http://zoobank.org/urn:lsid:zoobank.org:pub:9B04B65C-4508-4B57-AC5A-BDE8A3032EC2
Introduction
The family Romaleidae is a group of grasshoppers that is mainly distributed in the Neotropical region, with its species being medium to large sized and having rich wing coloration (Rehn & Grant, 1959a). Members of Romaleidae are characterized by having a hypognathous head with undivided fastigium, an immobile spine at the apex of the outer surface of posterior tibia and a highly specialized stridulatory system (Amédégnato, 1977; Dirsh, 1961). Romaleids are characterized by frequently exhibiting intraspecific variation in body coloration, which has led to the existence of a large number of species synonymies within the group (Pocco, 2013). However, some exhaustive morphological studies based on different character systems have provided valuable information for establishing species boundaries in several romaleid genera (Carbonell, 1986, 2004, 2007; Roberts & Carbonell, 1982).
The family Romaleidae takes its name from the monotypic genus RomaleaServille, 1831, which is only represented by R. microptera (Palisot de Beauvois, 1817), whose geographic distribution is restricted to southeastern USA (Hebard, 1925; Rehn & Grant, 1959a). This species has been suggested through its taxonomic history to be closely related with species of TaeniopodaStål, 1873 based on their similarity of external morphological and genitalia features and on their close geographical proximity (Mutun & Borst, 2004; Rehn & Grant, 1959a, b). Taeniopoda currently contains 12 recognized species that are distributed from southern USA to Panama, though most of its species occur in Mexico (Cigliano et al., 2021; Hebard, 1924). Members of Romalea and Taeniopoda were originally differentiated based on features of the pronotum (shape and size) and coloration (Brunner von Wattenwyl, 1893), and currently the same features are still employed to distinguish them.
Two recent molecular phylogenetic studies based on both genome wide (3RAD) and Sanger DNA sequence data, with the former also examining internal genitalia and external morphological features, consistently showed that Romalea is paraphyletic with respect to its morphologically similar genus Taeniopoda (De Jesús-Bonilla et al., 2017, 2019). Moreover, these studies also revealed that T. picticornis (Walker, 1870) and T. staliBruner, 1907 actually are conspecific, and showed the existence of an undescribed species from Guatemala that is morphologically similar to T. auricornis (Walker, 1870).
Here we carry out a taxonomic revision of Romalea, considering Taeniopoda as its junior synonym, based on examination of both external morphological and internal genitalia features and on the phylogenetic relationships obtained in the above phylogenetic studies. Based on the gathered evidence, we recognize 12 species for the genus, and formally describe a new species from Guatemala.
Material and methods
A total of 346 adult specimens originally assigned to Romalea and Taeniopoda were examined. Part of these specimens were collected by the authors along the known geographical distribution of both genera. The collected material is deposited at the Colección Nacional de Insectos (CNIN) of the Instituto de Biología, Universidad Nacional Autónoma de México (IB-UNAM). We also examined specimens deposited in the following entomological collections: Tecnológico Nacional de México-Instituto Tecnológico de Ciudad Victoria, Mexico (TecNM-ITCV); Universidad de San Carlos de Guatemala, Guatemala (USAC); Instituto Nacional de Biodiversidad, Costa Rica (INBio); Museo Nacional de Ciencias Naturales, Spain (MNCN); The Natural History Museum, United Kingdom (BMNH); and the Entomology Collection of the Academy of Natural Sciences of Drexel University, Philadelphia, USA (ANSP). We also reviewed research grade photographs of specimens assigned to Taeniopoda and Romalea from iNaturalist/GBIF (2022) and the Orthoptera Species File (Cigliano et al., 2022).
