Introduction
In recent years, there has been increasing interest in the extraction of phytochemicals from plant residues in the primary sector, due to the potential health benefits of these compounds (Kussmann et al., 2023). Among the most relevant phytochemicals are flavonoids, which are bioactive compounds present in plants derived from secondary metabolism (Nagula & Wairkar, 2019). Structurally, flavonoids consist of a C6-C3-C6 ring with several substitutes that create the different subclasses: flavones, flavanol, flavonol, isoflavones, and anthocyanins (Wang et al., 2018).
Flavonoids have mainly antioxidant (Alara et al., 2020), anti-inflammatory (Dong et al., 2019), antimicrobial (Saleem & Saeed, 2020), anticancer (Thamrongwatwongsa et al., 2024), photoprotective and anti-aging (Landa-Cansigno et al., 2023) properties. Numerous studies have analyzed the flavonoid profiles of plant materials from agro-industrial by-products (León-Roque et al., 2023), which are considered a viable alternative for the extraction of these compounds due to their abundance, low cost and easy accessibility (Oleszek et al., 2023). The search for new sources of bioactive compounds has gained interest due to their potential application in the cosmetic, pharmaceutical and agricultural industries.
The exploitation of Agave lechuguilla Torr. generates large amounts of waste from fiber extraction. This wild plant, common in the arid and semi-arid regions of the southern United States and northeastern Mexico, covers more than 20 million hectares in the latter country, corresponding to 10 % of the national territory. Its distribution is centered in the states of Coahuila, Chihuahua, Nuevo León, Durango, San Luis Potosí, Tamaulipas and Zacatecas (Castillo et al., 2011). The extraction of A. lechuguilla fiber is a livelihood activity for families in rural communities (Castillo et al., 2011). However, fiber represents only 15 % of the part of the plant that is used, while bagasse, or guishe, represents 85 % (Morreeuw et al., 2021b), which generates about 150 000 t∙year-1 nationally (Díaz-Jiménez et al., 2019).
Currently, there is no adequate and commercially valuable final disposal method for guishe, which contributes to environmental problems. For this reason, its valorization has been proposed through the extraction of phytochemicals, such as flavonoids, which have bioactive properties of interest to various industries (Sánchez-Robles et al., 2023). Recent studies have identified the presence of flavonoids in the leaves (Anguiano-Sevilla et al., 2018) and guishe of A. lechuguilla (Morreeuw et al., 2021a). However, the complexity of the guishe plant material is an important factor for the extraction of these compounds; therefore, the extraction process is key to their recovery (Bachtler & Bart, 2021; Rifna et al., 2023).
There are various techniques for the extraction of bioactive compounds; among the most commonly used are the Soxhlet method (continuous extraction with a hot solvent [Azwanida, 2015]), maceration (extraction by submersion in solvent [Nugrahani et al., 2024]), decoction (extraction by boiling the material in water [Perera et al., 2017]) and infusion (extraction using solvents at temperatures no higher than 100 °C [Soto-Maldonado et al., 2022]). However, these techniques require long times, high temperatures and large amounts of solvents, which increases production costs and may lead to the degradation of active compounds (Bachtler & Bart, 2021).
Recently, unconventional techniques have gained interest, such as extraction assisted by ultrasound (extraction by cavitation [Deng et al., 2017]), microwaves (extraction by microwave heating of the solvent [Rodsamrana & Sothornvita, 2019]), enzymes (extraction by addition of enzymes [Panja, 2018]) and supercritical fluids (extraction by solvents up to their critical temperature [Hu et al., 2023]). These techniques require less extraction time (Sonar & Rathod, 2020), reduce solvent and energy consumption (Feki et al., 2021), are environmentally friendly (Deng et al., 2017), are easy to use (Abbas et al., 202) and generate high extraction yields of compounds (Setyaningsih et al., 2019).
Among these techniques, microwave-assisted extraction stands out for its efficiency, generating higher yields in less time, with less solvent use and good final product quality (Pinela et al., 2016; Tapia-Quirós et al., 2023). Considering the above, the aim of the present research was to evaluate the effect of different parameters (time, temperature, mass:volume ratio and solvent concentration) in microwave-assisted extraction on the flavonoid content in Agave lechuguilla guishe biomass.
