Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.11 Tepic ene./dic. 2021 Epub 21-Mayo-2021
https://doi.org/10.21929/abavet2021.11
Review Article
Neurobiology and modulation of stress-induced hyperthermia and fever in animal
1Neurofisiología del dolor, comportamiento y evaluación de bienestar en animales domésticos, Departamento de Producción Agrícola y Animal. Universidad Autónoma Metropolitana (UAM), CDMX, México.
2Farmacología Clínica y Anestesia Veterinaria, Departamento de Ciencias Biológicas. Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Cuautitlán, FESC. Estado de México, México.
3Departamento de Ciencias Pecuarias. Universidad Nacional Autónoma de México, FESC. México.
Stress-induced hyperthermia is an acute response that occurs in the short term in individuals who are facing a stressful stimulus, considering that this response can provide significant information on stress degree. However, it is not yet clear whether the neurological pathway can be modified to the degree to which stress is perceived. Furthermore, there is not enough information as to how factors that modify perception stress degree act on stress-induced Hyperthermia. Besides, research indicates that the thermal response possibly has a greater cardiovascular influence by generating energy resource consumption. In the same way, the factors that induce this response have been questioned, since recent evidence indicates that social factors such as the presence of conspecifics attenuate the thermal response, but, when coexistence or some other action like parenting is prevented, the response is to the reverse. For this reason, the objective of this article was to analyze the neurobiology of stress-induced hyperthermia and its conceptual difference with infectious fever, as well as to integrate the factors that modulate it, analyzing recent scientific advances in stress-induced thermal response.
Keywords: temperature; stress; welfare; thermogenesis; thermal response
La hipertermia inducida por estrés es una respuesta aguda que se presenta a corto plazo en individuos que están frente a un estímulo estresante y que dicha respuesta puede aportar información significativa sobre el grado de estrés. Sin embargo, no es claro todavía si la vía neurológica pueda ser modificada al mismo grado en la que se percibe el estrés. Además, no se tiene suficiente claridad en cómo es que los factores que modifican el grado de percepción de estrés actúan sobre la Hipertermia Inducida por Estrés (SIH, por sus siglas en inglés). Asimismo, las investigaciones señalan que posiblemente la respuesta térmica tenga una mayor influencia cardiovascular al generar el consumo de recursos energéticos. De igual manera, los factores físicos que inducen dicha respuesta han sido cuestionados, ya que la evidencia reciente señala que además los factores sociales como la presencia de coespecíficos atenúan la respuesta térmica pero cuando se impide la convivencia o alguna otra conducta social como la crianza, la respuesta incrementa la SIH. Por tal motivo, el objetivo de este artículo es analizar la neurobiología de la hipertermia inducida por estrés y su diferencia conceptual con la fiebre infecciosa, así como integrar los factores que lo modulan, analizando los avances científicos recientes de la respuesta térmica inducida por estrés.
Palabras clave: temperatura; estrés; bienestar; termogénesis; respuesta térmica
INTRODUCTION
Stress-induced hyperthermia (SIH) is defined as an integral part of a physiological response, characterized by an increase in body temperature that is generated from threats to homeostasis, caused by stressful stimuli. This increases survival chances. This thermal response to acute stress and the associated factors that modify it, have been of great interest to determine the welfare of the animals, since it has been considered that the variations in temperature are a measure reliable and sensitive to determine stress degree perceived by animals (Lees et al., 2020). Recent scientific findings indicate that temperature control is essential for survival (Song et al., 2016; Fuller-Jackson et al., 2017; Wang et al., 2019; Casas-Alvarado et al., 2020; Mota-Rojas et al., 2020). Living beings have developed over thousands of evolution years a great variety of adaptive mechanisms for the multiple alterations that the environment or their habitat can undergo (Morrison and Nakamura, 2011; Villanueva-García et al., 2020).
Several studies have identified key elements of neurophysiological mechanisms responsible for the development of SIH. Their findings have determined that despite the existence of an important thermogenesis activation due to the consumption of brown adipose tissue (Brown adipose tissue; BAT), there is also an important thermogenesis of cardiac origin (Crestani, 2016). Which in both cases contributes to a decrease in the release of heat towards the external environment; however, the exact mechanism that intervenes in the thermal response modification is not entirely clear. However, a relationship between stressful stimuli and the deterioration of mediated baroreflex response has recently been described, through angiotensin receptors (Costa-Ferreira et al., 2016).
Another question that continues to be studied is the participation of factors that induce stress or that can modify its response. In this sense, it has been possible to identify that the environmental stimulus, such as cold, generates a significant increase in animal temperature that face this stimulus, which will be called stressful (Miyamoto et al., 2017a). On the other hand, not only stressful stimuli of a physical nature cause SIH, it has been seen that psychological or emotional stress increases body temperature through mechanisms other than those associated with the fever that animals develop during infectious or inflammatory processes. Furthermore, it has been determined that social factors exert a greater influence on SIH than environmental ones, since it has recently been observed that SIH can be attenuated in the presence of conspecifics (Oka, 2018). In fact, if coexistence between animal groups is prevented or breeding in females is impossible, it can trigger a thermal response similar to social and emotional factors (Faraji and Metz, 2020).
For this reason, the objective of this article is to analyze the neurobiology of stress-induced hyperthermia and its conceptual difference with infectious fever; as well as integrating the factors that modulate it, analyzing recent scientific advances associated with the thermal response induced by stress.