External morphological features were observed with a ZEISS Stemi DV4 stereomicroscope, and measurements were made with a digital caliper (Fig. 1A, B). Internal male genitalia were dissected by immersing the distal part of the abdomen in 10% KOH for 10 minutes and then removing it with a hook. After removal, the genitalia were kept immersed in 10% KOH for 20 minutes, muscle tissue was removed and the features of the internal genitalia were observed with a ZEISS Stemi DV4 stereo microscope (Fig. 1C, D). Digital photographs of the entire specimens were taken with a Fujifilm FinePix S1600 digital camera. Digital photographs of internal genitalia were taken with a Leica Z6 APO stereoscope. The terminology employed in this study followed Snodgrass (1935), except for the genitalia terminology, which followed Dirsh (1956).
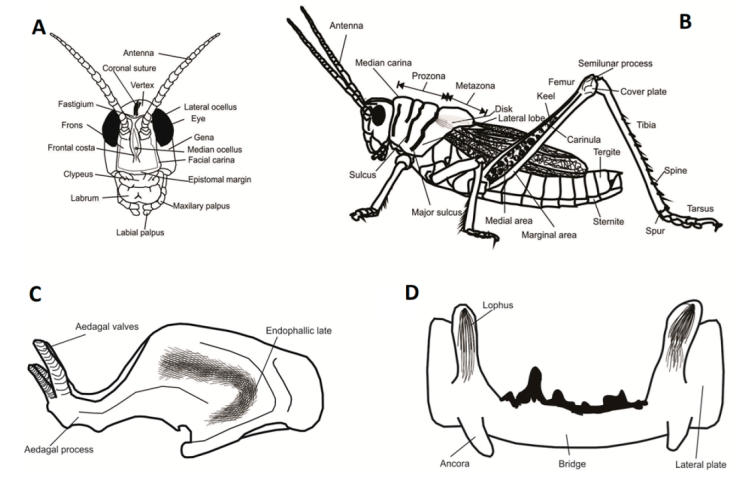
Figure 1 Morphological features of species of Romalea: A) head, B) body, C) endophallus, and D) ephiphallus.
We modelled the ecological niche of each species with our records and iNaturalist/GBIF (2022) research grade observations. We extracted data for each species from bioclimatic layers of WorldClim2 and altitude (Fick & Hijmans, 2017; Hijmans et al., 2005). We used SDMtoolbox (Brown, 2014) to retain layers without high collinearity (< 0.8). Ecological Niche Models (ENM) were reconstructed in MaxEnt 3.4.0 (Phillips et al., 2006) with 25% of subsample for training test model, 100,000 iterations and 20 replicates. ENM were projected to geographic space to generate potential distribution maps.
Results
Our taxonomic revision of the species originally assigned to Romalea and Taeniopoda allowed us to distinguish that they form a morphologically cohesive group that can be distinguished from the remaining members of Romaleidae by the following morphological features: 1) tectiform pronotum with a medial carina forming a pronotal crest, 2) lateral carinae with distinct lateral angles, 3) anterior margin of pronotum obtuse-angulate, posterior margin rectangular to acute-angulate, 4) prosternal process developed, spiniform and directed ventro-caudal, 5) head without marked rostral development, 6) first anal vein transversally dividing the tegmina into 2 sections, 7) reticulated veins and veinlets, and 8) fenestrated areas black or partially to completely bleeded by veins color. We also found morphological variation that is consistent with the species delineation revealed by the previously published molecular data, and that therefore can be employed to propose a stable phylogeny-based species classification for the group. Accordingly, we propose that Taeniopoda represents a junior synonym of Romalea, recognize 11 previously described species for the genus (see below their redescriptions), and describe a new species from Guatemala that is morphologically similar to R. auricornis.
Descriptions
Order Orthoptera Latreille, 1793 Superfamily Acridoidea MacLeay, 1821
Family Romaleidae Brunner von Wattenwyl, 1893
Subfamily Romaleinae Pictet & Saussure, 1887
Tribe Romaleini Pictet & Saussure, 1887
Genus Romalea Serville
RomaleaServille, 1831: 280; Hebard, 1925: 2; Rehn & Grant, 1959a: 252, 1961: 231; Amédégnato, 1974: 198; Eades, 2000: 204; Cigliano et al., 2021: online.