Materials and methods
Collection of Agave lechuguilla bagasse (guishe)
The A. lechuguilla guishe was obtained in February 2022 in the community of Cosme, belonging to the municipality of Ramos Arizpe, Coahuila, Mexico (25° 51’ 44’’ N and 101° 20’ 39’’ W), after carving to obtain the fiber. The guishe was dehydrated in a hot air oven (KL-10, Koleff, Mexico) at 50 °C for 24 h; subsequently, it was passed through a cutting mill (SM100 Industrial Mill, Retsch, Germany) to obtain 2-mm particles.
Microwave-assisted flavonoid extraction
The extraction was performed in a microwave oven (Ethos CFR, Milestone, Italy) with a 170 mL reactor, a 100-rpm stirring system, 50 Hz frequency and 2 500 W power. Different time (10, 15, 20, 25 and 30 min), temperature (35, 40, 45, 50 and 55 °C), mass:volume ratio (1:10, 1:15, 1:20, 1:25 and 1:30 g·mL-1) and solvent concentration (40, 50, 60, 70 and 80 % ethanol, v:v) conditions were evaluated. The extracts obtained were filtered, concentrated in a rotary evaporator (IKA, Willmington, USA) to dryness at 50 °C and recovered with 10 mL of distilled water. Finally, they were dried in a hot air dehydrator (Hamilton Beach, USA) at 40 °C until constant weight. The response variable was total flavonoid content. All the analysis was performed in triplicate. The extraction yield was calculated using the following equation:
Chemical characterization
Total flavonoid content
Total flavonoid content (TFC) was determined by the aluminum chloride method described by Morreeuw et al. (2021b), with some modifications. First, 200 µL of extract dilution (10 mg·mL-1), 75 µL of NaNO2 (5 %), 375 µL of AlCl3 solution (2 %), 500 µL of NaOH (4 %) and 500 µL of distilled water were placed in a test tube, with homogenization between each reagent added and 5 min incubation in the dark. Quantification was performed from the calibration curve (y = 0.0008x + 0.0111, R2 = 0.9923) obtained with quercetin as standard (0 - 1 000 mg·L-1). Samples were measured against an ethanol blank in a UV-Vis spectrophotometer (Cary 50, Varian, USA) at 510 nm. TFC was calculated based on Equation 2 and expressed in milligrams of quercetin equivalent per gram of dry sample (mgQE·g-1 ds).
Where the sample concentration was determined from the calibration curve, and the solvent volume and sample weight correspond to the amount used in the extraction process.
Total polyphenol content
Total polyphenol content (TPC) was determined by the Folin-Ciocalteu method described by Morreeuw et al. (2021a), with some modifications. Twenty µL of extract dilution (10 mg·mL-1), 800 µL of distilled water and 60 µL of Folin-Ciocalteu reagent (Sigma-Aldrich®, USA) were placed in a test tube. The mixture was shaken and incubated for 5 min in the dark at room temperature. Subsequently, 160 µL of Na2CO3 (20 %) was added and incubated at room temperature for 2 h in the dark. Absorbance was measured at 765 nm in a UV-Vis spectrophotometer (Cary 50, Varian, USA).
Quantification was performed from the calibration curve (y = 0.0023x - 0.0286, R2 = 0.9998) obtained with gallic acid (GA) used as standard (0 - 500 mg·L-1). Samples were measured against an ethanol blank in a UV-Vis spectrophotometer (Cary 50, Varian, USA) at 765 nm. TPC was calculated based on Equation 3 and expressed in milligrams of gallic acid equivalent per gram of dry sample (mgGAE·g-1 ds).
Where the sample concentration was determined from the calibration curve, and the solvent volume and sample weight correspond to the amount used in the extraction process.
