Artículos científicos
Acute toxicity assessment and subacute of compound alpha injectable (5-chloro-2-(methylthio)-6-(1naphthyloxy)-1H-benzimidazole) in rats
Zapata-Arenas, Abel1
Gracia-Mora, I.2
 http://orcid.org/0000-0001-9381-3673
http://orcid.org/0000-0001-9381-3673
Castillo, R.3
 http://orcid.org/0000-0003-4466-2340
http://orcid.org/0000-0003-4466-2340
Lezama-Ramírez, J.4
Rico-Morales, H.5
Bustamante-García, R.6
Ibarra-Velarde, F.1*
Gracia-Mora, I.2
 http://orcid.org/0000-0001-9381-3673
http://orcid.org/0000-0001-9381-3673Castillo, R.3
 http://orcid.org/0000-0003-4466-2340
http://orcid.org/0000-0003-4466-2340Lezama-Ramírez, J.4
Rico-Morales, H.5
Bustamante-García, R.6
Ibarra-Velarde, F.1*
Abstract
The objective of this work was to evaluate the acute and subacute toxicity of the injectable alpha compound to Hsd Han: WIST rats. For the acute toxicity assessment, rats were inoculated with doses of 2 000, 1 000, 500, 100, or 10 mg/kg alpha compound intramuscularly. The results of histopathological analysis did not show apparent changes with the doses used. Notably, 2 animals died after treatment with the 2 000 mg/kg dose, and one animal died in the 1 000 mg/kg group. In the subacute toxicity study, neither male nor female rats inoculated with repeated doses of 8, 24, and 40 mg/kg alpha compound showed significant changes in percent body weight (P = 0.5930). Among the females, significant differences were observed for the serum biochemical parameters glucose and cholesterol in the group inoculated with 8 mg/kg compared to the control. The hematocrit values in the 8, 24 and 40 mg/kg groups and the platelet count in the 24 mg/kg group were significantly different (P = 0.0110) among the females compared to the control group. For the male rats, significant differences were found in erythrocyte count and mean corpuscular volume (MCV) in the groups treated with 8 and 40 mg/kg. However, these values are normal according to the literature. The histopathological evaluations did not show apparent changes, so it is concluded that the alpha compound has a wide margin of safety after injection.
Keywords::
Alpha compound, Fasciolicide, acute and subacute Toxicity, Rats
Study contribution
Products formulated with benzimidazoles are not soluble, so they must be administered as a suspension at high doses. This alpha compound formulation (based on the solubility of benzimidazoles) has been studied for decades. Previous studies have determined the fasciolicidal activity of the alpha compound in sheep and cattle to reduce animal handling as well as to administer a lower dose of the active compound. In the present study, the acute and subacute toxicity of the injectable alpha compound was evaluated in Hsd Han: WIST rats by means of serum biochemical analysis, hematology and histomorphological study in terms of subacute toxicity. The data obtained indicated that there were no significant changes in the parameters analyzed; therefore, this drug has a wide margin of safety.
Introduction
Fasciola hepatica is a zoonotic trematode that affects different mammalian hosts, including humans.1,2 Fasciolosis is an important parasitic disease in veterinary medicine because it causes global economic losses in the livestock industry by decreasing milk productivity, meat and wool production, the growth of young animals and fertility. Fasciolosis also causes abortions and secondary bacterial infections, so the cost of the product rises and controlling parasitism in the animals is difficult.3,4 The prevalence of F. hepatica infection has risen due to climate change, the increased movement of animals, and changes in farming practices. On farms, it is necessary to improve the strategies to control this type of parasitosis to reduce drug resistance that can result from the constant use of drugs.5
-
1Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanidePLOS Neglected Tropical Diseases, 2013
-
2Human fasciolosis diagnosed in the acute phase: a first clinical report in MexicoRevista de Gastroenterología de México, 2016
-
3Occurrence of Fasciola hepatica (LINNAEUS, 1758) infection in Brazilian cattle in the State of Minas GeraisRevista Brasilera de Parasitología Veterinaria, 2009
-
4Chemotherapy of infections with Fasciolidae, 1994
-
5Modelling recent and future climatic suitability for fasciolosis in EuropeGeospatial Health, 2015
The method of controlling this parasite has been based on the application of fasciolicides in the definitive host.6 This has been achieved with, among other products, benzimidazoles, such as albendazole. Albendazole has broad-spectrum activity against flukes, but its activity is restricted to the mature parasite, and high doses are required.7 Another drug is triclabendazole, which is very effective against adult and juvenile flukes.8 On the other hand, since animals have developed parasite resistance, the efficacy of some chemical drugs, such as triclabendazole (TCBZ), closantel and clorsulon, against immature and adult parasites in sheep and cattle has decreased.9,10 However, since 1983, when launched triclabendazole on the market, the pharmaceutical industry has introduced several fasciolicides, some are imported and are expensive. So it is necessary to search alternatives on the development of new drugs for the control of this important parasitic disease.
