Artículos de revisión
L-Arginine, Aspartate and Glutamate, and their relationship with the ewes’ reproduction. Review.
Alvarez-Cardona, Fernanda1
 http://orcid.org/0000-0002-3079-3748
http://orcid.org/0000-0002-3079-3748
Maki-Díaz, Griselda2
 http://orcid.org/0000-0002-9851-8873
http://orcid.org/0000-0002-9851-8873
Franco-Robles, Elena3
 http://orcid.org/0000-0003-0345-7578
http://orcid.org/0000-0003-0345-7578
Cadena-Villegas, Said4
 http://orcid.org/0000-0002-7977-2796
http://orcid.org/0000-0002-7977-2796
Hernández-Marín, Antonio3*
 http://orcid.org/0000-0002-0255-0722
http://orcid.org/0000-0002-0255-0722
 http://orcid.org/0000-0002-3079-3748
http://orcid.org/0000-0002-3079-3748Maki-Díaz, Griselda2
 http://orcid.org/0000-0002-9851-8873
http://orcid.org/0000-0002-9851-8873Franco-Robles, Elena3
 http://orcid.org/0000-0003-0345-7578
http://orcid.org/0000-0003-0345-7578Cadena-Villegas, Said4
 http://orcid.org/0000-0002-7977-2796
http://orcid.org/0000-0002-7977-2796Hernández-Marín, Antonio3*
 http://orcid.org/0000-0002-0255-0722
http://orcid.org/0000-0002-0255-0722ABSTRACT
In most of livestock production systems, it is important to optimize reproductive activity to increase productive efficiency. This indicator depends on environmental factors such as nutrition, which regulates the onset of puberty, ovarian follicular development, oocyte quality and as a result, embryonic development. The purpose of animal nutrition strategies is to increase reproductive efficiency, to obtain better economic income in most of livestock production systems. Recent research reports that dietary supplementation with specific amino acids such as arginine, glutamine, leucine, glycine and methionine they have beneficial effects on survival and embryonic and fetal growth by regulating key signaling and metabolic pathways. In sheep production systems, supplementation with different routes with neurostimulatory amino acids such as L-Arginine, aspartate and glutamate, improves reproductive efficiency in females in a technical and economical way, with the aim of eliminating hormonal manipulation of the animals. Therefore, the objective of the present review of the literature is to describe the neurostimulatory function of amino acids and to know the neuroendocrine response in the hypothalamic-pituitary-ovarian axis in sheep to improve productive and reproductive variables.
Keywords::
neurostimulatory amino acids, neuroendocrinology, reproductive efficiency, gonadotropins, sheep production
INTRODUCTION
The ovarian activity responds to the adequate secretion of LH and FSH in the adenohypophysis, by the secretion of GnRH in the hypothalamus. This endocrine communication also occurs through the action of compounds that act as neurotransmitters, from the supply of neurostimulatory amino acids that favor the pulsatile secretion of GnRH and LH (Mahesh y Brann, 2005), such as glutamate (GLU; Brann y Mahesh, 1997), aspartate (ASP; Boni et al., 2006) and arginine (ARG; Recabarren et al., 1996).
-
Mahesh y
Brann, 2005Regulatory role of excitatory amino acids in reproductionEndocrine, 2005
-
GLU;
Brann y Mahesh, 1997Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretionEndocrine reviews, 1997
-
Boni et al., 2006D-aspartate and reproductive activity and sheepTheriogenology, 2006
-
Recabarren et al.,
1996Effect of arginine and ornithine infusions on luteinizing hormone secretion in prepubertal ewesJournal Animal Science, 1996
The action of amino acids such as glutamine, proline, and glycine regulate the functions of health, survival, growth, development, lactation and reproduction (Wu, 2010); or affect gene expression, fertility, neurotransmission and immunity in animals (Wu, 2014). In addition, GLU, glutamine, glycine, tryptophan, and tyrosine, D-alanine, D-aspartate and D-serine regulate the development and neurological function (Fernstrom, 2012). Neurotransmitters make up the neural networks and control cellular and synaptic functions in the central nervous system (CNS), excitatory and inhibitory neurotransmission is largely mediated by GLU and gamma-aminobutyric acid (GABA), which are excitatory and inhibitory neurotransmitters , respectively (Mayor y Tymianski, 2017).