Antioxidant activity
Free radical scavenging activity by DPPH
Free radical scavenging activity was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH•), according to the protocol described by Akbari et al. (2019). First, a 20 µL dilution of the extract (10 mg·mL-1) was added to a 96-well microplate, followed by 180 µL of DPPH• ethanolic solution (25 mg·L-1) (Sigma-Aldrich®, USA). Absorbance of the mixture was measured at 540 nm in a plate reader (Multiskan SkyHigh, Thermo Scientific, USA) after incubation in the dark for 30 min at room temperature. Ethanol was used as blank, DPPH• ethanolic solution as a control, and trolox as a standard (1 000 ppm). The percentage of inhibition of the DPPH• radical was determined from the following equation:
where Ao is the absorbance of the control and Am is the absorbance of the sample.
Free radical scavenging activity by ABTS+•
Free radical scavenging activity was determined using 2,2'-azino-bis-3(ethylbenzothiazoline-6-sulfonic acid) (ABTS+•), according to the protocol described by Akbari et al. (2019). The stock solutions of ABTS+• (7 mM) and potassium persulfate (2.45 mM) were prepared separately. To obtain the working solution, the stock solutions were mixed at a 1:1 ratio and incubated for 12 h in the dark at room temperature. Subsequently, 1 mL of the working solution was mixed with ethanol until obtaining an absorbance of 0.7 (± 0.02) at a wavelength of 734 nm.
For sample analysis, 10 µL of the extract dilution (10 mg·mL-1) was placed in a 96-well microplate followed by 190 µL of ABTS+• ethanolic solution. The absorbance of the mixture was measured at 734 nm on a plate reader (Multiskan SkyHigh, Thermo Scientific, USA). Ethanol was used as blank, ABTS+• ethanolic solution as a control and trolox as a standard (1 000 ppm). The percentage of inhibition of ABTS+• was obtained with the following equation:
where Ao is the absorbance of the control and Am is the absorbance of the sample.
Qualitative tests
Colorimetric tests for flavonoid determination
Qualitative identification of flavonoids was performed by colorimetric tests, based on the sodium hydroxide and sulfuric acid methodology described by Chauhan et al. (2018).
Sodium hydroxide (NaOH) test. First, 150 µL of extract solubilized in distilled water (10 mg∙mL-1) was placed in a 96-well microplate; subsequently, drops of NaOH were added. The color change of the extracts from blue to violet indicates the presence of anthocyanins, whereas a yellow to orange hue suggests the presence of flavonones, and a yellow coloration indicates the presence of flavones.
Concentrated sulfuric acid (H2SO4) test. First, 150 µL of extract solubilized in distilled water (10 mg∙mL-1) was placed in a 96-well microplate; subsequently, drops of H2SO4 were added. The color change of the extracts between yellow to orange indicates the presence of anthocyanins, and orange to red suggests the presence of flavones.
Statistical analysis
A single-factor design was used to evaluate the flavonoid extraction process. Statistical analysis of the results (standard deviation and Tukey's comparison of means) was performed using Minitab software (ver. 19), with a confidence level of 95 % (P ≤ 0.05). All analyses were performed in triplicate.
Results and discussion
Flavonoid extraction parameters
Irradiation time
Time is a fundamental parameter in the extraction process, as it directly influences the yield, quality, energy consumption and cost of the process (Vinatoru et al., 2017). Therefore, it is crucial to optimize the microwave irradiation time from the beginning of the process. To determine the effect of irradiation time on flavonoid extraction, 12 g of A. lechuguilla guishe and 120 mL of 70 % ethanol were used. The irradiation time varied from 10 to 30 min at a constant temperature of 40 °C. Figure 1a shows that the maximum extraction of total flavonoids (7.62 ± 0.05 mg QE·g-1 ds) was reached at 10 min of irradiation, which is attributed to the increase in molecular motions and the heat generated, factors influenced by the ionic condition and dielectric properties of the solvent (Sonar & Rathod, 2020).
