats, cattle, pigs).
The recommended dose in these species should not be less than 20 000 IU/kg of body weight, with 12 to 24 h of dosing intervals and for at least 4-6 days.
Other antimicrobials should not be combined with procaine BP-G in commercially available formulations unless a robust body of information on chemical stability and pharmacological evidence is presented and suggests otherwise. For example, including independent trials that show an increase or potentiation of antibacterial efficacy employing isobolograms vs. specific pathogens and at concentrations consistent with the PK/PD of the drugs involved; proof of augmented or improved clinical efficacy; pharmacokinetics of the components of a given formula to rationalize their joint activity, including dose rate, dosing interval and distribution to target sites.
Fully documented adjustments must be made to the milk and meat withdrawal times of the preparations to be marketed.
If an additional drug is combined in a new pharmaceutical preparation, a sufficiently robust body of proof on chemical stability, withdrawal times, and clinical test(s) must be made available.
Data availability
All relevant data are within the manuscript.
Acknowledgments
MVZ Ana María Román Díaz and MVZ Cristian López Montelongo for their help in finding information to carry out this work.
Funding statement
This research was funded by Universidad Nacional Autónoma de México (www.unam.mx). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
“The authors have no conflict of interest to declare in regard to this publication”.
Author contributions
Writing-original draft: Lilia Gutiérrez, Héctor Sumano
Conceptualization: Lilia Gutiérrez, Héctor Sumano
Investigation: Luis Ocampo, Agustín Nieto
Writing-review and editing: Itzcoatl Aquino











 nueva página del texto (beta)
nueva página del texto (beta)