RhomaleaBurmeister, 1838: 619 (misspelling); Saussure, 1859: 392; Pictet & Saussure, 1887: 348; Kirby, 1890: 588; Kirby, 1910: 369.
TaeniopodaStål, 1873: 50; Brunner von Wattenwyl, 1893: 134; Bolívar, 1901: 264; Rehn, 1904: 530; Bruner, 1907: 231; Kirby, 1910: 371; Hebard, 1924: 253, 1925: 7; Rehn & Grant, 1959a: 252, 1961: 240; Ortega & Márquez, 1988: 327; Eades, 2000: 204; Rowell, 2013: 106; Cigliano et al., 2021: online; De Jesús-Bonilla et al., 2017: 600-617, 2019: 64, syn. n.
TeniopodaBuzzetti & Barrientos-Lozano 2011: 210 (misspelling).
Type species. Romalea microptera (Palisot de Beauvois, 1817).
Size varies from medium to large, length of body (head-tegmina): females 70.00-31.83 mm, males 57.90-32.07 mm. Head: antennae filiform with 19-22 flagellar segments; first 7-17 antennal flagellar segments pale, the rest of antennal flagellar segments black. Antennal flagellar segments 1 and 2, and/or 5 and 6 tend to be fused (Fig. 2A, B). Gena rounded, vertex globose, fastigium declivent. Lateral ridges of fastigium obtuse-angulate. Frontal costa marked, narrow and sulcate from apical frons to medial ocellus, evanescent inferiorly. Frons with marked facial carina. Eye oval shaped and prominent. Fastigium and frons punctate; vertex sparsely punctate, gena and circumocular area sparsely punctate to smooth. Pronotum: tectiform with a medial carina forming a pronotal crest, and lateral carinae with distinct lateral angles (Fig. 2C, D). Lateral lobes at least 1.3 times wider than deep, the lower margin slightly emarginated. Anterior margin obtuse-angulate; posterior margin rectangular to acute-angulate. Pronotum with 4 transverse sulci, anterior sulcus does not cut the medial carina and do not touch the bottom margin of the lateral lobe of the pronotum, the second sulcus cut the medial carina and extends up to mid-length of the lateral lobe of pronotum, third sulcus cuts the medial carina and extends more than two-thirds of the lateral lobe of pronotum, fourth and major sulcus divides pronotum in prozona and metazona. Sulci are associated to scars in lateral part of pronotum. Pronotal disc and lateral lobes cribose-punctate to rugose-punctate. Prosternal process developed, spiniform. Tegmina: reduced (e.g., Fig. 3A) to macropterous (e.g., Figs. 3B, 4A), not brachypterous. Reticulated veins and veinlets, fenestrated areas black, or partially to completely bleeded by veins color. Costal margin arcuate, anal margin straight to arcuate. Apex semi-circular. Wings: wings as long as tegmina, with central red-pink area (Figs. 5A, 6A, C, E, G). Apex slightly curved. Costal and apical margin black, posterior margin narrowly black. Disc with central red area. Hind leg: femur almost equal to the abdomen in length, rather slender. Medial area of femur with 2 series of black spots (Fig. 1B). Tibia with 2 series of 8-10 posterior spines. Male genitalia: endophallic plates large. Dorsal and ventral aedeagal valves and aedeagal process relatively short. Aedeagal valves transverse sulcated (Figs. 7- 9).
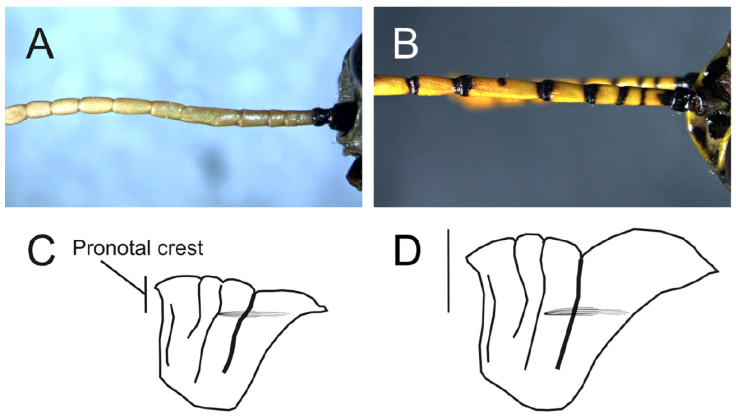
Figure 2 Morphological features of species of Romalea. Pronotum and antennae variation: Scheme representing antenna with A) antennal segments without black ring at apex, B) antennal segments with black ring at apex, C) pronotum with low pronotal crest, and D) pronotum with high elevated pronotal crest.