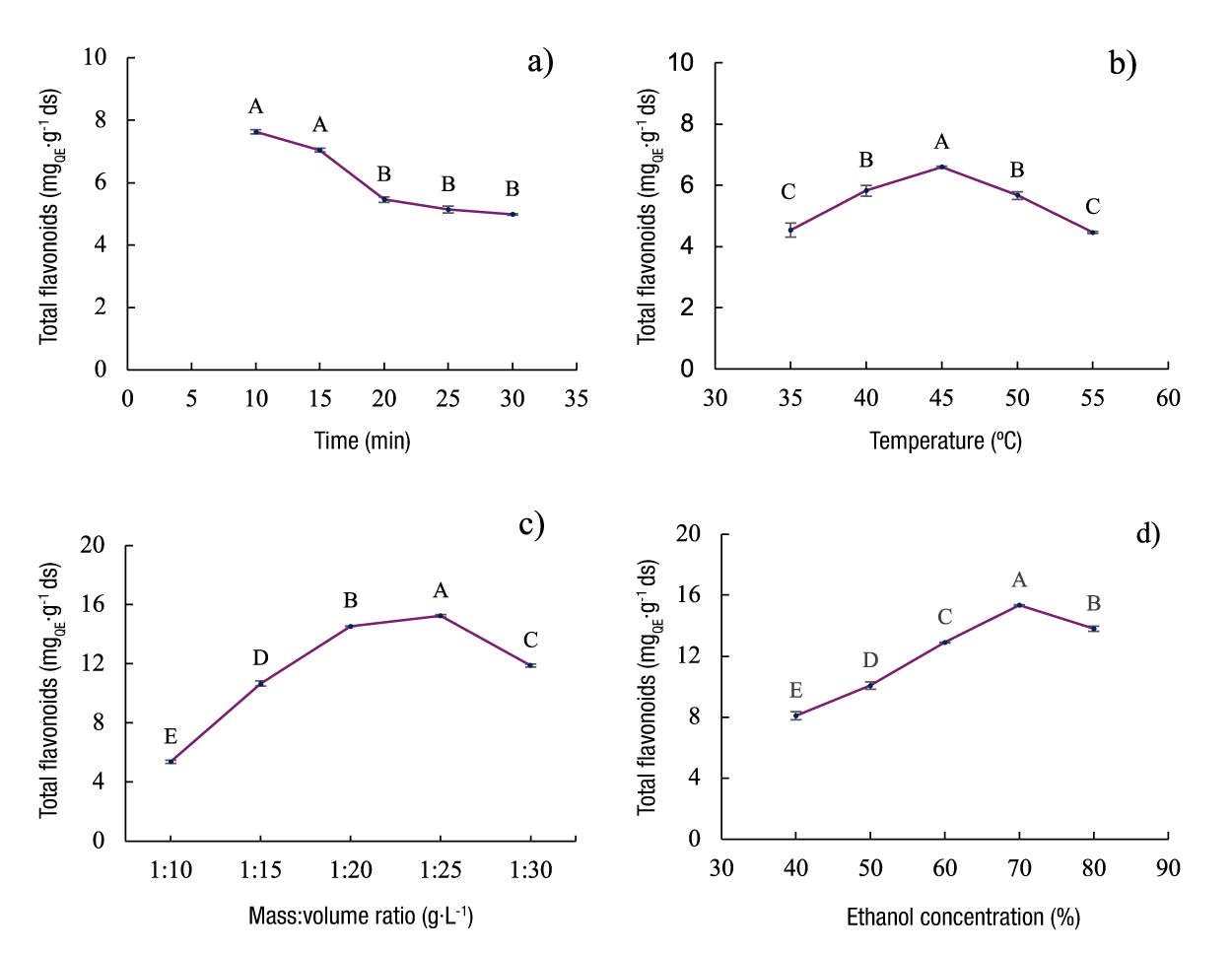
Figure 1 Effects of the flavonoid extraction process on Agave lechuguilla guishe biomass: a) time, b) temperature, c) mass:volume ratio and d) solvent concentration. Values with the same letters within each graph do not differ statistically (Tukey, P < 0.05).
