Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 spe 27 Texcoco ago./sep. 2022 Epub 31-Oct-2022
https://doi.org/10.29312/remexca.v13i27.3156
Articles
Influence of light on callus generation and in vitro plant culture
1Centro de Investigación en Biotecnología Aplicada-Instituto Politécnico Nacional. Ex-Hacienda San Juan Molino Carretera Estatal Tecuexcomac-Tepetitla km 1.5, Tlaxcala, México. CP. 90700. (mruizr1402@alumno.ipn.mx; ctellezv1800@alumno.ipn.mx; mmartinezn1202@alumno.ipn.mx; pverah1300@alumno.ipn.mx;).
2Centro de Ciencias Genómicas México-Campus Morelos-Universidad Nacional Autónoma de México. Av. Universidad s/n Col. Chamilpa, Cuernavaca, Morelos, México. CP. 62210. (esperanzaeriksson@yahoo.com.mx).
In vitro culture systems are important in the field of plant biotechnology. It has been observed that some factors, including light, affect tissue development under in vitro culture conditions. The objective of this work is to know the effect of light intensity on the development of tissues from seeds under in vitro culture conditions for the generation of seedlings and calluses, in order to favor the times and the regeneration of species of agronomic interest for their comprehensive use. In this work, different light intensities were tested, it was possible to obtain seedlings with calluses, demonstrating that the intensity of light influences the type and development of dedifferentiated structures and that this effect varies inter and intraspecies. By regenerating plant tissues from seedlings, it was found that apical meristem shoots are the most efficient type of explant for clonal regeneration of tobacco and tomato plants. This study is the first to present the effect of light intensity on seeds for the production of seedlings and calluses in different plant species, including a combination of different tissues and explants that could be used to obtain different plant structures for biotechnological purposes.
Keywords: callogenesis; light intensities; organogenesis
Los sistemas de cultivo in vitro son importantes en el área de la biotecnología vegetal. Se ha observado que algunos factores, incluyendo a la luz, afectan el desarrollo de los tejidos en condiciones de cultivo in vitro. El objetivo de este trabajo es conocer el efecto de la intensidad lumínica en el desarrollo de tejidos a partir de semillas bajo condiciones de cultivo in vitro para la generación de plántulas y callos, con la finalidad de favorecer los tiempos y la regeneración de especies de interés agronómico para su aprovechamiento integral. En este trabajo, se probaron diferentes intensidades lumínicas, se lograron obtener plántulas con callos, demostrando que la intensidad de la luz influye en el tipo y desarrollo de estructuras desdiferenciadas y que este efecto varía inter e intraespecie. Mediante la regeneración de tejidos vegetales de las plántulas, se encontró que los brotes de meristemos apicales son el tipo de explante más eficiente para la regeneración clonal de plantas de tabaco y tomate. Este estudio es el primero en presentar el efecto de la intensidad de la luz sobre semillas para la producción de plántulas y callos en diferentes especies vegetales, incluida una combinación de diferentes tejidos y explantes que podrían utilizarse para la obtención de diferentes estructuras vegetales con fines biotecnológicos.
Palabras clave: callogénesis; intensidades de luz; organogénesis
Introduction
The field of research for the induction of calluses and regeneration of plant species under in vitro conditions has been developed over the years due to the increase in their demand in the area of applied biotechnology. Currently, the industry has focused on in vitro micropropagation techniques for the multiplication of plants of superior elite varieties or with specific desirable characteristics on a large scale (Kumar and Reddy, 2011). In vitro plant culture represents a useful tool for the propagation and conservation of the gene pool, the obtaining of axenic plants and dedifferentiated structures, as well as for the genetic transformation of plants (Rajewski et al., 2019).
Several studies have focused on plant regeneration using leaves (Rahman et al., 2010; Khuong et al., 2013; Verma et al., 2016), epicotyls (Pal et al., 2013), hypocotyls (Le et al., 1998; Yaacob et al., 2012; Gajdošová et al., 2013; Pal et al., 2013), cotyledons (Bhatia et al., 2004), apical meristems (Scotton et al., 2013; Jamous and Abu-Qaoud, 2015), protoplasts (Ganapathi et al., 2004) and stems (Arya et al., 1993; Bennici et al., 1997; Kumar and Dube 1997). Likewise, the use of friable calluses is the first step towards the production of metabolites in vitro (Gourguillon et al., 2018), their use as bioreactors (Shibasaki et al., 1991; Ganapathi et al., 2004; Espinosa-Leal et al., 2018), among others.