-
Wu, 2010Functional amino acids in growth, reproduction, and healthAmerican Society for Nutrition. Advances in Nutrition, 2010
-
Wu, 2014Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutritionJournal of Animal Science and Biotechnology, 2014
-
Fernstrom, 2012Large neutral amino acids: dietary effects on brain neurochemistry and functionAmino Acids, 2012
-
Mayor y
Tymianski, 2017Neurotransmitters in the mediation of cerebral ischemic injuryNeuropharmacology, 2018
The GLU regulates the expression of sexual behavior, specifically in the medial preoptic area, through the action of dopamine due to its action on GnRH neurons (Iremonger et al., 2010). In the male, to regulate testosterone secretions, which is required as a mediator of baseline dopamine concentrations to increase copulatory ability (Will et al., 2014). Rodents increase neuronal activity that facilitates penile erection and mating behavior (Li et al., 2013). In the control of reproduction in sheep observed that, ovarian activity responds to neuronal changes in the brain and results from complementary alterations in the control of hypothalamic function, specifically in the regulation and secretion of GnRH (Weems et al., 2015). GnRH is the first messenger responsible for the initiation, restoration and cyclicity of reproductive activity in sheep and goats, and it is by different neurotransmitters regulated (Meza-Herrera, 2012). The control of the pulsatile secretion of GnRH by the hypothalamus and its ovarian response in the secretion of LH and FSH by the adenohypophysis is by the action of compounds favored that act as neurotransmitters (Brann y Mahesh, 1995) and they are improvement with the supply of neurostimulatory amino acids (AANE). Neurotransmitters and neuromodulators have stimulatory and inhibitory properties that depend on the composition of the neurocircuit. In addition, it depends on the state of development and the hormonal environment (Terasawa y Fernández, 2001). This classification is the characteristics of the control based on, of the pulsatile release of GnRH, in the adult animal and based on the classification AANEs can be as stimulators or inhibitors described. The main CNS neurotransmitters are AANE (Urbanski et al., 1994), which have specificity in the activation of postsynaptic CNS neurons. The neurotransmission of AANEs is an essential component in neuroendocrine transmission, which regulates the secretion of pituitary hormones. AANEs such as ASP and GLU are in large numbers in presynaptic areas found, of a variety of hypothalamic nuclei: arcuate, suprachiasmatic, supraoptic, paraventricular nucleus and preoptic area (Brann y Mahesh, 1994).
-
Iremonger et al.,
2010Glutamate regulation of GnRH neuron excitabilityBrain Research, 2010
-
Will et
al., 2014Influences of dopamine and glutamate in the medial preoptic area on male sexual behaviorPharmacololgy Biochemistry and Behavior, 2014
-
Li et al., 2013Effects of metabotropic glutamate receptor ligands on male sexual behavior in ratsNeuropharmacology, 2013
-
Weems et al., 2015Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamstersFrontiers in Neuroendocrinology, 2015
-
Meza-Herrera,
2012Puberty, kisspeptin and glutamate: A ceaseless golden braid. Chapter 3Advances in medicine and biology, 2012
-
Brann y Mahesh, 1995Glutamate: a major excitatory transmitter in neuroendocrine regulationNeuroendocrinology, 1995
-
Terasawa y Fernández, 2001Neurobiological mechanism of the onset of puberty in primatesEndocrine Reviews, 2001
-
Urbanski et al., 1994N-methyl-D-aspartate receptor gene expression in the hamster hypothalamus and in immortalized luteinizing hormone- releasing hormone neuronsJournal of Reproduction and Fertility, 1994
-
Brann y Mahesh,
1994Excitatory amino acids: function and significance in reproduction and neuroendocrine regulationFrontiers in Neuroendocrinology, 1994
Studies in sheep considered management practices to improve the productive efficiency of the herds in a technical and economic way, in which it is to eliminate the pharmacological manipulation of the animals intended (Martin et al., 2004). These methodologies are based on knowledge of reproductive events, socio-sexual factors and the effects of nutrition (Hawken y Martin, 2012; Scaramuzzi et al., 2013); or focused feeding, based on energy and protein supplements destined in the critical moments of reproduction (Somchit-Assavacheep, 2011). Therefore, the objective of the present review of the literature is to describe the neurostimulatory function of amino acids and to know the neuroendocrine response in the hypothalamic-pituitary-ovarian axis in sheep to improve the productive and reproductive variables.
-
Martin et al., 2004Natural methods of increasing reproductive efficiency in sheep and goatsAnimal Reproduction Science, 2004
-
Hawken y
Martin, 2012Sociosexual stimuli and gonadotropin-releasing hormone/luteinizing hormone secretion in sheep and goatsDomestic Animal Endocrinology, 2012
-
Scaramuzzi et
al., 2013The pattern of LH secretion and the ovarian response to the ‘ram effect’ in the anoestrous ewe is influenced by body condition but not by short-term nutritional supplementationReproduction Fertility and Development, 2013
-
Somchit-Assavacheep, 2011Influence of nutritional management on folliculogenesis in ewesThai Journal of Veterinary Medicine, 2011
L-ARGININE AND ITS NEUROENDOCRINE ACTION IN REPRODUCTION
The amino acid L-Arginine (ARG) was first isolated in 1886, from the seeds of the legume Lupinus sp. (Wu y Morris, 1998). It is from glutamine, glutamate (GLU) and intestinal proline synthesized through the renal axis in most mammals (Wu, 1998). It participates in the metabolism as a substrate for protein synthesis, because it is an intermediate in the urea cycle that is in the liver performed and as a precursor for the synthesis of several metabolic molecules, such as nitric oxide (NO) and polyamines (Kim et al., 2007). In the arginase pathway, polyamines are from ornithine synthesized to participate in embryogenesis and placental growth (Reynolds y Redmer, 2001).
-
Wu y
Morris, 1998Intestinal mucosal amino acid catabolismThe Journal of Nutrition, 1998
-
Wu, 1998Arginine metabolism: Nitric oxide and beyondBiochemical Journal, 1998
-
Kim et al.,
2007Functional amino acids and fatty acids for enhancing production performance of sows and piglets Asian-AustralasianJournal of Animal Sciences, 2007
-
Reynolds y Redmer, 2001Angiogenesis in the placentaBiology of Reproduction, 2001
In 1987, the scientific community discovered that the human body produces NO (Tsikas, 2007). It is known that NO is a regulator in the female reproductive process (Tamanini et al., 2003), such as the development and growth of the placenta. Besides, it participates in the maintenance of pregnancy and the physiology of delivery (Kwon et al., 2004), ovarian function, ovarian follicular development and ovulation; In addition, it participates in the regulation of blood pressure, immune response, platelet aggregation and neurotransmission.