-
6Drug resistance in liver flukesInt. J. Parasitol. Drugs Drug Resist., 2020
-
7Fasciolicides: efficacy, actions, resistance and its managementVeterinary Journal, 1999
-
8Anthelmintic efficacy of triclabendazole against Fasciola hepatica in sheepVeterinary Record, 1984
-
9Resistance of Fasciola hepatica against triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheepVeterinary Parasitology, 2013
-
10Triclabendazole-resistant Fasciola hepatica in southwest WalesVeterinary Record, 2000
In Mexico, F. hepatica is found mainly in grazing animals (sheep and cattle).11 In the early 1990s, the compound Fasciolinip-1 (2-amino-5(6)-chloro-1-methoxycarbonylbenzimidazole), a precursor of the alpha compound, eliminated limited numbers of 8-week-old flukes and 90.6 % of 12-week-old flukes in sheep.12 Subsequently, the alpha compound (5-chloro-2-(methylthio)-6-(1-naphthyloxy)-1H-benzimidazole) showed high efficacy against immature and adult F. hepatica after natural or artificial infection in calves, sheep and cattle, similar to that of TCBZ.13-15 Similar to other benzimidazoles, the alpha compound is poorly soluble in aqueous solvents, which is the greatest impediment for its administration via intramuscular or other routes.16
-
11Prevalence of Fasciola hepatica (ELISA and fecal analysis) in ruminants from a semi-desert area in the northwest of MexicoParasitology Research, 2007
-
12Fasciolinip-1: experimental fasciolicidal activity in sheepRevista Latinoamericana de Microbiología, 1995
-
13Eficacia comparativa de un fasciolicida experimental, triclabendazol y closantel en bovinos infectados en forma natural con Fasciola hepáticaVeterinaria México, 2002
-
15Efficacy of an experimental fasciolicide against immature and mature Fasciola hepatica in artificially infected calvesParasitoly Research, 2004
-
16Pharmacokinetic behavior in sheep and cattle of 5-chloro-2-(methylthio)-6-(1-naphthyloxy)-1H-benzimidazole, a new fasciolicide agentJournal of Veterinary Pharmacology and Therapeutics, 2009
Recently (2017), the water soluble prodrug of the alpha compound was synthesized by adding a phosphonooxymethyl group to improve its solubility (13 mg/ mL at pH = 7) and availability via in vivo hydrolysis by alkaline phosphatases in different tissues; this makes parenteral administration at reduced doses more favorable. The phosphate disodium salt demonstrated an efficacy of 87.8 % against adult parasites in artificially infected sheep using an intramuscular dose of 4 mg/kg compared to that reported for the alpha compound, which had an efficacy of 86.9 % at a dose of 15 mg/kg PO.17 Recently (2017), a new water soluble prodrug of TCBZ was reported. This prodrug was 88 000 times more soluble than TCBZ and had an efficacy close to 100 % in artificially infected sheep after an intramuscular dose of 8 mg/kg.18 However, before it can be applied for clinical use in animals, studies on its toxicity are needed to determine the safety of this new prodrug.
-
17A highly water soluble benzimidazole derivative useful for the treatment of fasciolosisBioorganic Medicine Chemestry Letter, 2014
-
18Novel triclabendazole prodrug: a highly water soluble alternative for the treatment of fasciolosisBioorganic Medical Chemestry Letter, 2017
Acute and subacute toxicity are based on the determination of toxicity after a single administration and repeated administrations of the drug, respectively. Both tests consider the following parameters: mortality, signs of toxicity, histopathology and weight change in accordance with the guidelines of the Organization for Economic Cooperation and Development (OECD). The guidelines stipulated by the OECD regulate the uniformity of biological assays both in vivo and in vitro for different purposes, guaranteeing the quality and effectiveness of the results obtained worldwide. However, in Mexico, no toxicity studies on these new fasciolicide compounds have been carried out. Therefore, the objective of this study was to evaluate the acute and subacute toxicity of alpha compound injection in experimental rats to determine its safety.
Material and methods
Ethical statement
The animal experimental protocols were approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL) of the Faculty of Chemistry of the UNAM (number FQ/CICUAL/165/16 and FQ/CICUAL/204/17). The study followed national and international standards for animal welfare and care.
Animals
In the acute toxicity studies, 18 female rats were used, and in the subacute toxicity studies, 24 female and 24 male Hsd Han: WIST rats aged 8 to 10 weeks were used, which were obtained from ENVIGO-Mexico (USA). All rats were identified by ear notches and housed in polysulfonate boxes with the following dimensions: 26.03 × 47.62 × 20.32 cm. Pine shavings were used, and the environmental conditions of temperature, relative humidity and light/dark cycle were 22 ±2 °C, 40-60 % and 12/12 h within the Animal Experimentation Unit (UNEXA) and in the Preclinical Research Unit (UNIPREC) of the Faculty of Chemistry, Group E of the National Autonomous University of Mexico (UNAM) in accordance with NOM-062ZOO-1999 of Technical Specifications for the Production, Care and Use of Animals of Laboratory.
All rats were fed Harlan Teklad 2018S mouse/rat chow and filtered water ad libitum for the acute toxicity study and 500 mL of each for three to four days for the subacute toxicity studies using an automatic sanitization module throughout the study period. The rats were acclimatized for five days before treatment. During this period, their health status was observed, and they were then randomized into an experimental group. The protocols for the use of experimental animals were approved by the CICUAL of the Faculty of Chemistry of the UNAM.
Synthesis of the alpha compound
The injectable alpha compound was synthesized in the Department of Pharmacy of the Faculty of Chemistry of the National Autonomous University of Mexico according to the methodology of Flores-Ramos et al. (2014).17
-
17A highly water soluble benzimidazole derivative useful for the treatment of fasciolosisBioorganic Medicine Chemestry Letter, 2014
Acute toxicity study
The acute toxicity test was carried out in accordance with the Guide of the Organization for Economic Co-operation and Development (OECD, No. 423). This guideline suggests using a higher than recommended dose series up to a maximum dose of 2 000 mg/kg body weight. Although the recommended dose for this injectable fasciolicide is 4 mg/kg body weight in ruminants,17 six groups of three nonpregnant nulliparous rats were used. The alpha compound doses used were 2 000 (Group 1), 1 000 (Group 2), 500 (Group 3), 100 (Group 4) and 10 (Group 5) mg/kg body weight diluted in sterile water, and inoculation was performed intramuscularly. Finally, Group 6 (control group) was inoculated intramuscularly with sterile water. Animals were observed individually for the first 30 minutes after inoculation and then periodically monitored for the first four hours. They were reviewed at 24 hours and then daily for 14 days. The behavior pattern, signs of toxicity (tremors, seizures, salivation, diarrhea, lethargy, and coma), and mortality were documented.
-
17A highly water soluble benzimidazole derivative useful for the treatment of fasciolosisBioorganic Medicine Chemestry Letter, 2014
Subacute toxicity study
Subacute toxicity tests adhered to the 28-day duration parameter according to the Guidance for Industry Target Animal Safety for Veterinary Pharmaceutical Products (No. 185, Food and Drug Administration, FDA) and OECD Guideline No. 407. Twenty-four females and 24 males were used, and both sexes were grouped into four groups with six animals each. The rats in the experimental groups were treated intramuscularly with the alpha compound at 8 mg/kg (Group 1), 24 mg/kg (Group 2) and 40 mg/kg (Group 3) body weight on Days 0, 7 and 14.