The energy generated during the irradiation process causes a build-up of heat in the solvent due to the absorption of microwave energy, which is efficiently transferred to the plant material through molecular interactions. This process facilitates the extraction of flavonoids (Jha & Kumar, 2021). Subsequently, a gradual decrease in the amount of flavonoids extracted was observed after 10 min of irradiation, probably due to the degradation of the compounds by prolonged exposure to microwaves (Alara et al., 2018; Antony & Farid, 2022; Gil-Martín et al., 2022). The comparison of means (Figure 1a) showed a significant difference (P < 0.05) between the evaluated times (R2 = 98.57 %). Therefore, for studies additional to the single-factor design, 10 min of irradiation was used for the extraction process.
Temperature
Temperature is a critical factor in the microwave-assisted extraction process in a closed vessel, as it contributes to a higher recovery of phytochemicals. To determine its effect on flavonoid extraction, the temperature was varied from 35 to 55 °C, with an irradiation time of 10 min. Figure 1b shows how flavonoid extraction increases as the temperature rises from 35 to 45 °C, which is attributed to intermolecular interactions in the solvent, which intensify at higher temperatures. This increases molecular motion and the solubility of the compounds within the reactor (Sonar & Rathod, 2020).
Increased temperature creates pressure on the cell wall, which facilitates the permeability and rupture of plant cells due to tissue softening and expansion, thus releasing bioactive compounds into the solvent (Arruda et al., 2017; Seremet et al., 2021). Furthermore, the elevated temperature reduces solvent viscosity and surface tension, which favors solvent diffusion through cells, improves mass transfer, and increases the solvent's ability to solubilize analytes from plant material (Vinatoru et al., 2017).
When experiments were carried out at a temperature above 45 °C, flavonoid extraction decreased, probably due to the degradation of thermolabile bioactive compounds under elevated temperature conditions (Alara et al., 2018; Lovrić et al., 2017; Seremet et al., 2021). The comparison of means (Figure 1b) showed a significant difference (P < 0.05) in flavonoid concentration in relation to the temperature conditions used (R2 = 98.47 %). Therefore, for further studies of the single-factor design, 45 °C was used for the extraction process, as it resulted in the highest TFC (6.59 ± 0.03 mgQE·g-1 ds).
Mass:volume ratio
The mass:volume ratio is another important parameter in microwave-assisted extraction, as it influences the recovery efficiency of compounds (Sai-Ut et al., 2023). The optimal value of this ratio varies in each extraction system, so it must be established experimentally (Alara et al., 2018). In this study, the ratio was adjusted from 1:10 to 1:30 (g·mL-1), and the irradiation time (10 min), temperature (45 °C), and ethanol concentration (70 %) were kept constant.
Figure 1c shows an increase in total flavonoid extraction up to a mass:volume ratio of 1:25 g·mL-1 (15.24 ± 0.09 mgQE·g-1 ds), which is attributed to an improvement in mass transfer during the process (Krishnan & Rajan, 2017), because the analytes tend to migrate from the plant material to the solvent by dissolution and diffusion (Latif et al., 2024). However, as the ratio increased, flavonoid extraction decreased, possibly due to reduced microwave penetration into the solvent, since excess solvent absorbs energy at the periphery and limits its effective penetration (Sonar & Rathod, 2020). The comparison of means (Figure 1c) confirmed the existence of significant differences (P < 0.05) between the ratios evaluated (R2 = 99.95 %). Therefore, the ratio of 1:25 g·mL-1 was established as optimal for further studies, as it resulted in the highest concentration of flavonoids in the extract obtained.
Solvent concentration
Ethanol is a widely used solvent in the extraction of plant compounds, and is considered a “green solvent” due to its low toxicity and its ability to be mixed with water in any proportion (Alara et al., 2018). The use of a binary solvent, such as a mixture of ethanol and water, improves extraction efficiency compared to the use of a monosolvent (Kaderides et al., 2019). Ethanol was evaluated at different concentrations (40, 50, 60, 70 and 80 %, v:v), and the irradiation time (10 min), temperature (45 °C) and mass:volume ratio (1:25 g·mL-1) were kept constant.