-
Tsikas, 2007Analysis of the L-arginine/NO pathwayJournal of Chromatography B, 2007
-
Tamanini et al., 2003Nitric oxide and the ovaryJournal Animal Science, 2003
-
Kwon et al., 2004Developmental changes in nitric oxide synthesis in the ovine placentaBiology of Reproduction, 2004
ARG is the only substrate of all isoforms of nitric oxide synthetase (NOS; Wiesinger, 2001). The production of the NO is by oxidation of the amino group of ARG, which uses molecular oxygen as a co-substrate, and as a secondary product of the reaction, L- Citrulline is obtained (CIT; Tsikas, 2007). The CIT can be recycled to ARG by synthetic arginosuccinate and arginosuccinate lyase, which forms the CIT-NO cycle (Mori y Gotoh, 2004). The mechanism of action of the NO as a regulator of said processes, responds to the fact that it stimulates the soluble guanylate cyclase enzyme to synthesize cyclic guanosine monophosphate (cGMP), which is responsible for such regulation (Figure 1).
-
Wiesinger, 2001Arginine metabolism and the synthesis of nitric oxide in the nervous systemProgress in Neurobiology, 2001
-
Tsikas, 2007Analysis of the L-arginine/NO pathwayJournal of Chromatography B, 2007
-
Mori y Gotoh,
2004Arginine metabolic enzymes, nitric oxide and infectionThe Journal of Nutrition, 2004
Thumbnail
Figure 1
Action of nitric oxide (NO) in the control and release of gonadotropin-releasing hormone (GnRH).
Action of nitric oxide (NO) in the control and release of gonadotropin-releasing hormone (GnRH).
In the hypothalamus, the neurons of the NO are close to those of the GnRH, which suggests that the NO may be a regulator in the secretion of the GnRH. These neurons are located in several hypothalamic nuclei (preoptic nucleus, ventromedial hypothalamic nucleus and aquatic nucleus), and in other sites (vascular organ of the terminal lamina, preoptic area and middle eminence) related in the regulation of GnRH secretion (Grossmann et al., 1994). The NO controls the action of hormones and neurotransmitters essential to regulate reproduction, it is known by relating to the control of LH and ovulation. In addition, several inhibitory neurotransmitters and stimulators affect the NOS neurons in the hypothalamus and control the secretion of the NO (Dixit y Parvizi, 2001).
-
Grossmann et al., 1994The distribution of hypothalamic nitric oxide synthase mRNA in relation of gonadotropin- releasing hormone neuronsJournal of Endocrinology, 1994
-
Dixit y Parvizi, 2001Nitric oxide and the control of reproductionAnimal Reproduction Science, 2001
ARG supplementation in production animals improves the productive and reproductive variables. Include 1.0% of arginine hydrochloride (L-Arginine HCl, Ajinomoto) in the diet of 22 pregnant Camborough sows (30 to 114 d), increase piglet weight by 24% and increase litter size by 22% (Mateo et al., 2007). In prepubertal Suffolk sheep (2 months old) infusion of 200 mL of ARG (350 mM, pH 7.4) was applied intravenously via in the jugular venipuncture for 60 min increases the average concentration of LH for 285 min after infusion with amplitude> 1 ng mL-1 in 13 of 17 pulses of LH. It suggests that the infusion of ARG stimulates the secretion of LH in prepubertal sheep (Recabarren et al., 1996).
-
Mateo et al., 2007Dietary L-arginine supplementation enhances the reproductive performance of giltsThe Journal of Nutrition, 2007
-
Recabarren et al., 1996Effect of arginine and ornithine infusions on luteinizing hormone secretion in prepubertal ewesJournal Animal Science, 1996
In estrous synchronization protocols with intravaginal sponges in adult Awassi sheep (3.5 to 4.0 years of age) was supplemented ARG (0.5 g kg-1 body weight) for 15 days after sponge removal, increased the amount of luteal bodies (CL; 2.38±0.67), the concentrations of E2 (5.92±0.33 pg mL-1) and P4 (4.21±0.83 ng mL-1). It was compared to the response of the control sheep: 100±0.58 CL, 4.56±1.06 pg mL-1 of E2 and 1.79±0.31 ng mL-1 of P4, which improves the birth rate and twin deliveries due to the increase in the ovulatory rate (Al-Dabbas et al. 2008).
-
Al-Dabbas et al. 2008The effect of arginine supplementation on some blood parameters, ovulation rate and concentrations of strogen and progesterone in female Awassi sheepPakistan Journal of Biological Sciences, 2008
In adult hair sheep synchronized with 40 mg of fluorogestone acetate impregnated in intravaginal sponges (Cronolone-Chrono-Gest, Intervet®) for 12 d ARG supplementation (300 mg kg-1 body weight) for 3 days prior to Sponge removal improves the presentation of estrus (PE; 100%), ovulatory rate (TO; 1.7±0.13) and prolificacy (PROL; 1.4±0.16). It was compared to the response of sheep synchronized only with oil progesterone (PE: 28.6±18.4%, TO: 1.4±0.25 and PROL: 1.5±0.5), which improves estrogen synchronization protocols with progestogens, due to the positive effects of ARG supplementation on reproductive efficiency in sheep hair (Bulbarela-García et al., 2009).