Conceptual difference between SIH and infectious fever
SIH refers to a significant increase in basal body temperature, and its nature is usually short or medium in duration; followed by a gradual return to basal temperature, once the stimulus or the perceived stressful situation dissipates (Oka et al., 2001).
In this context, Bittencourt et al., (2015), with the objective of determining the thermal response to stress stimuli in birds through telemetric records; they evaluated pigeons (Columbia livia) exposed to stressful stimuli. It was observed that the transfer from the cage, visual isolation and tonic immobility, caused an increase in body temperature for 10-20 minutes and subsequently it was possible to decrease significantly. Thus, with this observation it was determined that temperature is a parameter associated with stress, but according to what the authors observed, it can also show specific attributes to characterize the stressor based on its type, direction and species. On the other hand, it has been observed that when the individual is repeatedly exposed to a stressful stimulus and can express a behavioral pattern similar to depression, chronic hyperthermia occurs that is low-grade and persistent (Oka, 2018).
This has been related to a conditioned hyperthermia form, which refers to the increase in temperature caused by previous experiences during early or youthful age, due to an aversive memory between a certain stimulus and situation (Oka, 2018). An example of this is that if an animal receives an electric shock that is unfamiliar, a behavioral and autonomic response associated with fear is triggered when it is exposed again to the same stimulus ((Thompson et al., 2012; Wellman et al., 2016). In contrast, hyperthermia caused by infectious processes is called fever and it is a cardinal response typically related to sepsis or microorganism presence in the body (Evans et al., 2015). Unlike SIH, fever involves a high energy cost, since to produce a 1 ºC increase in body temperature, a 10- 15% increase in metabolic rate is required (Young and Saxena, 2014 ).
As can be seen, it is clear that from a conceptual analysis, there is a difference between the possible causes of temperature increase in the body; however, both signaling tracks share a neuronal pathway that modulates thermal response.
Temperature hypothalamic modulation in SIH and fever
Although stress encompasses a series of both behavioral and physiological responses in order to face a stressful event (Yaribeygi et al., 2017), to understand the response to stress, whether infectious or emotional in origin; it is necessary to understand the physiological response that is triggered to assess how animal welfare is compromised (Lees et al., 2020). In other words, when an individual faces a stressful event, different related physiological responses can be triggered, including an increase in body temperature ((Vinkers et al., 2009) and metabolic consequences could be different. proposed that both in humans and animals, stress perception seems to correlate with high activity in the Autonomous Nervous System (ANS) and with stress high levels (such as anxiety or fear), generating an increase in the frequency heart rate and body temperature level (Bi, 2014; Houtepen et al., 2011). For this reason, it has been considered as a physiological response associated with stress degree experienced by the body (Lees et al., 2020).
Emotional stress or fever increases body temperature through independent cytokine and prostaglandin E2 (PGE2) mechanisms. Thus, the systemic administration of non-steroidal analgesics (NSAIDs), such as phenylbutazone or indomethacin, fails to inhibit this type of stress-induced hyperthermia (Zhang et al., 2010). In contrast, drugs that possess anxiolytic properties, such as benzodiazepines and serotonin (5-HT) receptor agonists, such as buspirone and flesinoxane, do have effects on reducing the magnitude of stress- induced hyperthermia (Rygula et al., 2008; Vinkers et al., 2010).
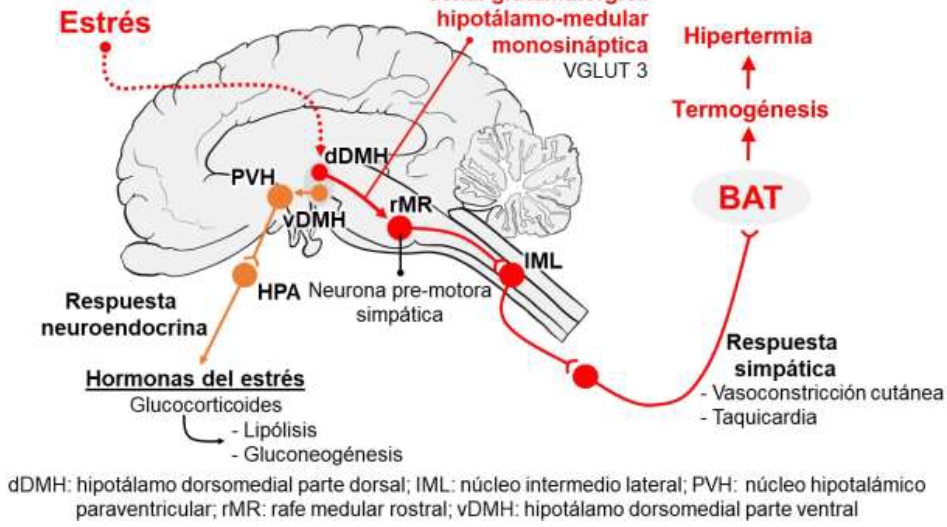
These findings have shown that ANS, over the whole sympathetic nervous system (SNSi) influences temperature modulation while main effector organs are BAT and blood vessels (Nakamura, 2015). In the first case it is controlled by SNSi innervation through β3 adrenoreceptors, which are predominantly expressed, and in some studies it has been shown that the hypothalamic-medullary glutamatergic signal is the one that drives sympathetic thermogenesis in BAT (Kataoka et al., 2014). On the contrary, in the blood vessels there is a decrease in heat loss by radiation, due to cutaneous vasoconstriction, which is measured by a sympathetic response of α adrenoreceptors that generate the decrease in dermal blood flow ((Nakamura, 2015; Ikoma et al., 2018)
Additionally, the hypothalamic-pituitary-adrenal (HPA) axis is activated, generating the stimulating hormone neurosecretion of the adrenal cortex, which in turn increases the secretion of glucocorticoids in the adrenal cortex; action that stimulates two catabolic events: gluconeogenesis and lipolysis, which contributes to increasing thermogenic activity (Oka, 2018; Wang et al., 2015). Likewise, during stress perception, a moderate tachycardia is induced without decreasing the stroke volume, thereby providing support to increase necessary oxygen supply for BAT consumption and distribute heat to the rest of the body. This process has been called “cardiac thermogenesis” (Morrison, 2011).