As in vitro culture is a closed system, there are external factors that influence the development of plant tissues, an example of this is light; which is a signal that photoreceptors receive to regulate the growth, development, differentiation and metabolism of plants (Wang et al., 2001; Ouyang et al., 2003; Chen et al., 2020). The response is affected by molecular mechanisms that include transcription factors (Casal et al., 2004), phytochromes (Lau and Deng, 2010; Bian et al., 2015; Galvão and Fankhauser, 2015) and photoreceptors (Casal et al., 2004; Nhut et al., 2015).
The effect of light on callus induction has been studied in plants such as Brassica napus (40.5 μMol m-2 s-1) (Afshari et al., 2011), Cistanche deserticola (46.5 μmol m-2 s-1) (Ouyang et al., 2003), Cymbidium orchid (45 μmol m-2 s-1) (Huan and Tanaka 2004), Panax vietnamensis (20-25 μmol m-2 s-1) (Nhut et al., 2015) and Allium hirtifolium (40 μmol m-2 s-1) (Nasrin et al., 2017).
Light intensity and spectral quality have been shown to influence callus growth (Ouyang et al., 2003; Bian et al., 2015). However, there is little information on the effect of a high light intensity (greater than 50 μmol m-2 s-1). In addition, LED light sources have been recommended to regulate light environments in the cultivation of plants under controlled environments because they provide optimal spectral integration (Wheeler, 2008; Bian et al., 2015; Yu et al., 2020).
Therefore, it is of great interest to determine the effect of light intensity on the generation of seedlings and calluses of commercial and agricultural importance. For this reason, the objective of this study was to analyze the effect of different light intensities on seeds for the generation of calluses and seedlings in three plant species, as well as the use of seedlings to obtain explants for plant regeneration and clone formation.
Material and methods
Plant material
Amaranthus hypochondriacus variety Gabriela (donated by the Technological Institute of the Altiplano of Tlaxcala, Tlaxcala, Mexico), Amaranthus hypochondriacus variety CIBA-2 (obtained in the Center for Research in Applied Biotechnology of the National Polytechnic Institute), Nicotiana tabacum (donated by CINVESTAV, Irapuato, Mexico) and Solanum lycopersicum for commercial use (Vita® variety Lycopersicon esculentum, France) were used in this study. The seeds were sterilized by immersing them in a solution composed of 20 ml of sterile distilled water and 60 μl of detergent (Axion Tricloro®) in a 50 ml conical tube, which was stirred in a vortex for 5 min. Then, they were washed four times with 70% ethanol, interspersed with washes with sterile water. Subsequently, the seeds were stirred in a vortex for 2 min with 1% sodium hypochlorite solution and washed five times with sterile distilled water.
Media preparation
Murashige and Skoog (MS) medium (M5519, Sigma-Aldrich, Saint Louis, USA) with 30 g L-1 of sucrose (Meyer, CAT 2240, Mexico) solidified with 2 g L-1 of phytagel (Sigma-Aldrich 5519, Saint Louis, USA) was used as a basal medium. With this basal medium, the medium for the generation of calluses and seedlings (NB medium) was formulated and supplemented with 3 mg L-1 (13.2 μmol) of N6-benzyladenine (BA) and 0.2 mg L-1 (1.08 μmol) of naphthalene acetic acid (NAA). The medium used for the elongation of shoots from explants (SE medium) was formulated with the basal medium supplemented with 5 mg L-1 (5.8 μmol) of GA. The medium used for root elongation (RO medium) was the basal medium supplemented with 2 mg L-1 (5.7 μmol) of IAA and 1 ml L-1 of Nitsch solution. The pH of the medium was adjusted to 5.8 using NaOH 0.1 N and sterilized for 20 min at 120 °C and 10.8 kPa.
Culture conditions and callus induction
Thirteen seeds were placed in each magenta bottle that contained 35 ml of NB medium. The bottles were exposed to 40.5 μmol m-2 s-1 (3 000 luxes), 108 μmol m-2 s-1 (8 000 luxes) or 162 μmol m-2 s-1 (12 000 luxes), incubated at 30 ±3 °C, 40 ±3% of relative humidity and a photoperiod of 16 h light/8 h darkness. Cold LED light lamps (JWJ, Mexico City, Mexico) provided the light intensity. All treatments were performed in triplicate, each replication contained thirteen seeds or explants per culture container. The appearance of calluses in the root zone of the seedlings from 20 days after sowing in NB medium was analyzed. The weight, number and type of calluses generated in the different treatments were monitored.
The calluses generated in six bottles for each treatment were counted and the average was determined. The calluses were separated from the NB medium and rinsed with water to remove the phytagel, before being dried briefly with sterilized paper towels. Subsequently, the calluses were weighed on a semi-analytical scale (Velab, Mexico City, Mexico). Some parameters such as coloration, opacity, tissue consistency and presence of shoots were considered to evaluate the type of callus. For the consistency of the tissue, it was determined whether it was friable or compact. The morphology of the callus was analyzed using a Zeiss Stemi 508 stereoscopic microscope, with Zeiss Axiocam ERC 5s Rev 2.0 camera, this was visualized with the ZEN lite software (Zeiss, Jena, Germany).