-
Bulbarela-García
et al., 2009Efecto de L-arginina y aceite de pescado en el comportamiento reproductivo de ovejas de pelo sincronizadas con un progestágenoAgrociencia, 2009
In Rambouillet sheep, treated with 27 mg of L-Arginine HCl/kg of intravenous weight during maternal recognition of pregnancy was observed that the pregnancy rate was by 24% improved that suggests that ARG is related to the synthesis of NO. The treatment prior to maternal recognition of pregnancy in sheep improves early embryonic survival through the synthesis of polyamines and NO (Saevre et al., 2011).
-
Saevre et al., 2011Impacts of arginine on ovarian function and reproductive performance at the time of maternal recognition of pregnancy in ewesSheep Research Report, 2011
ASPARTATE AND ITS NEUROENDOCRINE ACTION IN REPRODUCTION
D-aspartic acid is a neurotransmitter that acts via the GLU receptor to stimulate the secretion of GnRH. It is naturally in the pituitary, thyroid, and ovary, adrenal and pineal; in the brain, in excretory organs such as liver and kidney, in muscle and deep tissues. At present, this D-amino acid can be to N-methyl-D-aspartic acid (NMDA) converted. It is a neuromodulator related to sexual activity, which causes the release of hypothalamic and pituitary hormones and possibly the administration of D-aspartic acid. Increase NMDA concentrations in the nervous system; because D-aspartic acid is naturally present and is stored in the pituitary, brain and pineal gland (Boni et al., 2006).
-
Boni et al., 2006D-aspartate and reproductive activity and sheepTheriogenology, 2006
NMDA is biosynthesized endogenously from D-Aspartate by an enzyme dependent on S- adenosylmethionine, NMDA synthase and it is a potent agonist of the activity of aspartic and glutamic acids. These have a neuromodulatory activity that causes release of pituitary hormones, in vivo (D´aniello et al., 2000a; 2000b) and in vitro (Barb et al., 1993), and belongs to the group of ionotropic GLU receptors.
-
D´aniello et al., 2000aThe role of D-aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin releaseEndocrinology, 2000
-
2000bOccurrence of D- Aspartic acid and N-metil-D-aspartic acid in in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone releaseThe Federation of American Societies for Experimental Biology Journal, 2000
-
Barb et al., 1993N-methyl-DL-aspartate modulation of luteinizing hormone and growth hormone secretion from pig pituitary cell in cultureLife Sciences, 1993
Estienne et al.(1989 a) administered intravenously NMDA (12 mg kg-1 body weight; racemic mixture, Sigma Chemical co., St. Louis, MO) in castrated Hampshire rams (4 months old and 28.1±1.3 kg of weight). It observed an increase in growth hormone (GH; 185.1±20.7 ng mL-1) instead of LH secretion at 15 minutes after injecting the dose, which was in the range that stimulated secretion of LH in monkeys. Therefore, they concluded that it is possible that the ram is less sensitive to NMDA and requires a larger dose to evoke the secretion of LH.
-
Estienne et al.(1989 aN-methyl-d, l- aspartate stimulates growth hormone but not luteinizing hormone secretion in the sheepLife Sciences, 1989
On the contrary, in ovarian-ectomized sheep, Estienne et al. (1989 b) demonstrated that the supply of estradiol subcutaneously (1 pg mL-1 of E2; Silastic implant, polyethylene tube; Portex Ltd, Hythe, Kent) decreases the serum concentration of LH. However, intravenous application of 6, 12 or 24 mg NMDA kg-1 body weight (dissolved in 0.9% saline) increases the average LH concentrations by 326% (P <0.03), 1125% (P < 0.02) and 441% (P <0.0001). Therefore, these results demonstrate that exogenous E2 suppresses the secretion of LH in ovarian-ectomized sheep in an antagonized manner by the effect of NMDA.
-
Estienne et al. (1989 bEffect of N-methyl- d, l-aspartate on luteinizing hormone secretion in ovariectomized ewes in the absence and presence of estradiolBiology of Reproduction, 1989
Sheep nutrition improved the plasma concentration of D-aspartic acid in the brain can be increased, to stimulate an increase in GnRH secretion, due to the effect of NMDA on pituitary hormone concentrations and the positive effects of D-aspartic acid on the ovulatory rate and pituitary hormone concentrations. Thus, applying D-aspartic acid (intravenously) for five days in the luteal phase of the estrous cycle does not affect the ovulatory rate, but reduces the plasma concentrations of LH and FSH in cycling sheep (Downing et al., 1996). Therefore, the decrease in gonadotropin secretion in cycling sheep treated with D-aspartic acid is due to the response in the hypothalamus or adenohypophysis, which are not to the secretions of ovarian retroaction related, although it is possible that, these changes decrease the secretion of GnRH.
-
Downing et al., 1996The effects of N-methyl-D-L-aspartic acid and aspartic acid on the plasma concentration of gonadotrophins, GH and prolactin in the eweJournal of Endocrinology, 1996
GLUTAMATE AND ITS NEUROENDOCRINE ACTION IN REPRODUCTION
GLU acts in the control of brain functions, because it is in large numbers at the synapses of the brain found, and by the numerous subtypes of GLU receptors found in the CNS (Brann y Mahesh, 1997). GLU and ASP are as predominant AANE classified of the CNS in mammals (Kalb, 1995). Because of GLU receptors are distributed in the hippocampus, cerebral cortex, and cerebellum; this amino acid influences various physiological processes (Brann, 1995), such as the control of gonadotropin secretion and the ovulation of the female (Brann y Mahesh, 1997).