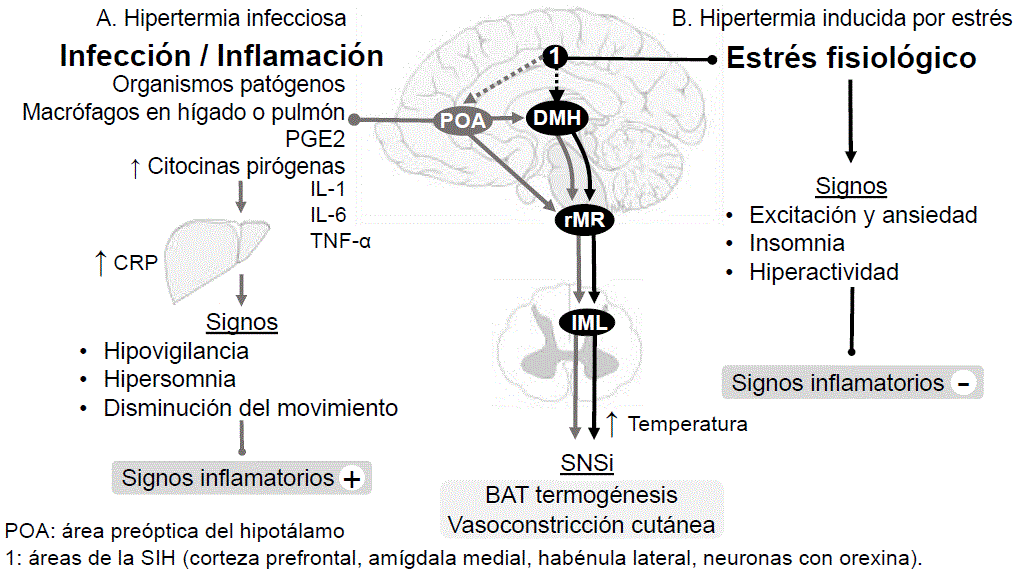
In this sense, SNSi neurons integrate signals from different brain regions, so that neurons specialized in thermogenesis for BAT and vasoconstriction are predominantly found in the rostral medullary raphe region (rMR), which involves the nucleus of raphe pallidus rostral and raphe magnus (Nakamura, 2004; Nakamura et al., 2005). Likewise, Nakamura (2015) reports that through nanoinjections use of drugs in vivo in the rat brain and evaluations by thermotelemetry, it was demonstrated that both rMR and the dorsomedial hypothalamus (DMH) mediate stress-induced thermogenesis. Possible brain regions that are involved in SIH include the prefrontal cortex, medial amygdala, lateral habenula, and orexin-containing neurons (Oka, 2018). Therefore, being these regions in which neurons that contain the vesicular glutamate transporter (VGLUT 3) are expressed, they have been identified as glutamatergic neurons (Nakamura, 2004).
Stornetta et al., (2005) observed that through the histological and immune-reactive detection of VGLUT 3 mRNA in medullary raphe, 89% of neurons showed both marker expression; therefore, VGLUT 3 neurons contain receptors for both serotonin and GABA. This observation indicates that glutamatergic receptor activation participate in thermal response modulation to acute stress (Horiuchi et al., 2004). In contrast, when glutamate receptor blockade in rMR is exerted with the GABA inhibitor use as such as muscimol, not only thermogenesis, but also hyperthermia and tachycardia due to stress are inhibited (Kataoka et al., 2014; Nakamura, 2015) (Figure 1).
On the contrary, in fever induced by infection and inflammation, the increase in temperature is considered a common response in sick patients, through interaction of exogenous pyrogens by pathogenic microorganism presence with interleukin (IL) - 1, IL- 6 and tumor necrosis factor α (TNF-α) (Walter et al., 2016). These inducers stimulate the production of pro-inflammatory cytokines, which act directly in the preoptic area of the hypothalamus (POA), the organum vasculosum neuronal pathway of the terminalis lamina (Schortgen, 2012). An area that is highly vascularized and lacks a blood-brain barrier, which allows it to be stimulated very easily (Walter et al., 2016).