Generation of shoots from seedling explants
Twenty days after sowing, the seedlings obtained in in vitro culture were used to obtain explants for organogenesis and clonal micropropagation assays. 5 and 10 mm segments of cotyledons with petioles and apical meristem shoots (AMS), respectively, were used. An average of 12 explants with axial polarity were placed in bottles with NB medium and exposed to a luminous intensity of 54 μmol m-2 s-1 for a photoperiod of 16 h light/8 h darkness, at 22 ±3 °C and relative humidity of 44 ±3%. After 15 days in NB medium, the generated shoots were transferred to SE medium. Once the roots were generated, the seedlings were placed in the RO medium. Subsequently, the plants were acclimatized to greenhouse conditions.
Generation, maintenance and acclimatization of plants to greenhouse conditions
Twenty days after sowing in NB medium at 108 μmol m-2 s-1, 30 ±3 °C and relative humidity of 40 ±3%, the seedlings were transferred to SE or RO medium and monitored for 40 to 50 days under in vitro conditions. The regenerated plants were placed in plastic pots or black bags that contained 1.6 L of sterile substrate LM-GPS Lambert® with 7 g L-1 of osmocote. The plants were grown in greenhouse conditions at 28 ±10 °C. They were covered with a transparent plastic bag for their acclimatization. After three days, the bags were removed. Irrigation was applied every third day until the end of their life cycle and seeds were collected for their subsequent propagation.
Statistical analysis
The experiments had a completely randomized design. Data were analyzed with Anova using the Statistical Analysis Software System (SAS) (SAS Institute, Cary, NC, USA). Differences in treatment mean were compared by the Duncan Test (GLM), a p-value less than or equal to 0.05 was used as an indicator of statistical significance.
Results and discussion
Effect of light intensity on seed germination and callus generation
The seeds in NB medium of the three species were subjected to three different light intensities (40.5, 108 and 162 μmol m-2 s-1), germination occurred after four, seven and nine days for amaranth, tomato and tobacco, respectively. The germination percentage was 100% in amaranth, 92% in tobacco and 90% in tomato. The generation of seedlings with calluses under the different light intensities was observed (Figure 1). Tomato and tobacco species generated calluses under the different light intensities. In the case of the Gabriela variety of amaranth, the generation of calluses was observed at 108 and 162 μmol m-2 s-1; however, in the CIBA-2 variety no calluses were generated (Figure 1). Overall, the size of the callus and the robustness of the seedlings were proportional to the intensity of the light (Figure 1).
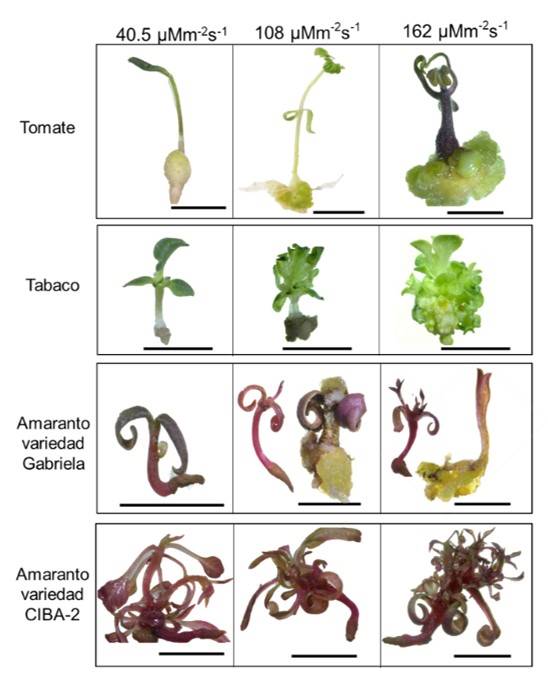
Figure 1 Effect of light intensity on the generation of calluses and seedlings for different plant species. Structures generated in NB medium after 15 days of sowing under different light intensity conditions. The scale bar corresponds to 1 cm.
In this study, tobacco exhibited the highest number of calluses with 108 μmol m-2 s-1, followed by tomato (Table 1). In amaranth, we detected different behaviors between the two varieties (Figure 1), similar to that reported with phytohormones (Bennici et al., 1997). The highest average in the weight of calluses was generated in tomato at 162 μmol m-2 s-1 (Table 1). In tomato and tobacco, the weight of the callus was proportional to the intensity of the light. Also, the calluses showed different shades in color, the calluses of tomato showed an opaque white coloration at 45 and 108 μmol m-2 s-1 and a green coloration at 162 μmol m-2 s-1. Tobacco calluses exhibited a white coloration with green shoots, and calluses of the Gabriela variety of amaranth showed a yellow-brown coloration (Figure 2a). This last finding is similar to that reported by Yaacob et al. (2012); Comia-Yebron et al. (2017); due to the presence of betacyanins (Yaacob et al., 2012; Comia-Yebron et al., 2017).