-
Brann y Mahesh, 1997Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretionEndocrine reviews, 1997
-
Kalb, 1995Current excitement about the glutamate receptor familyThe Neuroscientist, 1995
-
Brann, 1995Glutamate: a major excitatory transmitter in neuroendocrine regulationNeuroendocrinology, 1995
-
Brann y Mahesh,
1997Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretionEndocrine reviews, 1997
The administration of GLU agonists stimulates the release of GnRH and LH, while GLU antagonist receptors decrease steroid induction and the pre-occupational increase of LH (Dhandapani y Brann, 2000). Thus, AANE receptors are the most abundant stimulatory neurotransmitter receptors in the CNS, also called “GLU receptors” since it is known to be the largest endogenous ligand. Brann y Mahesh (1997) reported two groups of receptors:
-
Dhandapani y Brann,
2000The role of glutamate and nitric oxide in the reproductive neuroendocrine systemBiochemistry and Cell Biology, 2000
-
Brann
y Mahesh (1997)Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretionEndocrine reviews, 1997
Ionotropic: receptors coupled to ion channels, divided into the subtypes N-methyl-D- aspartate (NMDA), kainate and propionic acid DL-α-methyl-3-hydroxy-4-isoxazole (AMPA), where its main mode of action it is through the modulation of the channels of the Na+, K+ and Ca2+ ions.
Metabotropic: receptors coupled to G proteins, which modulate the production of secondary messengers such as inositol phosphate and/or adenylate cyclase.
The GLU exists in four different forms: transmitter, metabolic, glial and precursor of GABA. GLU is to critical processes related such as puberty, the pulsatility of hormones and sexual behavior. Releasing the neurotransmitter NO, which potently stimulates GnRH by activating a heme-containing enzyme, guanylate cyclase (Dhandapani y Brann, 2000). GLU stimulates LH secretion Brann y Mahesh (1997) and that ionotropic GLU receptor agonists increase LH secretion after systemic or intracerebroventricular injections in rats (Zamorano et al., 1998), by stimulating the secretion of GnRH. The action of these receptors underlies the rapid stimulatory synaptic transmission mediated by GLU in the CNS (Brann y Mahesh, 1994).
-
Dhandapani y Brann, 2000The role of glutamate and nitric oxide in the reproductive neuroendocrine systemBiochemistry and Cell Biology, 2000
-
Brann y Mahesh
(1997)Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretionEndocrine reviews, 1997
-
Zamorano et al., 1998Excitatory amino acid receptors and pubertySteroids, 1998
-
Brann y Mahesh, 1994Excitatory amino acids: function and significance in reproduction and neuroendocrine regulationFrontiers in Neuroendocrinology, 1994
Recent studies indicate that GLU, ARG and glutamine play important roles in the regulation of gene expression, cell signaling, antioxidant responses and immunity. In addition, GLU, glutamine and ASP are important metabolic fuels for the small intestine and, together with glycine, regulate neurological function (Wu, 2013). In reproductive function, ARG supplementation during maternal recognition of pregnancy in sheep favors embryonic survival (Crane et al., 2016) and improves pregnancy and delivery rates (Luther et al., 2009). NMDA and LH increase after ASP administration, which suggests a role of this amino acid in reproductive activity in sheep (Boni et al., 2006). GLU is a primary mediator of excitatory synaptic transmission in the CNS and its receptors are located in a variety of hypothalamic nuclei, some of which are critical for reproduction and neuroendocrine function, due to their relationship with puberty, neurogenesis and reproductive behavior in the female (Meza-Herrera et al., 2011).
-
Wu, 2013Functional amino acids in growth, reproduction, and healthAmerican Society for Nutrition. Advances in Nutrition, 2010
-
Crane et
al., 2016Impacts of supplemental arginine on the reproductive performance of fall lambing ewesJournal of Animal Science, 2016
-
Luther et al., 2009Effects of arginine supplementation on reproductive performance in Rambouillet ewesSheep Research Report, 2009
-
Boni
et al., 2006D-aspartate and reproductive activity and sheepTheriogenology, 2006
-
Meza-Herrera et al., 2011Glutamate supply positively affects serum release of triiodothyronine and insulin across time without increases of glucose during the onset of puberty in the female goatAnimal Reproduction Science, 2011
CONCLUSION
The action of neurostimulatory amino acids stimulates the secretion of adenohypophyseal gonadotropins, and therefore regulates the control of gonadal physiological events. This knowledge can be to increase reproductive efficiency applied in sheep and improve productive and reproductive variables.