Likewise, prostaglandin PGE2, which is produced in endothelial cells at the brain level, becomes the main pyrogenic mediator of fever (Engström et al., 2012). However, this chemical mediator can also be produced by hematopoietic cells after the activation of the Toll 4 receptor (TLR4) mediated by lipopolysaccharides (LPS) of bacteria, which, when in contact with the blood-brain barrier, initiate the thermal elevation known as fever (Hasday et al., 2014; Saper et al., 2012). PGE2 acts on the POA by slowing down the firing speed of heat-sensitive neurons, causing an increase in body temperature, favoring febrile states (Clarke and Pörtner, 2010) (Figure 2) .This evidence makes infectious fever is associated with elevated inflammatory markers, which can be attenuated with non- opioid NSAIDs, such as paracetamol, by blocking cyclooxygenase 3 at the brain level, thus decreasing the synthesis of PGE2 (Olivier et al., 2003; Jahr and Lee, 2010).
Therefore, there is a great similarity between infectious fever and SIH, since in both cases the mediation pathway is given by POA, due to the abundance of excitatory glutamatergic neurons. However, the difference between the two phenomena is the origin that will trigger the hyperthermia response, which can be serotoninergic and glutamatergic, as in SIH; while for infectious origin fever, the temperature increases will correspond to the presence of exogenous pyrogens (Figure 2).
Modulating factors of stress-induced thermal response
There are several factors that must be taken into account for the stress-induced thermal cascade to be generated, including:
a) Nature and intensity of the stressor
In a study by Watanabe (2015), they evaluated 40 mice by infrared thermography, which were under three different social conditions: alone, immobilized and restrained alone mice with cage mates that moved freely; found that those animals that remained alone had a lower SIH thermal response, compared to immobilized and restrained single mice with free-moving cage mates (Watanabe, 2015).
On the other hand, Hayashida et al., (2010), who tried to confirm that SIH is typically monophasic; that is, after the stress, the body temperature returns to the baseline. They evaluated Wistar strain male rats, exposed to emotional experiences such as social defeat and periods of darkness; this last group was considered as control. The authors reported that at the time of being under social defeat, the rats presented a significant increase of 0.2 ºC in temperature, compared to the rats that were exposed to darkness. It was concluded that depending on the type of stressor and its nature, it was social, light or spatial, a sustained thermal response can be triggered and even, after stimulus habituation, the hyperthermia is reversed until reaching the basal temperature.
b) Species and sex
Similar to what occurs in the face of stressor nature, the morpho-physiological and behavioral differences also have an effect on thermal response modulation (Oka, 2018).
Dymon and Fewell (1998), evaluated the thermal response of male and female guinea pigs, against the exposure of a simulated open field, it was observed that neither the males nor the females developed SIH; however, in the case of females, there was a lower value of body temperature. This observation is in contrast to that reported in the study by Dallmann et al., (2006), who found that social confrontation generates SIH, due to the increase in corticosterone, approximately between 10 to 30 minutes after exposure to the stressor. It should be noted that other authors have determined that SIH can be prolonged 60-120 minutes after the noxious stimulus, which was presented by performing an immune-staining analysis for the Fos receptor in the preoptic and periolivar nuclei (Veening et al., 2004). This last evidence agrees with what was recently observed by Lees et al., (2020), investigated the relationship between temperament traits, handling and SIH. To do this, they recorded the rectal temperature of 60 pure Angus breed steers, which were exposed to a standardized manipulation such as immobilization in the box for 30 seconds; also having a retention per group and immobilization in the sleeve for 60 seconds.
In this study, the temperaments evaluated were: agitator score, crush score, and flight speed. Their findings report that there was a moderate correlation between rectal temperature with flight speed and crush score (r = 0.37, r = 0.31). It is worth mentioning that, as observed by the authors, regardless of sex and temperament traits; rectal temperature showed a more significant relationship with time. It was concluded that the degree of expression or the increase in temperature is related to the animal species that presents it, probably due to a difference in receptor expression in the POA.
However, despite the fact that both in the guinea pig and in cattle, the evidence shows that there is no sex significant influence on SIH expression. Some studies have indicated that SIH is expressed to a greater extent in females. In this sense, Rosinger et al., (2017) mention that female rats have 1.3 ºC higher temperature than males. This could be due to a differential response of the HPA axis, in the face of stressors; possibly because estrogen can improve the function of this axis and therefore of the corticotropin-releasing hormone, which has been associated with the thermal effect (Oka, 2018). In addition to this, it was recently observed in female mice that SIH occurred when the female was deprived of breeding; however, this effect did not show a correlation with circulating cortisol levels (Faraji and Metz, 2020).
In summary, a significant difference has been observed between SIH response, in relation to species and sex; which can be explained by a difference in receptor expression the responsible for signaling the thermal response, although some studies do not provide sufficient data to establish a clear answer. Therefore, it is necessary to continue developing studies to answer these questions.
c) Environmental factors (ambient temperature)
It has been pointed out that the magnitude in which the SIH is expressed may differ with the values of ambient temperature. In this regard, Herborn et al., (2015), demonstrated that rats exposed to a low temperature (8 ºC) presented higher SIH, than those animals kept at ambient temperature (23 ºC), or higher temperatures (30 ºC). It was concluded that exposure to cold can cause a higher SIH. On the other hand, it has been observed that in rats incubated at a temperature between 11 and 25 ºC, SIH response did not present a significant difference (Oka, 2018).