The animals in the control group (Group 4) were inoculated with sterile water. The welfare parameters (behavioral pattern, mortality, and signs of toxicity such as tremors, convulsions, salivation, diarrhea, lethargy, and coma) of the animals were observed during the first 30 minutes, at 4 and 24 hours after each inoculation, and then daily for 28 days. The body weight and mortality were recorded, and the food and water intake were noted and analyzed according to the following equations:
Serum biochemical analysis and hematological study
Blood samples were taken from each animal on Days 0 and 14 after treatment in the acute toxicity test, and for those undergoing the subacute toxicity test, blood samples were taken on Days 0, 14, and 28. The following methodology was used for both studies. Each blood sample (600 µL) was obtained humanely from puncture of the retroorbital plexus with the help of a capillary tube under anesthesia with isoflurane (5 %) using an anesthesia machine (VET EQUIP IMPAC6). The sample was deposited in two tubes, one with anticoagulant (EDTA) and one without, for biochemical and hematological studies. Serum biochemical parameters included determining glucose, blood urea nitrogen (BUN), creatinine, total cholesterol, triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total protein, urea and total bilirubin.
Hematological parameters, such as hematocrit (HCT), hemoglobin (HGB), erythrocyte count (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), leukocyte count (LE), neutrophil (NE), lymphocyte (LY), monocyte (MO) and eosinophil (EO), were measured using an automated biochemical analyzer (CS-T240, DIRUI model) and a hematology analyzer (S. MBC VET, KONTRO Lab model).
Pathological analysis
After completing the acute and subacute toxicity testing, animals in each study were euthanized by carbon dioxide (CO2) exposure in a euthanasia chamber. The death of the animals was verified by the absence of vital signs. Necropsies were performed to observe the presence or absence of changes in the skin and muscle (inoculation site). Brain, heart, lung, liver, spleen, kidney, and adrenal gland samples were collected from the animals for histopathological examination. Tissues were fixed in 10 % formalin solution (pH = 7), dehydrated and paraffin embedded using a histokinette and paraffin embedder (Leica model TP 1020 and Leica EG 1150 H and EG 1150 C). Subsequently, 4-6 µm sections were obtained with the aid of a microtome (Leica RM 2125 RTS) and stained with hematoxylin and eosin (H&E) for analysis by light microscopy.
Statistical analysis
Statistical analysis was carried out using SIGMA PLOT 13 software. Percent changes in body weight, food consumption and water intake, as well as serum biochemical and hematological parameters, were analyzed using one-way ANOVA (analysis of variance), Student’s t test and Tukey’s multiple comparison test for the different doses in the treated groups compared to the control group. All analyses were performed considering a confidence level of 95 % (P ≤ 0.05).
Results
Acute toxicity
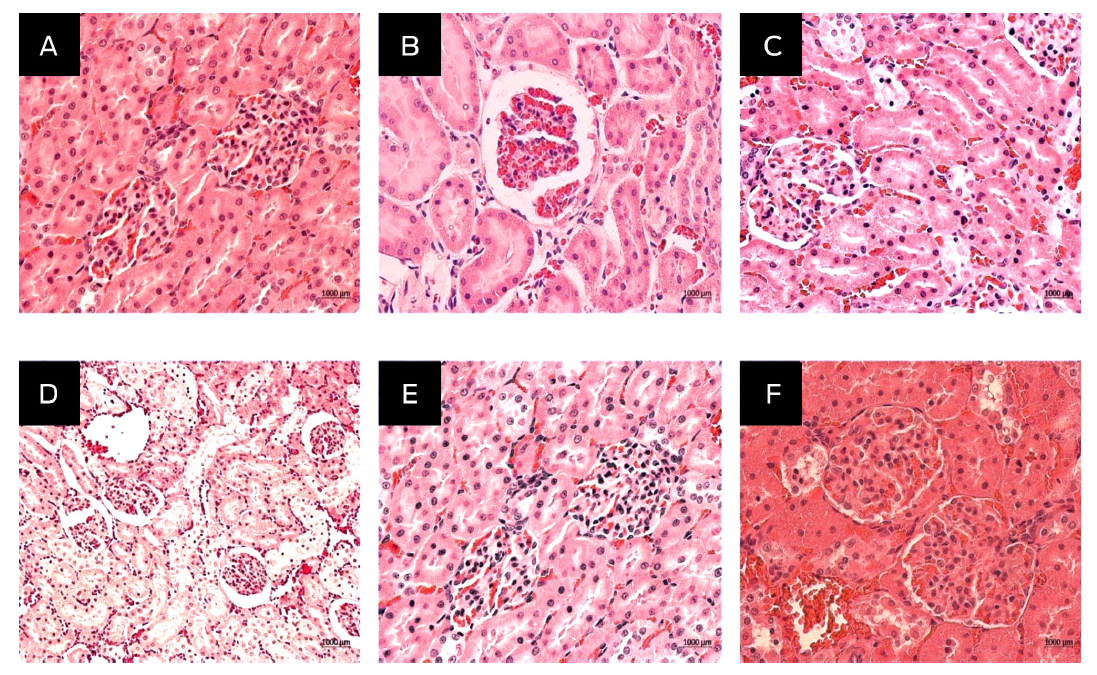
In the alpha compound toxicity test, statistical analysis of the weights and blood biochemical parameters could not be performed due to the mortality of two animals in the 2 000 mg/kg group and one animal in the 1 000 mg/kg group. This was because, as mentioned in the guide, the compound is considered toxic at the dose at which animal death occurs. Signs of toxicity, pathology and weight change were not assessed due to the lack of these data. Necropsy was not performed on these animals, since their death occurred after 48 h posttreatment during the night, and there were already signs of decomposition; in addition, the exact time of death was unknown. Histopathological analysis did not show apparent changes in body weight in the other animals inoculated with doses of 2 000 (Group 1), 1 000 (Group 2), 500 (Group 3), 100 (Group 4) and 10 (Group 5) mg/kg. The organs collected, such as muscle, brain, heart, lung, liver, spleen, and adrenal glands, had no apparent histomorphological changes compared with the control group. The renal tissue of the treated groups showed a normal cell morphology, similar to that in the control group (Figure 1).