An increase in flavonoid extraction was observed as the amount of ethanol increased, with significant differences (P < 0.05) between the different solvent concentrations (R2 = 99.76 %). Concentrations higher than 70% decreased flavonoid content (Figure 1d); therefore, 70 % was established as the optimal ethanol concentration. This increase is attributed to the increase in ethanol polarity when adding water, which enhances mass transfer and, consequently, extraction efficiency (Alara et al., 2018; Vetal et al., 2014). Additionally, water, due to its dielectric constant and loss factor, absorbs microwave energy better than ethanol, which favors cell expansion of plant material and the release of phytocompounds into the extraction solvent (Sonar & Rathod, 2020).
In summary, the optimal extraction parameters were: 10 min of irradiation, temperature of 45 °C, mass:volume ratio of 1:25 g·mL-1 and an ethanol concentration of 70 %. These conditions yielded a TFC of 15.34 ± 0.03 mgQE·g-1 ds and an extraction yield of 21.33 %. Finally, the extract obtained under these conditions was characterized.
Chemical characterization
The chemical characterization of the extract showed a concentration of 8.24 ± 0.35 mgGAE·g-1 ds of hydrolyzable polyphenols, 15.34 ± 0.03 mgQE·g-1 ds of total flavonoids and an antioxidant activity with a free radical scavenging capacity of 72.4 88 ± 0.90 % in the DPPH• assay and of 86.20 ± 0.09 % with ABTS+•, compared to the trolox control, which presented a free radical scavenging of 85.30 ± 0.38 and 100 ± 0.00 % with DPPH• and ABTS+•, respectively.
The results obtained were superior to those reported by Morreeuw et al. (2021a), Morreeuw et al. (2021c) and Quiroz-Guzmán et al. (2023) in A. lechuguilla guishe extracts obtained by enzymatic, ultrasound, supercritical fluid and Soxhlet extraction, as well as in hydrolyzed and fractionated extracts from A. lechuguilla leaves reported by Anguiano-Sevilla et al. (2018). The higher flavonoid content is attributed to the use of microwaves, which allows significantly improving the extraction efficiency by providing high yields in less time. This is due to the heating of the mixture, which causes the expansion of the plant cells and the rupture of their walls, facilitating the release of the active components (Sonar & Rathod, 2020). However, the values obtained were lower than the results reported by Anguiano-Sevilla et al. (2018) in A. lechuguilla leaf extracts using ultrasound, possibly due to variations in the sampling location and time, environmental factors that can affect the content of bioactive compounds in plants (Morreeuw et al., 2021a).
The antioxidant activity by DPPH• is similar to that reported by Carmona et al. (2017) in ethanolic extracts of buds (central part of the plant) of A. lechuguilla, and higher than that obtained by Morreeuw et al. (2021c) in guishe extracts. The increase in antioxidant activity is attributed to the higher content of flavonoids in the extracts and the ability of ethanol to improve the solubility of phenolic compounds (Kaur et al., 2019) in microwave-assisted extraction (Sonar & Rathod, 2020), as it enhances the antioxidant activity due to the hydroxyl groups present in the structures of the bioactive compounds (Huang et al., 2023).
Qualitative characterization of guishe extract
Qualitative tests of the extract showed a yellow coloration upon addition of NaOH, which indicated the presence of flavanones and flavones, and an orange to red coloration upon addition of H2SO4, which also indicated the presence of flavones (Table 1). These flavonoids are known for their antioxidant, anti-inflammatory, antimicrobial and anticancer properties (Nagula & Wairkar, 2019). Morreeuw et al. (2021b) reported the presence of flavonoids by HPLC-MS/MS of A. lechuguilla guishe extracts obtained by ultrasound. In these ethanolic and methanolic extracts, they identified different flavones (such as apigenin, apigenin-7-O-glucoside and apigenin-7-O-rutinoside) and flavanones (such as naringenin, naringenin-7-O-rutinoside, naringenin O-rutinoside, hesperidin and hesperidin methylchalcone).