The number and weight of tomato and amaranth calluses 15 days after sowing are considered. The mean values within a column followed by the same letter are not significantly different by Duncan’s multiple range test (p≤ 0.05). a) values represent the means (±SD) of three independent experiments, six flasks were used for each treatment and the average was determined; b) the upperrcase letter represents the effect on each species at different light intensities; y c) the lowercase letter represents the effect between species at different light intensities.
Table 1 Effect of light intensity on the weight and number of calluses generated from seeds germinated in NB medium.
| Species | Calluses | Light intensity (µmol m-2 s-1) | ||
| 40.5 | 108 | 162 | ||
| Tomato | Number of calluses | 8.5 ±2 Ce | 11.8 ±0.75 Ab | 10.0 ±0 Bc |
| Weight of calluses (g) | 0.33 ±0.14 Cf | 0.72 ±0.07 Bb | 0.89 ±0.12 Aa | |
| Tobacco | Number of calluses | 1.16 ±0.75 Cg | 12.2 ±0.75 Aa | 10.0 ±0 Bc |
| Weight of calluses (g) | 0.06 ±0.02 Ch | 0.14 ±0.06 Bg | 0.41 ±0.07 Ad | |
| Amaranth variety Gabriela | Number of calluses | 0 Ch | 9.5 ±1.87 Ad | 6.66 ±1.2 Bf |
| Weight of calluses (g) | 0 Ci | 0.47 ±0.1 Ac | 0.38 ±0.04 Be | |
| Amaranth variety | Number of calluses | 0 Ah | 0 Ah | 0 Ah |
| CIBA-2 | Weight of calluses (g) | 0 Ah | 0 Ah | 0 Ah |
Friable, compact calluses and calluses with shoots were identified. The total calluses of each species were grouped to identify the percentage of the type of callus. In tomato and amaranth (Gabriela variety), the calluses of compact and friable type predominated, while in tabaco the calluses with shoots predominated (Figure 2a). In tomato, 51% were friable calluses (at 162 μmol m-2 s-1) and 47% were compact calluses (at 40.5 μmol m-2 s-1). In tobacco, 55% were calluses with shoots. For amaranth variety Gabriela, 76% of the calluses were compact at 108 μmol m-2 s-1 (Figure 2b). The percentage of calluses with shoots for the cultures of amaranth variety Gabriela and tomato was very low (1.7 and 5.3%, respectively) (Figure 2b).
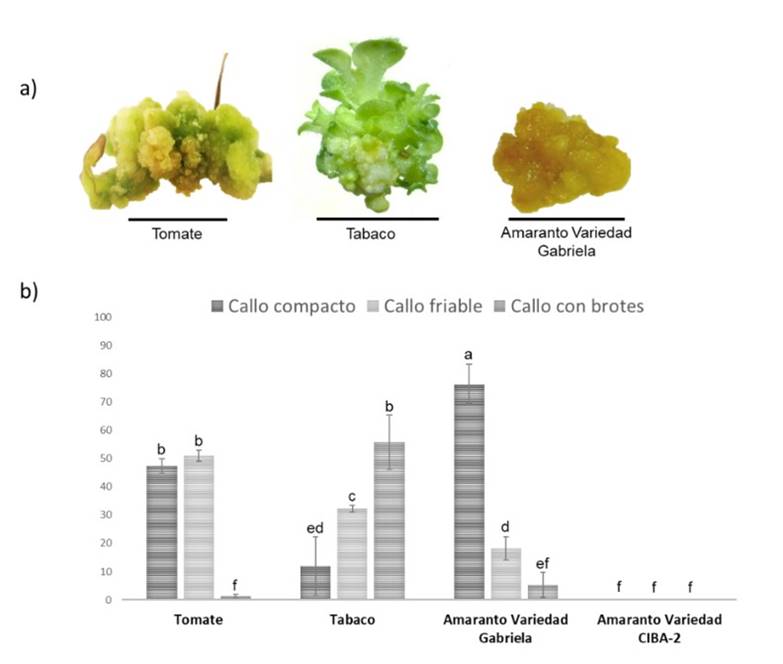
Figure 2 Calluses generated in NB medium under different light conditions for tomato, tobacco and amaranth. a) type of callus generated from tomato, tobacco and amaranth; and b) percentage of types of calluses generated. The scale bar corresponds to 1 cm.