Thumbnail
Figure 1
Histopathological finding in the acute toxicity of the injectable alpha compound in the kidney of Hsd Han: WIST inoculated with 2 000 (A), 1 000 (B), 500 (C), 100 (D) and 10 (E) mg/kg body weight of injectable alpha compound and control group (F). Histological sections were stained with H&E and viewed at 40X.
Histopathological finding in the acute toxicity of the injectable alpha compound in the kidney of Hsd Han: WIST inoculated with 2 000 (A), 1 000 (B), 500 (C), 100 (D) and 10 (E) mg/kg body weight of injectable alpha compound and control group (F). Histological sections were stained with H&E and viewed at 40X.
Subacute toxicity
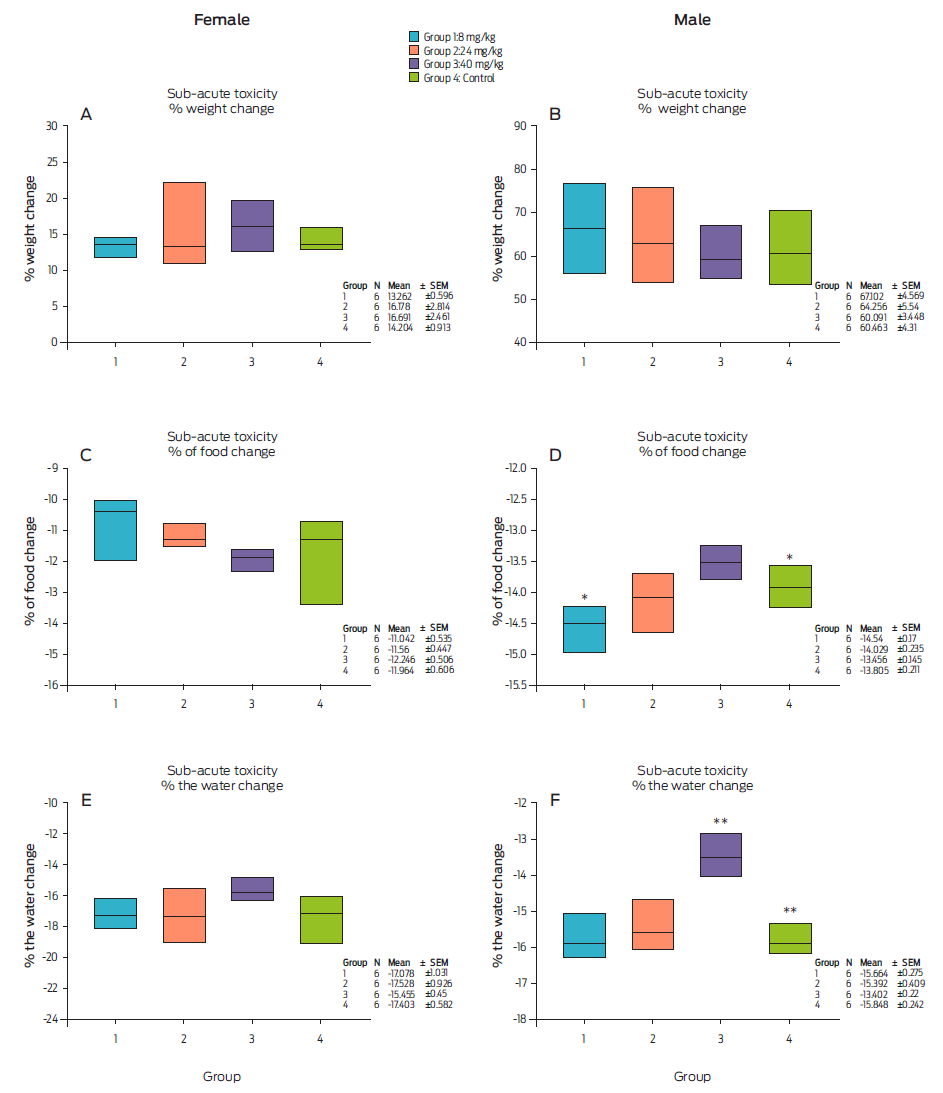
In Figure 2A, the percent body weight change among the female Hsd Han: WIST rats was observed for the groups dosed with 8 (Group 1), 24 (Group 2), and 40 (Group 3) mg/kg body weight alpha compound and injected water (Group 4). Changes were observed in the percent body weights of the rats in Group 2 (24 mg/kg), although no significant differences were found between the experimental groups (Kruskal‒Wallis: Hg.l. = 3; 0.05 = 1.9; P = 0.5930). For the males (Figure 2-B), the percentage body weight changed slightly in Groups 1 (8 mg/kg) and 2 (24 mg/kg), but this change was significantly different between groups (F = 0.541, P = 0.6630)
Thumbnail
Figure 2
Effect of compound alpha on 28-day subacute toxicity on percent body weight, food consumption, and water intake of female and male Hsd Han: WIST. * Statistical significance (Kruskal-Wallis: Hg.l. = 3; 0.05 = 14.102; P = 0.003); **Statistical differences between groups 4 and 3 (P ≤ 0.0010).
Effect of compound alpha on 28-day subacute toxicity on percent body weight, food consumption, and water intake of female and male Hsd Han: WIST. * Statistical significance (Kruskal-Wallis: Hg.l. = 3; 0.05 = 14.102; P = 0.003); **Statistical differences between groups 4 and 3 (P ≤ 0.0010).