In order to check whether exposure to cold alters SIH expression degree, Miyamoto et al., (2017a), evaluated mice housed at 5 °C (acclimatized to cold) and at 25 °C (controls) for 4 weeks . The SIH magnitude was observed to be greater in cold-acclimatized rats than in control rats. The explanation suggested by the researchers is that exposure to cold leads to the pigmentation of white adipose tissue and the consequent increase in thermogenesis in BAT, due to the accelerated activation of sympathetic β3 adrenoreceptors. These same authors report that the response and magnitude SIH is affected in mice previously stressed with exposure to cold, due to LPS stimulation effect; however, cold-induced stress did not alter baseline serum corticosterone levels, suggesting that exposure to cold increases susceptibility to LPS, leading to higher SIH (Miyamoto et al., 2017b). Therefore, the Environmental temperature below the comfort limit zone mainly affects the thermal response to stress and the susceptibility to pyrogens, compared to exposure to high temperatures.
d) Social factors
Another important aspect that influences SIH development are social factors, such as the presence of other individuals or the confrontations between them. Regarding the first case, it has been observed that the SIH can be higher when the animals are alone or in restriction, but with the presence of congeners that move freely (Watanabe, 2015). This increase in temperature can be attenuated when the individuals are paired after the perception of a stressful event (Kiyokawa et al., 2004; Kiyokawa et al., 2007; Kiyokawa et al., 2014). Even this response persists if there is a physical barrier, which has been explained by an influence of odoriferous substances released by conspecifics that are detected by the olfactory system, with this it is possible to carry out a measure of social damping, without the need for contact physical (Kiyokawa et al., 2009; Takahashi, 2014).
It is worth mentioning that another important social aspect is the presence of young animals or breeding opportunity for. In this sense, it has been pointed out that when the opportunity to breed is limited to females, SIH is accentuated compared to rats that did manage to carry out this behavior (Faraji and Metz, 2020). However, in this regard it is also necessary to consider the affective and emotional links that favor the release of substances, such as oxytocin, that counteract the stressful effects.
Participation of cardiac thermogenesis in the development and SIH modulation
Acute stress can affect cardiovascular functions, for example increasing blood pressure; therefore, it has been considered as a physiological impact factor in the development and modulation of SIH (Crestani, 2016).
In relation to this and with the objective of determining the angiotensin II participation on the type1 Ang-II (AT1) both in homotypic and heterotypic emotional dysfunctions, Costa- Ferreira et al., (2016) compared the effect of an AT1 receptor antagonist (Losartan 30- mg/ kg/ day orally), on automatic and cardiovascular changes in rats. They observed that sympathetic tone increased in response to heart stressor, decreasing the cardiac parasympathetic activity, in addition, when a selective AT1 receptor blocker such as Losartan was administered, and the baroreflex deterioration was inhibited, as was the autonomic activity. Likewise, it was possible to identify the increase in levels of circulating corticosterone and a reduction in body weight. It was concluded that there is an important participation of AT1 in the autonomic changes caused by acute stress. This new evidence is additional to the modification of the cardiovascular pattern, due to α adrenoreceptor stimulation that generate a tachycardia in aversive situations (dos Reis et al., 2014; Crestani, 2016).
On the other hand, it has been investigated whether social damping can inhibit SIH, since it has been observed that in male Wistar rats in the presence of a partner or a conspecific, stressor perception can be inhibited with the consequent reduction in SIH response (Kiyokawa et al., 2004; Lkhagvasuren and Oka, 2017). However, it has recently been discovered that without the need for social contact, SIH response is inhibited due to the uptake of odors (Kiyokawa, 2015); however, it is not yet clear whether the familiar odor effect could have the same answer to SIH.
In this context, Kiyokawa et al., (2014) studied familiarity effect with a conspecific on social damping intensity; for this, they evaluated the response of male Wistar rats housed with a family conspecific for 3 weeks. These same animals were subsequently exposed to a conditioned stimulus in a clean or scented control box with unknown or familiar conspecific. They observed that the subjects showed freezing and Fos expression in the paraventricular nucleus; but this response was nullified when they were exposed to a conspecific smell, showing a greater effect with the familiar smell. Thus, concluding that the smell of a familiar conspecific is more effective in socially dampening conditioned responses to fear.
For all the foregoing, the evidence indicates that probably the vascular changes produced by acute stress that affect the thermal response cannot be explained only with the HPA axis response and catecholamine secretion. Therefore, cardiovascular changes caused by stress may have more than one physiological pathway that can alter the temperature and worsen cardiovascular pathologies; however, these changes are inhibited by the conspecific presence, which in the future should be a field study to determine if inhibition follows the same feedback pathway at the neurological level.
CONCLUSIONS
SIH is a physiological response to situations perceived as threatening or distressing, which can be acute, chronic and even anticipatory or conditioned, related to aversive memories; thus, due to stress perception, energy resources are optimized for individual preparation, for the fight or the escape, reason why the thermogenesis is generated when using the BAT and the cardiogenic changes. For this reason, these factors cause a physiological difference between emotional hyperthermia and infectious origin fever, since in emotional hyperthermia there is no participation of cytokines released by the immune system.
With regard to the factors that influence SIH appearance, it is clear that physical and especially environmental factors play an important role; but recently there has been a greater interest in investigating the social components, since the presence of conspecifics can have a direct and important influence on SIH response.