The appearance of calluses began to be observed in all species 15 days after sowing (Figure 1), decreasing the times established in other studies in amaranth, where the times are 30 to 40 days (Yaacob et al., 2012; Comia-Yebron et al., 2017), 27 days in tomato (Bhatia et al., 2004; Osman et al., 2010; Jehan and Hassanein, 2013) and 21 days in tobacco (Shibasaki et al., 1991; Ganapathi et al., 2004), which at the industrial level would represent a reduction in costs. On the other hand, a variation in the size, weight, color and type of calluses was observed between species (Figure 2, Table 1), suggesting that light intensity is crucial in the induction and development of the callus and its effect will depend on the species, which could be associated with the type and number of photoreceptors of each species (Ouyang et al., 2003; Bian et al., 2015; Nhut et al., 2015).
Organogenesis assays and clone generation
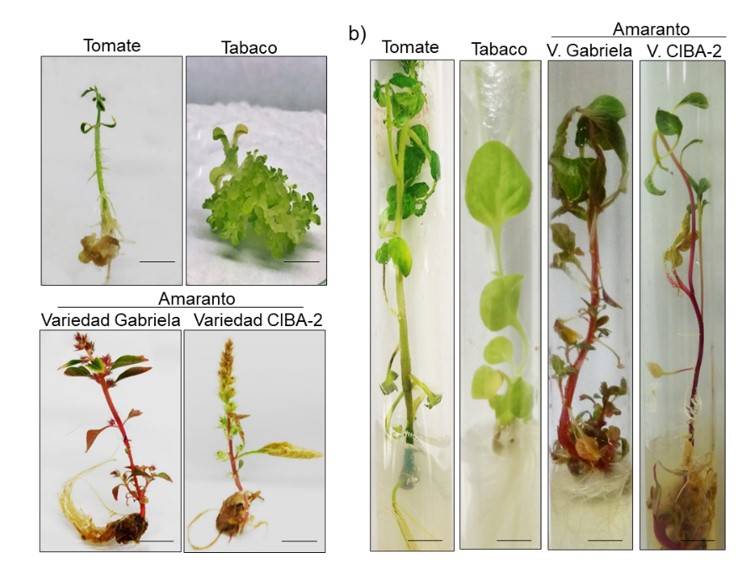
The presence of shoots with leaves was observed in the explants from nine days in NB medium (Figure 3a). AMS explants were more effective than cotyledons for plant regeneration in different species (Figure 3b). The highest percentage in regeneration from AMS explants was obtained in tobacco and tomato with approximately 80% (Figure 3b), higher than previously reported (Bhatia et al., 2004; Rahman et al., 2010; Jamous and Abu-Qaoud, 2015). Here, between 15% (CIBA-2 variety) and 30% (Gabriela variety) of AMS regeneration in amaranth was obtained, higher than previously reported in amaranth leaves and hypocotyls (Mnzava and Masam, 1985; Gajdošová et al., 2013; Pal et al., 2013). Our results suggest that AMS explants are an excellent alternative for plant regeneration and their efficiency depends on the species. In addition, a reduction in times for regeneration from explants was obtained, compared to what was reported in tomatoes and tobacco (Osman et al., 2010; Rahman et al., 2010).
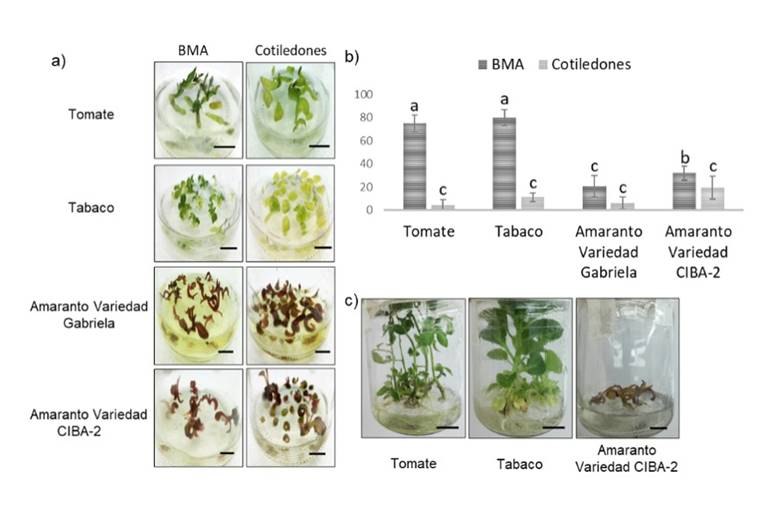
Figure 3 Regeneration of shoots and development of plants. a) regeneration of shoots of AMS explants and cotyledons in NB medium under a light intensity of 54 μmol m-2 s-1, 9 days after placement in NB medium; b) percentage of regeneration of AMS shoots and cotyledons in each species after 15 days in NB medium; and c) regeneration of AMS shoots after 15 days in RO medium. The scale bar corresponds to 1 cm.