In Figures 2C and D, food consumption by the treated females (six rats per group) and males (six rats per group) is shown and compared to that by the control group (six rats without treatment). Among the females, the percent food consumption was calculated with the following equation:
Group 4 (water injection) presented a change in food consumption (Figure 2C), but this difference was not significant compared with the treatment groups (Kruskal‒ Wallis: Hg.l. 3; 0.05 = 7.55; P = 0.0560). A similar trend in percent food consumption was observed (between 11 and 12 %).
Among the males (Figure 2D), a significant difference was observed in Group 3 (40 mg/kg) compared to Group 1 (8 mg/kg) (Kruskal‒Wallis: Hg.l. = 3; 0.05 = 14.102; P = 0.0030). Moreover, significant differences were found between Group 1 (8 mg/kg) (P = 0.0170), Group 2 (24 mg/kg) (P = 0.4420) and Group 3 (40 mg/kg) (P = 0.0650) compared to the control group (water injection). Statistical analysis indicated that there was a difference between Group 1 (8 mg/kg) and Group 4 (water injection), taking into account that the range of change in the percent food consumption in all groups was similar (13-14 %).
In Figure 2E, an increase in food consumption is observed, but no statistical differences were found between groups in female rats (P = 0.2370). However, water consumption was similar in all groups (15-17 %), although statistically significant differences were observed in males (Figure 2F). There were differences in Group 3 compared to the other groups (P ˂ 0.001).
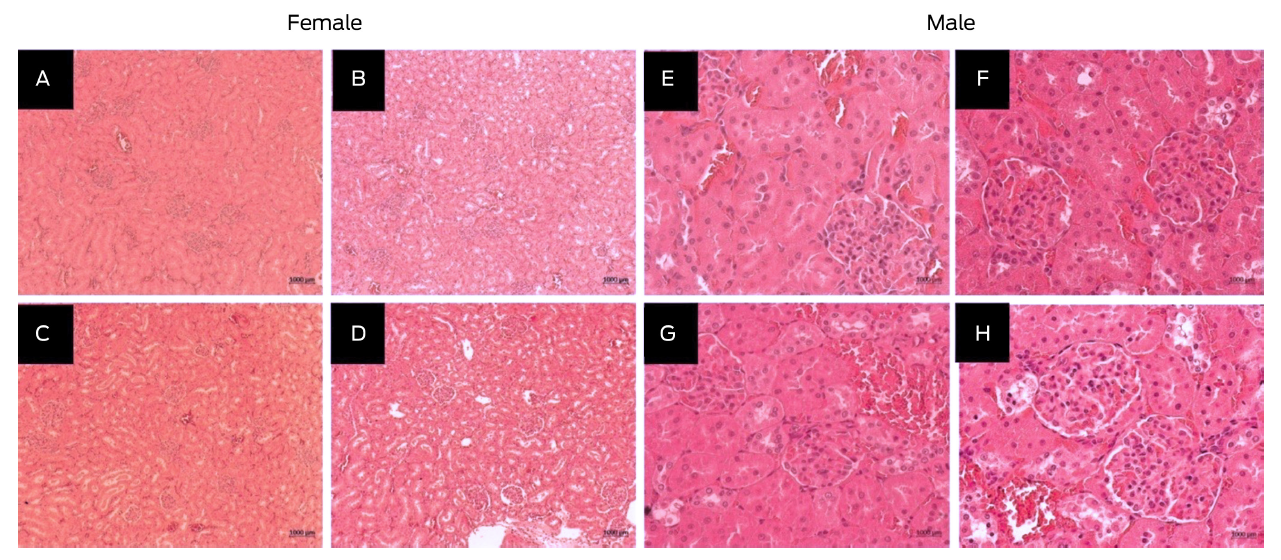
Student’s t test was performed to determine the significance of the differences between Groups 3 and 4 (P ≤ 0.001). In the other groups, there were no significant differences. The water intake in Groups 1, 2 and 4 was approximately 15 %, while Group 3 displayed a reduction to 13 %. Therefore, food and water consumption did not affect body weight in all groups. No apparent changes were observed in the histological findings of the muscles and organs analyzed in both the treated and the control groups of animals. From the histopathological examination, the morphological structure of the kidney was normal (Figure 3).
Thumbnail
Figure 3
Histological finding in subacute kidney toxicity of female rats (A, B, C, and D) and male rats (E, F, G, and H) Hsd Han: WIST inoculated with 8 (A, E), 24 ( B, F), 40 (C, G) mg/kg per body weight of the alpha compound and the control group (D, H; water for injection). Histological sections were stained with H & E and were observed at 10X and 40X.
Histological finding in subacute kidney toxicity of female rats (A, B, C, and D) and male rats (E, F, G, and H) Hsd Han: WIST inoculated with 8 (A, E), 24 ( B, F), 40 (C, G) mg/kg per body weight of the alpha compound and the control group (D, H; water for injection). Histological sections were stained with H & E and were observed at 10X and 40X.
The effect of alpha compound treatment on serum biochemical parameters are shown in Table 1. A statistical difference with the glucose analyte was found between groups 1 and 2, compared to group 3 using a one-way ANOVA (P = 0.0010) according to the following equation:
Table 1
Results of serum biochemical parameters in the subacute toxicity study in female Hsd Han: WIST rats
Results of serum biochemical parameters in the subacute toxicity study in female Hsd Han: WIST rats
| Media ± SEM+ | ||||
| Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
| Glucose (mmol/L) | -25.88 ±1.43* | -17.60 ±5.41* | -13.19 ±7.13* | 11.22 ±6.78 |
| BUN (mmol/L) | 17.72 ±7.83 | 17.36 ±9.59 | 17.30 ±6.76 | 1.36 ±4.75 |
| Creatinine (mmol/L) | 2.30 ±3.77 | -0.29 ± 2.2 | 2.55 ±3.64 | -6.70 ±4.49 |
| Cholesterol (mmol/L) | -9.85 ±2.54** | 0.68 ±3.77 | 1.55 ±4.49 | 5.92 ±1.94 |
| Triglycerides (mmol/L) | -39.89 ±10.34 | 12.11 ±13.32 | 11.99 ±23.69 | 37.45 ±24.85 |
| ALT (U/L) | 2.13 ±8.05 | 8.41 ±7.51 | 12.61 ±11.13 | 19.65 ±9.81 |
| AST (U/L) | 0.80 ±7.95 | -8.77 ±11.82 | 20.36 ±11.67 | 9.58 ±15.23 |
| ALP (U/L) | -15.2 ±2.74 | -15.63 ±4.82 | -25.51 ±4.68 | -4.27 ±6.77 |
| Proteins (mmol/L) | 3.47 ±1.61 | 2.89 ±3.12 | 7.59 ±2.2 | 4.70 ±2.35 |
| Urea (mmol/L) | 20.23 ±7.22 | 18.76 ±9.7 | 16.27 ±6.05 | 1.28 ±5.67 |
| Bilirubin (mmol/L) | 35.97 ±25.92 | 12.31 ±14.89 | 71.15 ±30.83 | 16.11 ±12.74 |
To corroborate this difference, Tukey’s test showed a statistical difference between groups 1, 2 and 3 compared to group 4. In addition, cholesterol was significant in two groups: group 1 and the control group, using Tukey’s test. (P = 0.0220). In contrast, in Table 2, no statistical difference was found in male rats.