Table 1 Qualitative characterization of Agave lechuguilla guishe extract.
| Test | Observation | Result |
|---|---|---|
| Sodium hydroxide | Yellow coloration | + |
| Sulfuric acid | Orange coloration | + |
FTIR analysis
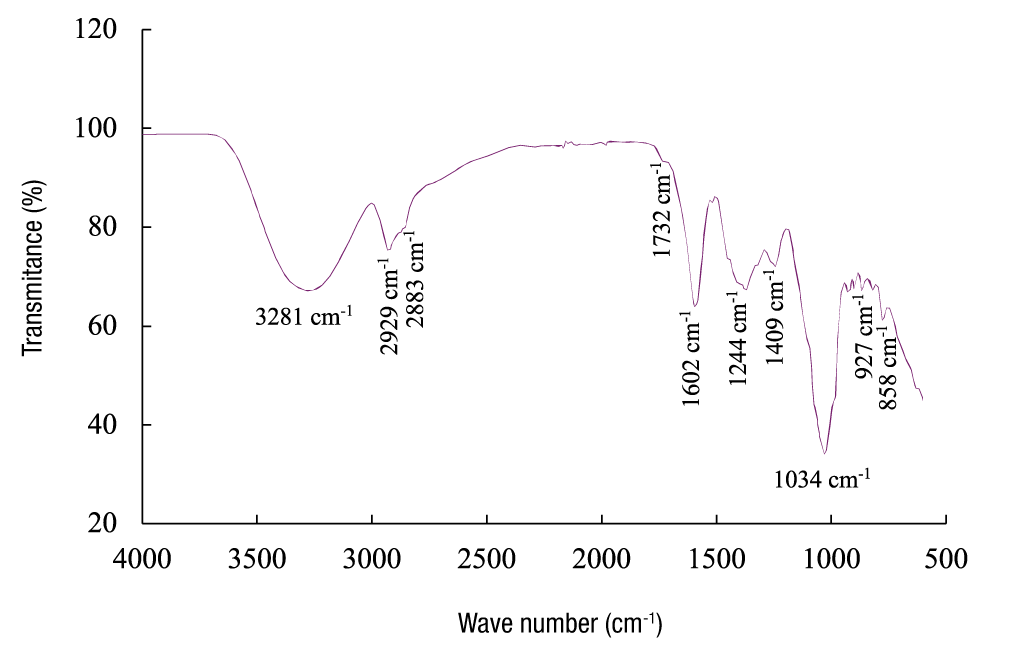
The FTIR spectrum of the guishe extract showed several characteristic bands (Figure 2). The band at 3 281 cm-1 (Figure 2) is associated with O-H stretching, while the bands at 2 929 and 2 883 cm-1 correspond to C-H stretching. The band at 1 732 cm-1 is probably related to the vibration of carboxyl groups (C=O stretching) present in flavonoids. A band at 1 602 cm-1 attributed to the stretching of C=C groups, related to deformations of aromatic rings, was also observed. The band at 1 409 cm-1 is related to CH3, CH2 groups, flavonoids and aromatic rings, and reflects the C-H bending vibration (δ) and stretching of aromatic rings. The band at 1 244 cm-1 is attributed to the vibration of the C-O group of hydroxyflavonoids. The band at 1 034 cm-1 is related to secondary alcohols and C-O stretching of ester groups. Finally, bands at 927 and 858 cm-1 were found, corresponding to out-of-plane C-H bending vibrations, indicating substitutions in the aromatic ring (Carlota-da Silva et al., 2020; Krysa et al., 2022; Nunes et al., 2016).
Conclusions
Microwave-assisted extraction is a viable option for obtaining flavonoids from Agave lechuguilla guishe, as it achieves a higher concentration (15.34 ± 0.03 mgQE·g-1 ds) than that reported with other techniques. The optimal conditions for extraction were irradiation time of 10 min, temperature of 45 °C, mass:volume ratio of 1:25 g·mL-1 and ethanol concentration of 70 %. The parameters studied showed their effect on flavonoid extraction efficiency; therefore, future research should focus on optimizing the process to maximize extraction yield. Finally, the valorization of guishe as a source of bioactive compounds adds value to this waste and contributes to mitigating the environmental impacts resulting from its disposal.











 texto en
texto en