Generation of plants in vegetative or reproductive phase under in vitro conditions
Seven days after the transfer of the clonal shoots to SE medium, the generation of roots in tobacco and tomato was observed. For amaranth, the shoots began an oxidation process, which limited the generation of roots. The seedlings that were transferred to RO medium showed true leaves and roots in tobacco and tomato (Figure 3c), achieving 100% of acclimatization in substrate (Figure 4).
Obtaining calluses, seedlings and adult plants in the same system
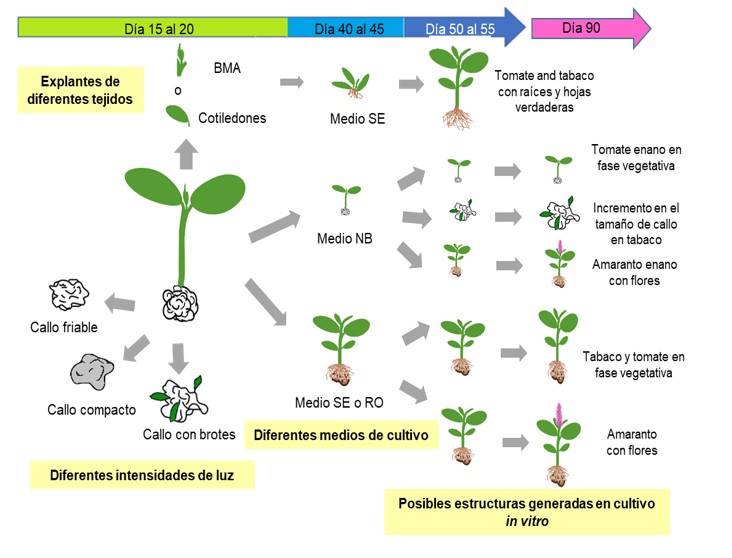
The structures generated in in vitro culture of the different species using different tissues and culture media are summarized in Figure 5. The germination of seeds in NB medium under different light intensities allowed the generation of seedlings with calluses at the same time, except for the CIBA-2 variety. The seedlings of 20 days after sowing transferred to SE or RO reached the vegetative or reproductive stage after 90 days (Figure 4b, 5). Simultaneously, seedlings with callus in NB medium at 90 days after sowing were monitored. For tobacco, it was possible to obtain calluses with shoots and friable calluses, which allowed obtaining calluses of up to four centimeters without showing signs of oxidation.
For amaranth, small plants were obtained, but they completed their life cycle, this is the first work to demonstrate that it is possible to conclude the life cycle of amaranth in an in vitro system, which leads to the production of aseptic anthers that can be used for the generation of haploid cells. In summary, this work highlights the possibility of generating calluses, seedlings or adult plants in the same system; such parts could be widely used for various biotechnological applications, for example, to obtain transgenic plants or cultures in suspension for the introduction of molecules of commercial interest, propagate tissues, obtain clones, obtain sterile anthers for genetic manipulation and even generate haploid plants.
Conclusions
This study is the first to demonstrate that light intensity affects the development and type of callus and this effect varies within and between species. The combination of species, media and light intensity allow obtaining different types of calluses, seedlings and plants in the vegetative and reproductive phases, which gives us the possibility of generating these tissues in the same in vitro culture system and that could be used for biotechnological purposes. Finally, the reduced culture time and minimal use of culture media for the generation of structures such as axenic plants or calluses, suggests that it could be proposed as a simple protocol for in vitro culture of seedlings with calluses.