Table 2
Results of the biochemical parameters from sera of the subacute toxicity study in male rats Hsd Han: WIST
Results of the biochemical parameters from sera of the subacute toxicity study in male rats Hsd Han: WIST
| Parameters | Males | |||
| Media ± SEM | ||||
| Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
| Glucose (mmol/L) | -6.48 ±3.01 | -12.78 ±4.88 | 5.98 ±8.7 | 2.02 ±5.75 |
| BUN (mmol/L) | 3.99 ±3.19 | 3.7 ±2.25 | 1.32 ±2.18 | -2.41 ±6.36 |
| Creatinine (mmol/L) | 7.06 ±3.9 | 4.77 ±3.98 | 6.26 ±4.12 | 6.81 ±4.06 |
| Cholesterol (mmol/L) | -18.10 ±1.99 | -16.31 ±2.32 | -21.46 ±1.84 | -11.72 ±3.76 |
| Triglycerides (mmol/L) | 29.35 ±10.35 | 48.26 ±14.84 | 47.80 ±18.93 | 73.59 ±24.83 |
| ALT (U/L) | -2.67 ±3.54 | -6.80 ±4.47 | -14.27 ±2.27 | -8.09 ±5.93 |
| AST (U/L) | -17.65 ±3.7 | -7.75 ±10.52 | -14.21 ±3.82 | -10.92 ±5.29 |
| ALP (U/L) | -31.80 ±1.27 | -29.29 ±3.21 | -37.17 ±2.21 | -36.09 ±2.37 |
| Proteins (mmol/L) | 7.79 ±2.49 | 8.18 ±1.94 | 4.61 ±0.61 | 7.3 ±1.03 |
| Urea (mmol/L) | 4.16 ±4.51 | 3.85 ±2.88 | 1.31 ±1.92 | -2.73 ±5.63 |
| Bilirubin (mmol/L) | 13.18 ±7.44 | 11.40 ±15.7 | 23.67 ±17.66 | 15.99 ±7.96 |
Table 3 shows the significant differences from the hematological analysis of the female rats. There were changes in hematocrit in the female rats in the 8 mg/kg, 24 mg/kg and 40 mg/kg groups compared to the control group and a change in the platelet count between the 24 mg/kg and control groups, showing a significant difference by means of a one-way ANOVA (P = 0.0370), and Tukey’s test corroborated this difference (P = 0.0110).
Table 3
Results of the hematological parameters in the subacute toxicity study in female rats Hsd Han: WIST
Results of the hematological parameters in the subacute toxicity study in female rats Hsd Han: WIST
| Parameters | Females | |||
| Media ± SEM+ | ||||
| Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
| HCT (L/L) | 9.92 ±1.66* | 12.09 ±3* | 8.52 ±0.38* | 1.33 ±1.33 |
| HGB (g/L) | 7.38 ±1.12 | 5.30 ±2.09 | 8.59 ±1.17 | 3.89 ±2.04 |
| RBC (×1012/L) | 19.03 ±5.57 | 26.74 ±6.89 | 24.91 ±4.5 | 21.62 ±2.81 |
| MCV (fL) | -6.65 ±4.23 | -10.54 ±4.59 | -12.82 ±3.08 | -13.57 ±1.28 |
| MCHC (g/L) | -2.49 ±1.07 | 6.143 ±2.28 | 6.02 ±6.04 | -0.57 ±1.55 |
| PL (×109/L) | 57.47 ±27.77 | 75.15 ±25.65* | -23.46 ±14.33 | -3.40 ±15.28 |
| LE (×109/L) | -35.18 ±4.58 | -30.14 ±13.26 | -22.34 ±10.97 | -27.68 ±9.7 |
| NE (×109/L) | 9.91 ±33.59 | -36.27 ±21.75 | -19.98 ±30.04 | -14.96 ±35.14 |
| LY (×109/L) | -37.55 ±5.82 | -29.14 ±12.53 | -20.11 ±13.03 | -25.75 ±10.07 |
| MO (×109/L) | -11.67 ±17.97 | 11.67 ±4.01 | -3.33 ±19.94 | -16.67 ±16.67 |
| EO (×109/L) | -33.33 ±21.08 | -50.0 ±22.36 | -15.0 ±17.08 | -45 ±24.73 |
In Table 4, it can be seen how the RBC and MCV in male rats were significantly different between Groups 1 and 3 compared to the control group by a oneway ANOVA (P = 0.0200 and P = 0.0120), the significance of which was verified Tukey’s test.