LITERATURA CITADA
- AL-DABBAS FM, Hamra AH, Awawdeh FT. 2008. The effect of arginine supplementation on some blood parameters, ovulation rate and concentrations of strogen and progesterone in female Awassi sheep. Pakistan Journal of Biological Sciences. 11(20): 2389-2394. ISSN: 1028-8880. https://www.ncbi.nlm.nih.gov/pubmed/19137847 Links
- BARB CR, Barrett JB, Rampacek GB, Kraeling RR. 1993. N-methyl-DL-aspartate modulation of luteinizing hormone and growth hormone secretion from pig pituitary cell in culture. Life Sciences. 53: 1157-1164. ISSN: 0024-3205. https://doi.org/10.1016/0024- 3205(93)90552-E Links
- BONI R, Santillo R, Macchia G, Spinelli P, Ferrandino G, D´Aniello A. 2006. D-aspartate and reproductive activity and sheep. Theriogenology. 65: 1265-1278. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2005.07.019 Links
- BRANN DW, Mahesh VB. 1994. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Frontiers in Neuroendocrinology. 15: 3-49. ISSN: 0091-3022. https://doi.org/10.1006/frne.1994.1002 Links
- BRANN DW, Mahesh VB. 1995. Glutamate: a major neuroendocrine excitatory signal mediating steroid effects on gonadotropin secretion. The Journal of Steroid Biochemistry and Molecular Biology. 53(1-6): 325-329. ISSN: 0960-0760. https://doi.org/10.1016/0960- 0760(95)00070-G Links
- BRANN DW, Mahesh VB. 1997. Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocrine reviews. 18(5): 678- 700. ISSN: 0163-769X. https://doi.org/10.1210/edrv.18.5.0311 Links
- BRANN DW. 1995. Glutamate: a major excitatory transmitter in neuroendocrine regulation. Neuroendocrinology. 61: 213-225. ISSN: 0028-3835 https://doi.org/10.1159/000126843 Links
- BULBARELA G, Pro-Martínez A, Becerril-Pérez CM, Días-Rivera P, Rosendo-Ponce A, Gallegos-Sánchez J. 2009. Efecto de L-arginina y aceite de pescado en el comportamiento reproductivo de ovejas de pelo sincronizadas con un progestágeno. Agrociencia. 43(4): 371-377. ISSN: 1405-3195. http://www.colpos.mx/agrocien/Bimestral/2009/may-jun/art-4.pdf Links
- CRANE AR, Redden RR, Van Emon ML, Neville TL, Reynolds LP, Caton JS, Schauer CS. 2016. Impacts of supplemental arginine on the reproductive performance of fall lambing ewes. Journal of Animal Science. 94: 3540-3549. ISSN: 1525-3163. https://doi.org/10.2527/jas.2016-0379 Links
- D’ANIELLO G, Tolino A, D’aniello A, Errico F, Fisher GH, Di-Fiore MM. 2000a. The role of D-aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin release. Endocrinology. 141(10): 3862-3870. ISSN: 1945-7170. https://doi.org/10.1210/endo.141.10.7706 Links
- D´ANIELLO G, Di-Fiore MM, Fisher GH, Milone A, Seleni AD. 2000b. Occurrence of D- Aspartic acid and N-metil-D-aspartic acid in in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. The Federation of American Societies for Experimental Biology Journal. 14(5): 699-714. ISSN: 0892-6638. https://doi.org/10.1096/fasebj.14.5.699 Links
- DHANDAPANI KM, Brann DW. 2000. The role of glutamate and nitric oxide in the reproductive neuroendocrine system. Biochemistry and Cell Biology. 78(3): 165-79. https://doi.org/10.1139/o00-015 Links
- DIXIT VD, Parvizi N. 2001. Nitric oxide and the control of reproduction. Animal Reproduction Science. 65(1-2): 1-16. ISSN: 0378-4320. https://doi.org/10.1016/S0378-4320(00)00224-4 Links
- DOWNING JA, Joss J, Scaramuzzi RJ. 1996. The effects of N-methyl-D-L-aspartic acid and aspartic acid on the plasma concentration of gonadotrophins, GH and prolactin in the ewe. Journal of Endocrinology . 149: 65-72. ISSN: 0022-0795. https://doi.org/10.1677/joe.0.1490065 Links
- ESTIENNE MJ, Schillo KK, Green MA, Hileman SM, Boling JA. 1989a. N-methyl-d, l- aspartate stimulates growth hormone but not luteinizing hormone secretion in the sheep. Life Sciences . 44(21): 1527-1533. ISSN: 0024-3205. https://doi.org/10.1016/0024-3205(89)90445-1 Links
- ESTIENNE MJ, Schillo KK, Hileman SM, Green MA, Hayes SH. 1989b. Effect of N-methyl- d, l-aspartate on luteinizing hormone secretion in ovariectomized ewes in the absence and presence of estradiol. Biology of Reproduction. 42: 126-130. ISSN: 0006-3363. https://doi.org/10.1095/biolreprod42.1.126 Links
- FALETTI AG, Mastronardi CA, Lomniczi A, Seilicovich A, Gimeno M, McCann SM, Rettori V. 1999. β-Endorphin blocks luteinizing hormone-releasing hormone release by inhibiting the nitricoxidergic pathway controlling its release. Proceedings of the National Academy of Sciences. U.S.A. 96: 1722-1726. ISSN: 0027-8424. https://doi.org/10.1073/pnas.96.4.1722 Links