Literatura citada
Afshari, R. T.; Angoshtari, R. and Kalantari, S. 2011. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics J. 4(2):6067. https://doi.org/10.3316/informit.873216546192644. [ Links ]
Arya, I. D.; Chakravarty, T. N. and Sopory, S. K. 1993. Development of secondary inflorescences and in vitro plantlets from inflorescence cultures of Amaranthus paniculatus. Plant Cell Reports. 12(5):286-288. https://doi.org/10.1007/BF00237137. [ Links ]
Bennici, A.; Grifoni, T.; Schiff, S. and Bovelli, R. 1997. Studies on callus growth and morphogenesis in several species and lines of Amaranthus. Plant Cell, Tissue and Organ Culture. 49(1):29-33. https://doi.org/10.1023/A:1005882322044. [ Links ]
Bhatia, P.; Ashwath, N.; Senaratna, T. and Midmore, D. 2004. Tissue culture studies of tomato (Lycopersicon esculentum). Plant Cell, Tissue and Organ Culture . 78(1):1-21. https://doi.org/10.1023/B:TICU.0000020430.08558.6e. [ Links ]
Bian, Z. H.; Yang, Q. C. and Liu, W. K. 2015. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review. J. Sci. Food Agric. 95(5):869-877. https://doi.org/10.1002/jsfa.6789. [ Links ]
Casal, J. J.; Fankhauser, C.; Coupland, G. and Blázquez, M. A. 2004. Signalling for developmental plasticity. Trends in Plant Sci. 9(6):309-314. https://doi.org/10.1016/j.tplants.2004.04.007. [ Links ]
Chen, L. L.; Zhang, K.;Gong, X.; Wang, H. Y.; Gao, Y. H.; Wang, X. Q.; Zeng, Z. H. and Hu, Y. G. 2020. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integrative Agric. 19(1):108-119. https://doi.org/ 10.1016/S2095-3119(19)62633-X. [ Links ]
Comia-Yebron, R.; Aspuria, E. T. and Bernardo, E. L. 2017. Callus induction in Amaranthus tricolor and Amaranthus spinosus. J.Inter. Soc. Southeast Asian Agric. Sci. 23(1):12-23. [ Links ]
Espinosa-Leal, C. A.; Puente-Garza, C. A. and García-Lara, S. 2018. In vitro plant tissue culture: means for production of biological active compounds. Planta. 248(2018):1.18. https://doi.org/10.1007/s00425-018-2910-1. [ Links ]
Gajdošová, A.; Libiaková, G.; Iliev, I. and Hricová, A. 2013. Adventitious shoots induction of Amaranthus cruentus L. in vitro. Propagation of Ornamental Plants. 13(1):33-49. [ Links ]
Galvão, V. C. and Fankhauser C. 2015. Sensing the light environment in plants: photoreceptors and early signaling steps. Current Opinion in Neurobiology. 34:46-53. https://doi.org/10.1016/j.conb.2015.01.013. [ Links ]
Ganapathi, T. R.; Suprasanna, P.; Rao, P. S. and Bapat, V. A. 2004. Tobacco (Nicotiana tabacum L.) A model system for tissue culture interventions and genetic engineering. Indian J. Biotechnol. 3(2004):171-184. [ Links ]
Gourguillon, L.; Rustenholz, C.; Lobstein, A. and Gondet, L. 2018. Callus induction and establishment of cell suspension cultures of the halophyte Armeria maritima (Mill.) willd. Sc. Hortic. 233(2018):407-411. https://doi.org/10.1016/j.scienta.2017.08.001. [ Links ]
Huan, L. and Tanaka, M. 2004. Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ. Control Biol. 42(1):57.64. https://doi.org/10.2525/ecb1963.42.57. [ Links ]
Jamous, F. and Abu-Qaoud, H. 2015. In vitro regeneration of tomato (Lycopersicon esculentum Mill). Plant Cell Biotechnol. Mol. Biol.16(3-4):181-190. [ Links ]
Jehan, S. and Hassanein, A. 2013. Hormonal requirements trigger different organogenic pathways on tomato nodal explants. Am. J.Plant Sci. 4(11):2118-2125. https://doi.org/10.4236/ajps.2013.411263 . [ Links ]
Khuong, T. T. H.; Crété, P.; Robaglia, C. and Caffarri, S. 2013. Optimisation of tomato Micro-tom regeneration and selection on glufosinate/basta and dependency of gene silencing on transgene copy number. Plant Cell Reports . 32(9):1441-1454. https://doi.org/10.1007/s00299-013-1456-8 . [ Links ]
Kumar, N. and Reddy, M. P. 2011. In vitro plant propagation: a review. J. Forest Sci. 27(2):61-72. [ Links ]
Kumar, P. and Dube, S. D. 1997. In vitro plant regeneration from shoot tips of Amaranthus hypochondriacus. Indian J. Plant Physiol. 2(2):142-144. [ Links ]
Lau, O. S. and Deng, X. W. 2010. Plant hormone signaling lightens up: integrators of light and hormones. Current Opinion in Plant Biol. 13(5):571-577. https://doi.org/10.1016/ j.pbi.2010.07.001. [ Links ]