Table 4
Hematological parameters of the male Hsd Han: WIST rats in the subacute toxicity study
Hematological parameters of the male Hsd Han: WIST rats in the subacute toxicity study
| Parameters | Males | |||
| Media ± SEM+ | ||||
| Group 1
|
Group 2
|
Group 3
|
Group 4
|
|
| HCT (L/L) | 9.43±1.86 | 10.64±1.57 | 12.38±3.06 | 9.90±1.43 |
| HGB (g/L) | 12.01±1.80 | 14.64±1.40 | 13.95±1.56 | 13.34±1.37 |
| RBC (×1012/L) | 49.85±5.71** | 40.57±4.91 | 50.71±7.59** | 23.61±6.36 |
| MCV (fL) | -27.07±2.60** | -20.75±2.89 | -25.41±2.34** | -9.02±5.93 |
| MCHC (g/L) | 3.19±2.07 | 3.93±1.50 | 1.84±1.89 | 2.94±1.36 |
| PL (×109/L) | 23.53±35.59 | 8.35±11.39 | -15.01±6.34 | -2.27±8.26 |
| LE (×109/L) | -4.53±17.66 | 14.62±20.04 | -25.76±7.20 | -25.51±6.93 |
| NE (×109/L) | 14.07±39.65 | 51.81±26.79 | -2.88±29.65 | -18.36±19.01 |
| LY (×109/L) | -0.70±17.69 | 18.59±22.3 | -26.44±6.74 | -25.57±6.79 |
| MO (×109/L) | -94.44±5.56 | -66.67±21.08 | -46.67±23.90 | -50.0±22.36 |
| EO (×109/L) | -11.67±17.97 | -16.67±16.67 | -33.33±33.33 | -15.0±17.08 |
Discussion
In the acute toxicity study, there were three animal deaths, which transpired in Groups 1 (2 000 mg/kg) and 2 (1 000 mg/kg), occurring more than 48 h posttreatment. OECD guide 423 mentions that due to these deaths, experiments should be carried out to find the lethal dose, because these deaths occurred with the highest doses, it is possible that the lethal dose is between 2 000 and 1 000 mg/kg. Thus, for the subacute toxicity study, it was decided to use the 40 mg/kg dose, which represents 10 times the recommended dose (4 mg/kg).
Hematological evaluations of the animals in the subacute toxicity test treated with the injectable alpha compound showed significant differences for four parameters (P ˂ 0.01) compared to the control group. However, these values are within the normal values according to the literature. Such variations may be affected by the normal fluctuations in all treated groups. Therefore, these data suggest that the injected alpha compound did not show toxicity to the hematopoietic system. The glucose and cholesterol values were low and significantly different among females in the serum biochemical test, although they were in the normal ranges. 19,20 In the treated males, there were no differences in these analytes.
-
19Biochemical and hematological parameters of Wistar rats and Swiss mice in the Professor Thomas George Animal LaboratoryRevista Brasileira de Ciências da Saúde, 2011
-
20Differences in hematologic variables in rats of the same strain but different originVeterinary Clinical Pathology, 2012
Upon histopathological examination, the common lesions found in the animals in the subacute test were interstitial changes, congestion, hemorrhages, and inflammatory cell infiltration.21,22 Despite this, in this acute toxicity study, there were no changes in the muscles, brain, heart, lung, spleen, liver, kidney, or adrenal glands. Moreover, the changes in the cell structure and others mentioned above were not related to the serum biochemical analysis. Thus, the injectable alpha compound is emerging as a future alternative fasciolicide for the control of fasciolosis in ruminants. However, it is necessary to carry out additional studies to determine the lethal dose, corroborate its efficacy in cabinet and field tests, and verify its tolerance, safety index and retention period in ruminants to verify its true fasciolicide potential in the target species.
-
21Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar ratsToxicology, 2017
-
22Acute and sub-acute toxicity study of a Pakistani polyherbal formulationBMC Complementary and Alternative Medicine, 2017
Conclusions
After intramuscular injection into Hsd Han: WIST rats, the alpha compound did not produce significant changes in serum biochemistry, hematology, or histomorphology in the subacute toxicity test when compared to data reported in the literature. The information obtained here serves as preliminary data to indicate that this compound has a wide margin of safety.
Acknowledgments
The authors wish to thank the Preclinical Research Unit (UNIPREC) for their permission to use their facilities and MVZ Lucía Macias Rosales, MC Claudia P. Rico Torres and Dr. Miguel Angel Flores Ramos for their excellent technical support.
Funding statement
This research was funded by the National Autonomous University of Mexico (www.unam.mx), PAPIIT DGAPA-UNAM RT201015 grant, awarded to F. Ibarra-Velarde. The funder had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Author contributions
Conceptualization: A. Zapata-Arenas, F. Ibarra-Velarde.
Data curation: A. Zapata-Arenas, F. Ibarra-Velarde.
Formal analysis: A. Zapata-Arenas, F. Ibarra-Velarde.
Funding acquisition: F. Ibarra-Velarde.
Investigation: A. Zapata-Arenas.
Methodology: A. Zapata-Arenas, R. Castillo, J. Ramírez-Lezama, H. Rico-Morales, R. Bustamante-García.
Project administration: A. Zapata-Arenas, F. Ibarra-Velarde.
Resources: F. Ibarra-Velarde, I. Gracia-Mora.
Software: R. Bustamante-García.
Supervision: F. Ibarra-Velarde, I. Gracia-Mora.
Validation: A. Zapata-Arenas.
Visualization: F. Ibarra-Velarde, I. Gracia-Mora.
Writing-original draft: A. Zapata-Arenas.
Writing-review and editing: A. Zapata-Arenas, F. Ibarra-Velarde, R. Bustamante-García, R. Castillo, H. Rico-Morales.