- FERNSTROM JD. 2012. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids (in this issue). ISSN: 1438-2199.https://doi.org/10.1007/s00726-012-1330-y Links
- GROSSMANN AB, Rossmanith WG, Kabigting E, Cadd G, Clifton D, Steiner R. 1994. The distribution of hypothalamic nitric oxide synthase mRNA in relation of gonadotropin- releasing hormone neurons. Journal of Endocrinology . 140(2): R5-R8. ISSN: 0022-0795. https://doi.org/10.1677/joe 0.140R005 Links
- HAWKEN PAR, Martin GB. 2012. Sociosexual stimuli and gonadotropin-releasing hormone/luteinizing hormone secretion in sheep and goats. Domestic Animal Endocrinology . 43: 85-94. ISSN: 0739-7240. https://doi.org/10.1016/j.domaniend.2012.03.005 Links
- IREMONGER KJ, Constaintin S, Liu X, Herbison AE. 2010. Glutamate regulation of GnRH neuron excitability. Brain Research. 1364: 35-43. ISSN: 0006-8993. https://doi.org/10.1016/j.brainres.2010.08.071 Links
- KALB RG. 1995. Current excitement about the glutamate receptor family. The Neuroscientist. 1:60-63. ISSN: 1073-8584. https://doi.org/10.1177/107385849500100201 Links
- KIM SW, Mateo RD, Yin YL, Wu G. 2007. Functional amino acids and fatty acids for enhancing production performance of sows and piglets Asian-Australasian. Journal of Animal Sciences. 20(2): 295-306. ISSN: 1011-2363. https://doi.org/10.5713/ajas.2007.295 Links
- KWON H, Wu G, Meininger CJ, Bazer FW, Spencer TE. 2004. Developmental changes in nitric oxide synthesis in the ovine placenta. Biology of Reproduction . 70(3): 679-686. ISSN: 0006-3363. https://doi.org/10.1095/biolreprod.103.023184 Links
- LI X, Higley A, Song R, Xi ZX. 2013. Effects of metabotropic glutamate receptor ligands on male sexual behavior in rats. Neuropharmacology. 66: 373-381. ISSN: 0028-3908. https://doi.org/10.1016/j.neuropharm.2012.08.006 Links
- LUTHER JS, Windorski EJ, Caton JS, Wu G, Kirsch JD, Vonnahme KA, Reynolds LP, Schauer CS. 2009. Effects of arginine supplementation on reproductive performance in Rambouillet ewes. Sheep Research Report. No. 50. North Dakota State Univertity, Fargo. p. 11-13. https://www.ag.ndsu.edu/archive/hettinge/livestock/2009%20Sheep%20Reserch%20Report/Arginine%20Supplementation%20to%20Ewes%20for%20Increased%20Reproductive%20Performance.pdf Links
- MAHESH VB, Braan DW. 2005. Regulatory role of excitatory amino acids in reproduction. Endocrine. 28: 271-280. ISSN: 1559-0100. https://link.springer.com/article/10.1385/ENDO:28:3:271 Links
- MARTIN GB, Milton JTB, Davidson RH, Banchero GE, Lindsay DR, Blache D. 2004. Natural methods of increasing reproductive efficiency in sheep and goats. Animal Reproduction Science . 82-83: 231-46. 0378-4320. https://doi.org/10.1016/j.anireprosci.2004.05.014 Links
- MATEO RD, Wu G, Bazer FW, Park JC, Shinzato I, Kim SW. 2007. Dietary L-arginine supplementation enhances the reproductive performance of gilts. The Journal of Nutrition. 137(3): 652-656. ISSN: 0022-3166. https://doi.org/10.1093/jn/137.3.652 Links
- MAYOR D, Tymianski M. 2018. Neurotransmitters in the mediation of cerebral ischemic injury. Neuropharmacology . 134(B): 178-188. ISSN: 0028-3908. https://doi.org/10.1016/j.neuropharm.2017.11.050 Links
- MEZA-HERRERA CA, Torres-Moreno M, Lopez-Medrano JI, Gonzalez-Bulnes A, Veliz FG, Mellado M, Wurzinger M, Soto-Sánchez MJ, Calderón-Leyva MG. 2011. Glutamate supply positively affects serum release of triiodothyronine and insulin across time without increases of glucose during the onset of puberty in the female goat. Animal Reproduction Science . 125:74-80. ISSN: 0378-4320. https://doi.org/10.1016/j.anireprosci.2011.03.011 Links
- MEZA-HERRERA CA. 2012. Puberty, kisspeptin and glutamate: A ceaseless golden braid. Chapter 3. In: Advances in medicine and biology. Benhardt, L.V. (ed). Nova Science Publishers, NY, USA. 97-124. ISBN: 978-1-62081-339-3. https://www.researchgate.net/publication/235933762_Puberty_kisspeptin_and_glutamat e_A_ceaseless_golden_braid Links
- MORI M, Gotoh T. 2004. Arginine metabolic enzymes, nitric oxide and infection. The Journal of Nutrition . 134(10): 2820s-2825s. ISSN: 0022-3166. https://doi.org/10.1093/jn/134.10.2820S Links
- RECABARREN SE, Jofré A, Lobos A, Orellana P, Parilo J. 1996. Effect of arginine and ornithine infusions on luteinizing hormone secretion in prepubertal ewes. Journal Animal Science. 74: 162-166. ISSN: 0021-8812. https://doi.org/10.2527/1996.741162x Links
- REYNOLDS LP, Redmer DA. 2001. Angiogenesis in the placenta. Biology of Reproduction . 64(4): 1033-1040. ISSN: 0006-3363. https://doi.org/10.1095/biolreprod64.4.1033 Links