Le B.; Do, N. T.; Jeanneau, M.; Sadik, S.; Tu, S.; Vidal, J. and Van, K. T. T. 1998. Rapid plant regeneration of a C4dicot species: Amaranthus edulis. Plant Sci. 132(1):45-54. https://doi.org/10.1016/S0168-9452(97)00262-8. [ Links ]
Mnzava, N. A and Masam, A. M. 1985. Regeneration potential, leaf and seed yield of vegetable amaranth, (Amaranthus cruentus (L.), as a function of initial topping heights. Acta Hortic.9(153):151-160. https://doi.org/10.17660/ActaHortic.1985.153.20. [ Links ]
Nasrin, F.; Jaber, P.; Alireza, M. A. and Saeideh, A. S. 2017. Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of persian shallot (Allium hirtifolium). Sci. Hortic. 218(2017):80-86. https://doi.org/10.1016/j.scienta.2016.11.056 . [ Links ]
Nhut, D. T.; Huy, N. P.; Tai, N. T.; Nam, N. B.; Luan, V. Q.; Hien, V. T.; Tung, H. T.; Vinh, B. T. and Luan, T. C. 2015. Light-emitting diodes and their potential in callus growth, plantlet development and saponin accumulation during somatic embryogenesis of Panax vietnamensis ha et grushv. Biotechnol. Biotechnol. Equip. 29(2):299-308. https://doi.org/10.1080/13102818.2014.1000210. [ Links ]
Osman, M. G.; Elhadi, E. A. and Khalafalla, M. M. 2010. Callus formation and organogenesis of tomato (Lycopersicon esculentum Mill, C. V. Omdurman) induced by thidiazuron. Afr. J. Biotechnol. 9(28):4407-4413. https://doi.org/10.5897/AJB10.1516. [ Links ]
Ouyang, J.; Wang, X.; Zhao, B. and Wang, Y. 2003. Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci. 165(3):657-661. https://doi.org/10.1016/S0168-9452(03)00255-3. [ Links ]
Pal, A.; Swain, S. S.; Das, A. B.; Mukherjee. A. K. and Chand P. K. 2013. Stable germ line transformation of a leafy vegetable crop amaranth (Amaranthus tricolor L.) mediated by Agrobacterium tumefaciens. In vitro Cell. Dev. Bio. Plant. 49(2):114-128. https://doi.org/10.1007/s11627-013-9489-9 . [ Links ]
Rahman, M. A.; Alam, M. A.; Hossain, M. R.; Hossain, A. and Afroz, R. 2010. In vitro regeneration of popular tobacco varieties of Bangladesh from leaf disc. Bangladesh J. Agric. Res.35(1):125-134. https://doi.org/10.3329/bjar.v35i1.5873. [ Links ]
Rajewski, A. C.; Elkins, K. B.; Henry, A.: Eck, J. V. and Litt, A. 2019. In vitro plant regeneration and Agrobacterium tumefaciens mediated transformation of Datura stramonium (Solanaceae). Appl. Plant Sci. 7(2):1-5. https://doi.org/10.1002/aps3.1220. [ Links ]
Scotton, D. C.; Benedito, V. A.; B de Molfetta, J. and Rodrigues, Benedita, I. F. P. 2013. Response of root explants to in vitro cultivation of marketable garlic cultivars. Hortic. Bras. 31(1):80-85. https://doi.org/10.1590/S0102-05362013000100013. [ Links ]
Shibasaki, N.; Hirose, K.; Yonemoto, T. and Tadaki, T. 1991. Suspension culture of Nicotiana tabacum cells in a rotary-drum bioreactor. J. Chem. Technol. Biotechnol. 53(4):359-363. https://doi.org/10.1002/jctb.280530407. [ Links ]
Verma, S. K.; Das, A. K.; Cingoz, G. S.; Uslu, E. and Gurel, E. 2016. Influence of nutrient media on callus induction, somatic embryogenesis and plant regeneration in selected Turkish crocus species. Biotechnology Reports. 10(2016):66-74. https://doi.org/10.1016/j.btre.2016.03.006. [ Links ]
Wang, Y.; Zhang, H.; Zhao, B. and Yuan, X. 2001. Improved growth of Artemisia annua L hairy roots and artemisinin production under red light conditions. Biotechnology Letters. 23(2001):1971-1973. https://doi.org/10.1023/A:1013786332363. [ Links ]
Wheeler, R. M. 2008. A historical background of plant lighting: an introduction to the workshop. HortSci. 43(7):1942-1943. https://doi.org/10.21273/HORTSCI.43.7.1942. [ Links ]
Yaacob, J. S.; Hwei, L. C. and Taha, R. M. 2012. Pigment analysis and tissue culture of Amaranthus cruentus L. Acta Hortic. 958(2012):171-178. https://doi.org/10.17660/ActaHortic.2012. 958.20. [ Links ]
Yu, L.: Song, C.; Sun, L.; Li, L.; Xu, Z. and Tang, C. 2020. Effects of light-emitting diodes on tissue culture plantlets and seedlings of rice (Oryza sativa L.). J. Integrative Agric. 19(7):1743-1754. https://doi.org/10.1016/S2095-3119(19)62793-0. [ Links ]
Received: January 01, 2022; Accepted: May 01, 2022











 texto en
texto en