Finally, it should be noted that the vascular changes produced by acute stress can affect the thermal response in SIH, so further research is required in the future to explain the participation level of the HPA axis and catecholamines. This situation could complement the idea that cardiovascular changes caused by stress may have more than one physiological pathway that modulates SIH response.
LITERATURA CITADA
Bi S. 2014. Stress Prompts Brown Fat into Combustion. Cell Metab. 20:205-207. https://doi.org/10.1016/j.cmet.2014.07.017 [ Links ]
Bittencourt M de A, Melleu FF, Marino-Neto J. 2015. Stress-induced core temperature changes in pigeons (Columba livia). Physiol. Behav. 139:449-458. https://doi.org/10.1016/j.physbeh.2014.11.067 [ Links ]
Casas-Alvarado A, Mota-Rojas D, Hernández-Avalos I, Mora-Medina P, Olmos-Hernández A, Verduzco-Mendoza A, Reyes-Sotelo B, Martínez-Burnes J. 2020. Advances in infrared thermography: surgical aspects, vascular changes and pain monitoring in veterinary medicine. J. Therm. Biol. 92:102664. https://doi.org/10.1016/j.jtherbio.2020.102664 [ Links ]
Clarke A, Pörtner H-O. 2010. Temperature, metabolic power and the evolution of endothermy. Biol. Rev. 85(4):703-727. https://doi.org/10.1111/j.1469-185X.2010.00122.x [ Links ]
Costa-Ferreira W, Vieira JO, Almeida J, Gomes-de-Souza L, Crestani CC. 2016. Involvement of Type 1 Angiontensin II Receptor (AT1) in Cardiovascular Changes Induced by Chronic Emotional Stress: Comparison between Homotypic and Heterotypic Stressors. Front. Pharmacol. 7. https://doi.org/10.3389/fphar.2016.00262 [ Links ]
Crestani CC. 2016. Emotional Stress and Cardiovascular Complications in Animal Models: A Review of the Influence of Stress Type. Front. Physiol. 7. https://doi.org/10.3389/fphys.2016.00251 [ Links ]
Dallmann R, Steinlechner S, Von Hörsten S, Karl T. 2006. Stress-induced hyperthermia in the rat: Comparison of classical and novel recording methods. Lab. Anim. 40: 186-193. https://doi.org/10.1258/002367706776319015 [ Links ]
Dos Reis DG, Fortaleza EAT, Tavares RF, Corrêa FMA. 2014. Role of the autonomic nervous system and baroreflex in stress-evoked cardiovascular responses in rats. Stress. 17: 362-372. https://doi.org/10.3109/10253890.2014.930429 [ Links ]
Dymond KE, Fewell JE. 1998. Gender Influences the Core Temperature Response to a Simulated Open Field in Adult Guinea Pigs. Physiol. Behav. 65: 889-892. https://doi.org/10.1016/S0031-9384(98)00198-X [ Links ]
Engström L, Ruud J, Eskilsson A, Larsson A, Mackerlova L, Kugelberg U, Qian H, Vasilache AM, Larsson P, Engblom D, Sigvardsson M, Jönsson J-I, Blomqvist A. 2012. Lipopolysaccharide-Induced Fever Depends on Prostaglandin E2 Production Specifically in Brain Endothelial Cells. Endocrinology153: 4849-4861. https://doi.org/10.1210/en.2012-1375 [ Links ]
Evans SS, Repasky EA, Fisher DT. 2015. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 15: 335-349. https://doi.org/10.1038/nri3843 [ Links ]
Faraji J, Metz GAS. 2020. Infrared Thermography Reveals Sex-Specific Responses to Stress in Mice. Front. Behav. Neurosci. 14. https://doi.org/10.3389/fnbeh.2020.00079 [ Links ]
Fuller-Jackson JP, Clarke IJ, Henry BA. 2017. Chapter 12: Animal Models for Manipulation of Thermogenesis. Animals Models for the Study of Human Disease. Elsevier, Australia, pp. 281-312. http://doi.org/10.1016/b978-0-12-809468-6.00012-7 [ Links ]
Hasday JD, Thompson C, Singh IS. 2014. Fever, Immunity, and Molecular Adaptations, in: Comprehensive Physiology. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 109-148. https://doi.org/10.1002/cphy.c130019 [ Links ]
Hayashida S, Oka T, Mera T, Tsuji S. 2010. Repeated social defeat stress induces chronic hyperthermia in rats. Physiol. Behav. 101: 124-131. https://doi.org/10.1016/j.physbeh.2010.04.027 [ Links ]
Herborn KA, Graves JL, Jerem P, Evans NP, Nager R, McCafferty DJ, McKeegan DEF. 2015. Skin temperature reveals the intensity of acute stress. Physiol. Behav. 152: 225-230. https://doi.org/10.1016/j.physbeh.2015.09.032 [ Links ]
Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MAP, Dampney RAL. 2004. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am. J. Physiol. Integr. Comp. Physiol. 287: R824-R832. https://doi.org/10.1152/ajpregu.00221.2004 [ Links ]
Houtepen LC, Peterse DP, Westphal KGC, Olivier B, Vinkers CH. 2011. The autonomic stress-induced hyperthermia response is not enhanced by several anxiogenic drugs. Physiol. Behav. 102: 105-109. https://doi.org/10.1016/j.physbeh.2010.09.002 [ Links ]