- SAEVRE, C, Meyer AM, Van Emon ML, Redmer DA, Caton JS, Kirsch JD, Luther JS, Schauer CS. 2011. Impacts of arginine on ovarian function and reproductive performance at the time of maternal recognition of pregnancy in ewes. Sheep Research Report . North Dakota State University, Fargo. 52: 13-16. https://pdfs.semanticscholar.org/c540/63c9f5742fd22198d92d5cf367aed3343f01.pdf Links
- SCARAMUZZI RJ, Oujagir L, Menassol JB, Freret F, Piezel A, Brown HM, Cognié J, Fabre-Nys C. 2013. The pattern of LH secretion and the ovarian response to the ‘ram effect’ in the anoestrous ewe is influenced by body condition but not by short-term nutritional supplementation. Reproduction Fertility and Development. 26 (8): 1154-1165. ISSN: 1448-5990. https://doi.org/10.1071/RD13139 Links
- SOMCHIT-ASSAVACHEEP A. 2011. Influence of nutritional management on folliculogenesis in ewes. Thai Journal of Veterinary Medicine. (Suppl.). 41: 25-29. ISSN: 0125-6491. https://pdfs.semanticscholar.org/2aa1/26edb54f9699e68162edcff1b537947bcc3a.pdf Links
- TAMANINI C, Basini G, Grasselli F, Tirelli M. 2003. Nitric oxide and the ovary. Journal Animal Science . 81(2): E1-E7. ISSN: 0021-8812. https://doi.org/10.2527/2003.8114_suppl_2E1x Links
- TERASAWA E, Fernández DL. 2001. Neurobiological mechanism of the onset of puberty in primates. Endocrine Reviews. Rev. 22: 111-151. ISSN: 0163-769X. https://doi.org/10.1210/edrv.22.1.0418 Links
- TSIKAS D. 2007. Analysis of the L-arginine/NO pathway. Journal of Chromatography B. 851: 1-2. ISSN: 1570-0232. https://www.sciencedirect.com/journal/journal-of- chromatography-b/vol/851 Links
- URBANSKI HF, Fahy MM, Daschel M, Mashul C. 1994. N-methyl-D-aspartate receptor gene expression in the hamster hypothalamus and in immortalized luteinizing hormone- releasing hormone neurons. Journal of Reproduction and Fertility. 100: 5-9. ISSN: 0022-4251. http://www.reproduction-online.org/content/100/1/5.long Links
- WEEMS PW, Goodman RL, Lehman MN. 2015. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Frontiers in Neuroendocrinology. 37: 43-51. ISSN: 0091-3022. https://doi.org/10.1016/j.yfrne.2014.12.002 Links
- WIESINGER H. 2001. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Progress in Neurobiology. 64(4): 365-391. ISSN: 0301-0082. https://doi.org/10.1016/S0301-0082(00)00056-3 Links
- WILL RG, Hull EM, Dominguez JM. 2014. Influences of dopamine and glutamate in the medial preoptic area on male sexual behavior. Pharmacololgy Biochemistry and Behavior. 121: 115-123. ISSN: 0091-3057. https://doi.org/10.1016/j.pbb.2014.02.005 Links
- WU G, Morris SM. 1998. Arginine metabolism: Nitric oxide and beyond. Biochemical Journal. 336: 1-17. ISSN: 0264-6021. https://doi.org/10.1042/bj3360001 Links
- WU G. 1998. Intestinal mucosal amino acid catabolism. The Journal of Nutrition . ISSN: 0022-3166. https://doi.org/10.1093/jn/128.8.1249 Links
- WU, G. 2010. Functional amino acids in growth, reproduction, and health. American Society for Nutrition. Advances in Nutrition. 1: 31-37. ISSN: 2156-5376. https://doi.org/10.3945/an.110.1008 Links
- WU G. 2013. Functional amino acids in nutrition and health. Amino Acids . 45: 407-411. ISSN: 1438-2199. https://doi.org/10.1007/s00726-013-1500-6 Links
- Wu G. 2014. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of Animal Science and Biotechnology. 5: 34. ISSN: 2049-1891. https://jasbsci.biomedcentral.com/articles/10.1186/2049-1891-5-34 Links
- ZAMORANO PL, Mahesh VB, De Sevilla L, Brann DW. 1998. Excitatory amino acid receptors and puberty. Steroids. 63(5-6): 268-270. ISSN: 0039-128X. https://doi.org/10.1016/S0039-128X(98)00033-6 Links
- ZARAZAGA LA, Celi I, Guzmán JL, Malpaux B. 2011. The role of nutrition in the regulation of LH secretion by the opioidergic, dopaminergic and serotonergic systems in female Mediterranean goats. Biology of Reproduction . 84: 447-454. ISSN: 1529-7268. https://doi.org/10.1095/biolreprod.110.086520 Links

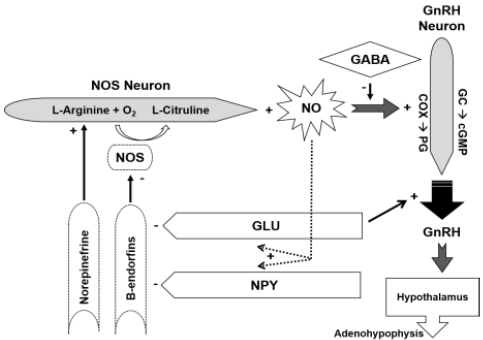 Positive effect [+], negative effect [-], GABA: Aminobutyric acid
range, GC: Guanilyl cyclase, cGMP: Guanidine methyl cyclic phosphate,
COX: Cyclooxygenase, PG: Prostaglandin, Glu: Glutamate, NPY:
Neuropeptide, NOS: Oxide nitric synthetase (Modified by Faletti et al.,
1999).
Positive effect [+], negative effect [-], GABA: Aminobutyric acid
range, GC: Guanilyl cyclase, cGMP: Guanidine methyl cyclic phosphate,
COX: Cyclooxygenase, PG: Prostaglandin, Glu: Glutamate, NPY:
Neuropeptide, NOS: Oxide nitric synthetase (Modified by Faletti et al.,
1999).