References
-
1Zumaquero, RJL, Sarracent, PJ, Rojas, GR, Rojas, RL, Martínez, TY, Valero, AM. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: epidemiology and treatment with nitazoxanide. PLOS Neglected Tropical Diseases. 2013;7(11):e2553. doi: 10.1371/journal.pntd.0002553. Links
-
2Cruz, LOR, Gómez, VE, Cárdenas, PME, Gutiérrez, DA, Tamariz, COJ. Human fasciolosis diagnosed in the acute phase: a first clinical report in Mexico. Revista de Gastroenterología de México. 2016;81(2):111-113. doi: 10.1016/j.rgmx.2015.06.006. Links
-
3Lima, WS, Soares, LR, Barçante, TA, Guimaraes, MP, Barçante, JM. Occurrence of Fasciola hepatica (LINNAEUS, 1758) infection in Brazilian cattle in the State of Minas Gerais. Revista Brasilera de Parasitología Veterinaria. 2009;18(2):27-30. doi: 10.4322/rbpv.01802006. Links
-
4Boray JC. Chemotherapy of infections with Fasciolidae. In: ICOPA VIII, Round Table Conference: Immunology, Pathobiology and Control of Fasciolosis, Izmir, Turkey; 1994. pp. 83-97. Links
-
5Caminade, C, van Dijk, J, Baylis, M, Williams, D. Modelling recent and future climatic suitability for fasciolosis in Europe. Geospatial Health. 2015;9(2):301-308. doi: 10.4081/gh.2015.352. Links
-
6Fairweather, I, Brennan, GP, Hanna, REB, Robinson, MW, Skuce, PJ. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Resist. 2020;12, 39-59. Links
-
7Fairweather, I, Boray, JC. Fasciolicides: efficacy, actions, resistance and its management. Veterinary Journal. 1999;158(2):81-112. doi:10.1053/tvjl.1999.0377. Links
-
8Turner, K, Armour, J, Richards, RJ. Anthelmintic efficacy of triclabendazole against Fasciola hepatica in sheep. Veterinary Record. 1984;114:41-42. doi: 10.1136/vr.114.2.41. Links
-
9Ortiz, P, Scarcella, S, Cerna, C, Rosales, C, Cabrera, M, Guzmán, M, et al. Resistance of Fasciola hepatica against triclabendazole in cattle in Cajamarca (Peru): a clinical trial and an in vivo efficacy test in sheep. Veterinary Parasitology. 2013;195(1-2):118-121. doi: 10.1016/j.vetpar.2013.01.001. Links
-
10Thomas, I, Coles, GC, Duffus, K. Triclabendazole-resistant Fasciola hepatica in southwest Wales. Veterinary Record. 2000;146(7):200. Links
-
11Munguía, XJA, Ibarra, VF, Ducoing, WA, Montenegro, CN, Quiroz, RH. Prevalence of Fasciola hepatica (ELISA and fecal analysis) in ruminants from a semi-desert area in the northwest of Mexico. Parasitology Research. 2007;101(1):127-130. doi: 10.1007/s00436-006-0438-y. Links
-
12Ibarra, VF, Vera, MY, Olazarán, JS, Hernández, CA, Castillo, BR. Fasciolinip-1: experimental fasciolicidal activity in sheep. Revista Latinoamericana de Microbiología. 1995;37(2):171-178. Links
-
13Ibarra, VF, Montenegro, CN, Vera, MY, Castillo, BR, Hernández, CA, Ochoa, GP. Eficacia comparativa de un fasciolicida experimental, triclabendazol y closantel en bovinos infectados en forma natural con Fasciola hepática. Veterinaria México. 2002;3(33):237-245. Links
-
14Vera, MY, Ibarra, VF, Quiroz, RH, Hernández, CA, Castillo, R. Field trial on the efficacy of an experimental fasciolicide compared with some commercial compounds in naturally infected cattle. Parasitoly Research. 2003;91(1):1-4. doi: 10.1007/s00436-003-0901-y. Links
-
15Vera, MY, Ibarra, VF, Liébano, HE, Quiroz, RH, Castillo, BR, Hernández, CA. Efficacy of an experimental fasciolicide against immature and mature Fasciola hepatica in artificially infected calves. Parasitoly Research . 2004;92(3):211-214.doi: 10.1007/s00436-003-1007-2. Links
-
16Ramírez, N, Mayet, L, Del Rivero, L, Ibarra, VF, Castillo, R, Hernández, CA. Pharmacokinetic behavior in sheep and cattle of 5-chloro-2-(methylthio)-6-(1-naphthyloxy)-1H-benzimidazole, a new fasciolicide agent. Journal of Veterinary Pharmacology and Therapeutics. 2009;32(2):154-159. doi: 10.1111/j.1365-2885.2008.01014.x. Links
-
17Flores Ramos, M, Ibarra, VF, Hernández, CA, Vera, MY, Jung, CH, Cantó, AGJ, et al. A highly water soluble benzimidazole derivative useful for the treatment of fasciolosis. Bioorganic Medicine Chemestry Letter. 2014;24(24):5814-5817. doi: 10.1016/j.bmcl.2014.10.017. Links
-
18Flores Ramos, M, Ibarra, VF, Jung, CH, Hernández, CA, Vera, MY, Castillo, R. Novel triclabendazole prodrug: a highly water soluble alternative for the treatment of fasciolosis. Bioorganic Medical Chemestry Letter. 2017;27(3):616-619. doi: 0.1016/j.bmcl.2016.12.004. Links
-
19Camillo, DA, Castello, BS, De Fátima, FM, Melo, DR, De Almeida, N, Bandeira, SH, et al. Biochemical and hematological parameters of Wistar rats and Swiss mice in the Professor Thomas George Animal Laboratory. Revista Brasileira de Ciências da Saúde. 2011;15(2):209-214. Links
-
20Kampfmann, I, Bauer, N, Johannes, S, Moritz, A. Differences in hematologic variables in rats of the same strain but different origin. Veterinary Clinical Pathology. 2012;41(2):228-234. doi: 10.1111/j.1939-165X.2012.00427.x. Links
-
21Faria, J, Barbosa, J, Leal, S, Afonso, LP, Lobo, J, Moreira, R, et al. Effective analgesic doses of tramadol or tapentadol induce brain, lung and heart toxicity in Wistar rats. Toxicology. 2017;385:38-47. doi: 10.1016/j.tox.2017.05.003. Links
-
22Ishtiaq, S, Akram, M, Kamran, SH, Hanif, U, Afridi, MSK, Sajid-Ur-Rehman. Acute and sub-acute toxicity study of a Pakistani polyherbal formulation. BMC Complementary and Alternative Medicine. 2017;17(1):387. doi: 10.1186/s12906-017-1889-7. Links