Ikoma Y, Kusumoto-Yoshida I, Yamanaka A, Ootsuka Y, Kuwaki T. 2018. Inactivation of Serotonergic Neurons in the Rostral Medullary Raphé Attenuates Stress-Induced Tachypnea and Tachycardia in Mice. Front. Physiol. 9. https://doi.org/10.3389/fphys.2018.00832 [ Links ]
Jahr JS, Lee VK. 2010. Intravenous acetaminophen. Anesthesiol Clin. 28: 619-645. https://doi.org/10.1016/j.anclin.2010.08.006 [ Links ]
Kataoka N, Hioki H, Kaneko T, Nakamura K. 2014. Psychological Stress Activates a Dorsomedial Hypothalamus-Medullary Raphe Circuit Driving Brown Adipose Tissue Thermogenesis and Hyperthermia. Cell Metab. 20: 346-358. https://doi.org/10.1016/j.cmet.2014.05.018 [ Links ]
Kiyokawa Y. 2015. Social Odors: Alarm Pheromones and Social Buffering. pp. 47-65. https://doi.org/10.1007/7854_2015_406 [ Links ]
Kiyokawa Y, Honda A, Takeuchi Y, Mori Y. 2014. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav. Brain Res. 267: 189-193. https://doi.org/10.1016/j.bbr.2014.03.043 [ Links ]
Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. 2004. Partner’s Stress Status Influences Social Buffering Effects in Rats. Behav. Neurosci. 118: 798-804. https://doi.org/10.1037/0735-7044.118.4.798 [ Links ]
Kiyokawa Y, Takeuchi Y, Mori Y. 2007. Two types of social buffering differentially mitigate conditioned fear responses. Eur. J. Neurosci. 26: 3606-3613. https://doi.org/10.1111/j.1460-9568.2007.05969.x [ Links ]
Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. 2009. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur. J. Neurosci. 29: 777-785. https://doi.org/10.1111/j.1460-9568.2009.06618.x [ Links ]
Lees AM, Salvin HE, Colditz IG, Lee C. 2020. The Influence of Temperament on Body Temperature Response to Handling in Angus Cattle. Animals. 10: 172. https://doi.org/10.3390/ani10010172 [ Links ]
Lkhagvasuren B, Oka T. 2017. The histaminergic system is involved in psychological stress-induced hyperthermia in rats. Physiol. Rep. 5: e13204. https://doi.org/10.14814/phy2.13204 [ Links ]
Miyamoto T, Funakami Y, Kawashita E, Nomura A, Sugimoto N, Saeki H, Tsubota M, Ichida S, Kawabata A. 2017a. Repeated Cold Stress Enhances the Acute Restraint Stress-Induced Hyperthermia in Mice. Biol. Pharm. Bull. 40: 11-16. https://doi.org/10.1248/bpb.b16-00343 [ Links ]
Miyamoto T, Funakami Y, Kawashita E, Tomita S, Nomura A, Sugimoto N, Saeki H, Miyazakia T, Tsubota M, Ichida S, Kawabata A. 2017b. Enhanced Hyperthermic Responses to Lipopolysaccharide in Mice Exposed to Repeated Cold Stress. Pharmacology. 99: 172-178. https://doi.org/10.1159/000454815 [ Links ]
Morrison SF. 2011. Central neural pathways for thermoregulation. Front. Biosci. 16: 74. https://doi.org/10.2741/3677 [ Links ]
Morrison SF, Nakamura K. 2011. Central neural pathways for thermoregulation. Front. Biosci. 16: 74-104. https://doi.org/10.2741/3677 [ Links ]
Mota‐Rojas D, Olmos‐Hernández A, Verduzco‐Mendoza A, Lecona‐Butrón H, Martínez‐Burnes J, Mora‐Medina P, Gómez‐Prado J, Orihuela A. 2020. Infrared thermal imaging associated with pain in laboratory animals. Exp. Anim. 70: 20‐0052. https://doi:10.1538/expanim.20‐0052 [ Links ]
Nakamura K, 2015. Neural circuit for psychological stress-induced hyperthermia. Temperature. 2: 352-361. https://doi.org/10.1080/23328940.2015.1070944 [ Links ]
Nakamura K. 2004. Identification of Sympathetic Premotor Neurons in Medullary Raphe Regions Mediating Fever and Other Thermoregulatory Functions. J. Neurosci. 24: 5370- 5380. https://doi.org/10.1523/JNEUROSCI.1219-04.2004 [ Links ]
Nakamura K, Matsumura K, Kobayashi S, Kaneko T. 2005. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci. Res. 51: 1-8. https://doi.org/10.1016/j.neures.2004.09.007 [ Links ]
Oka T. 2018. Stress-induced hyperthermia and hypothermia. pp. 599-621. https://doi.org/10.1016/B978-0-444-64074-1.00035-5 [ Links ]
Oka T, Oka K, Hori T. 2001. Mechanisms and Mediators of Psychological Stress-Induced Rise in Core Temperature. Psychosom. Med. 63: 476-486. https://doi.org/10.1097/00006842-200105000-00018 [ Links ]
Olivier B, Zethof T, Pattij T, Van Boogaert M, Van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, Van der Gugten J, Groenink L. 2003. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur. J. Pharmacol. 463: 117-132. https://doi.org/10.1016/S0014-2999(03)01326-8 [ Links ]
Ootsuka Y, Blessing WW, Nalivaiko E. 2008. Selective blockade of 5-HT2A receptors attenuates the increased temperature response in brown adipose tissue to restraint stress in rats. Stress. 11: 125-133. https://doi.org/10.1080/10253890701638303 [ Links ]
Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, Zuloaga DG. 2017. Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: A novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience. 361: 167-178. https://doi.org/10.1016/j.neuroscience.2017.08.016 [ Links ]
Rygula R, Abumaria N, Havemann-Reinecke U, Rüther E, Hiemke C, Zernig G, Fuchs E, Flügge G. 2008. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav. Pharmacol. 19: 183-196. https://doi.org/10.1097/FBP.0b013e3282fe8871 [ Links ]
Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. 2006. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc. Natl. Acad. Sci. 103: 2904-2908. https://doi.org/10.1073/pnas.0510960103 [ Links ]
Saper CB, Romanovsky AA, Scammell TE. 2012. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 15: 1088-1095. https://doi.org/10.1038/nn.3159 [ Links ]
Schortgen F. 2012. Fever in sepsis. Minerva Anestesiol. 78: 1254-64. https://www.minervamedica.it/en/journals/minerva-anestesiologica/article.php?cod=R02Y2012N11A1254 [ Links ]
Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J. 2016. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 353(6306): 1393-1398. https://doi.org/10.1126/science.aaf7537 [ Links ]
Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. 2005. Coexpression of vesicular glutamate transporter-3 and γ-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J. Comp. Neurol. 492: 477-494. https://doi.org/10.1002/cne.20742 [ Links ]
Takahashi LK. 2014. Olfactory systems and neural circuits that modulate predator odor fear. Front. Behav. Neurosci. 8. https://doi.org/10.3389/fnbeh.2014.00072 [ Links ]
Thompson RS, Strong PV, Fleshner M. 2012. Physiological Consequences of Repeated Exposures to Conditioned Fear. Behav. Sci. (Basel). 2: 57-78. https://doi.org/10.3390/bs2020057 [ Links ]
Veening JG, Bouwknecht JA, Joosten HJJ, Dederen PJ, Zethof TJJ, Groenink L, Van der Gugten J, Olivier B. 2004. Stress-induced hyperthermia in the mouse: c-fos expression, corticosterone and temperature changes. Prog. Neuro-Psychopharmacology Biol. Psychiatry 28: 699-707. https://doi.org/10.1016/j.pnpbp.2004.05.007 [ Links ]
Villanueva-García D, Mota-Rojas D, Martínez-Burnes J, Olmos-Hernández A, Boscato L, Gomez J, González LM. Hypothermia in newly born piglets: mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometeorol. 2020(8):2101. https://doi.org/10.31893/jabb.21001 [ Links ]
Vinkers CH, Groenink L, Van Bogaert MJV, Westphal KGC, Kalkman CJ, Van Oorschot R, Oosting RS, Olivier B, Korte SM. 2009. Stress-induced hyperthermia and infection- induced fever: Two of a kind?. Physiol. Behav. 98: 37-43. https://doi.org/10.1016/j.physbeh.2009.04.004 [ Links ]
Vinkers CH, Olivier B, Bouwknecht JA, Groenink L, Olivier JDA. 2010. Stress-induced hyperthermia, the serotonin system and anxiety. Open Pharmacol. J. 4: 15-29. https://benthamopen.com/contents/pdf/TOPHARMJ/TOPHARMJ-4-15.pdf [ Links ]
Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. 2016. The pathophysiological basis and consequences of fever. Crit. Care. 20: 200. https://doi.org/10.1186/s13054-016-1375- 5 [ Links ]
Wang TA, Teo CF, Åkerblom M, Chen C, Tynan-La Fontaine M, Greiner VJ, Diaz A, McManus MT, Jan YN, Jan LY. 2019. Thermoregulation via Temperature-Dependent PGD2 Production in Mouse Preoptic Area. Neuron. 103: 309-322. E7. http://doi.org/10.1016/j.neuron.2019.04.035 [ Links ]
Wang L, Liu F, Luo Y, Zhu L, Li G. 2015. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed. Reports. 3: 425-429. https://doi.org/10.3892/br.2015.445 [ Links ]
Watanabe S. 2015. Social factors modulate restraint stress induced hyperthermia in mice. Brain Res. 1624: 134-139. https://doi.org/10.1016/j.brainres.2015.07.019 [ Links ]
Wellman LL, Fitzpatrick ME, Hallum OY, Sutton AM, Williams BL, Sanford LD. 2016. Individual Differences in Animal Stress Models: Considering Resilience, Vulnerability, and the Amygdala in Mediating the Effects of Stress and Conditioned Fear on Sleep. Sleep. 39: 1293-1303. https://doi.org/10.5665/sleep.5856 [ Links ]
Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. 2017. The impact of stress on body function: A review. Excli J.16: 1057-1072. https://doi.org/10.17179/excli2017-480 [ Links ]
Young PJ, Saxena M. 2014. Fever management in intensive care patients with infections. Crit. Care. 18: 206. https://doi.org/10.1186/cc13773 [ Links ]
Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. 2010. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J. Physiol. 588: 4117-4129. https://doi.org/10.1113/jphysiol.2010.195099 [ Links ]
Received: November 03, 2019; Accepted: February 10, 2020; Published: February 27, 2021











 texto en
texto en