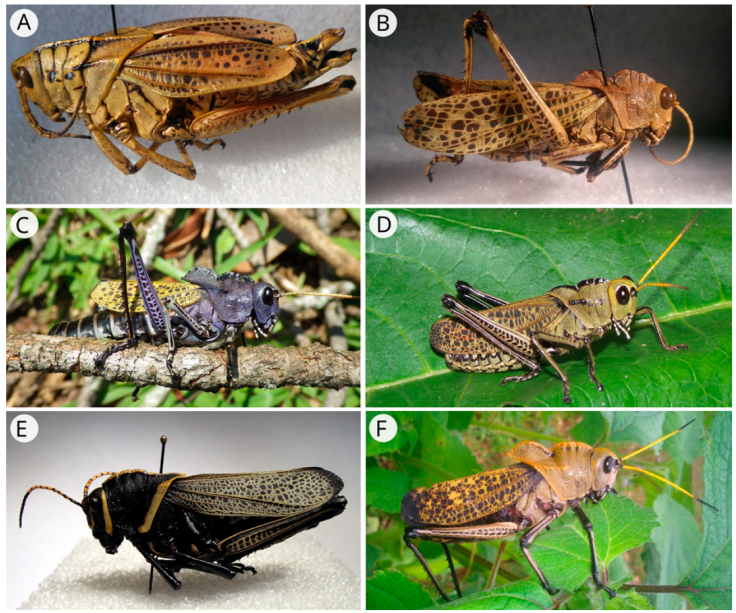
Figure 3 Lateral view of A) Romalea microptera, female; B) R. auricornis, male; C) R. centurio, female; D) R. citricornis, male; E) R. eques male, and F) R. guatemalensis, male.
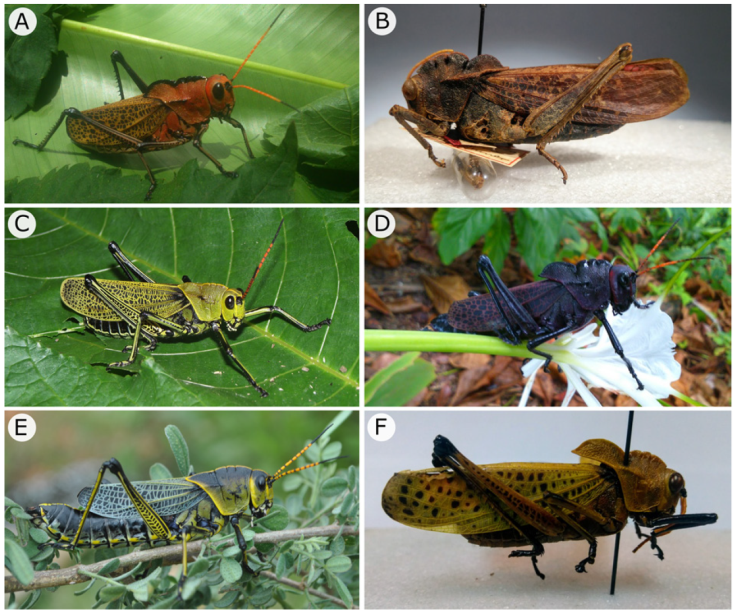
Figure 4 Lateral view of A) Romalea gutturosa, male; B) R. obscura, male; C) R. picticornis, male; D) R. reticulata, female; E) R. tamaulipensis female, and F) R. varipennis, male.
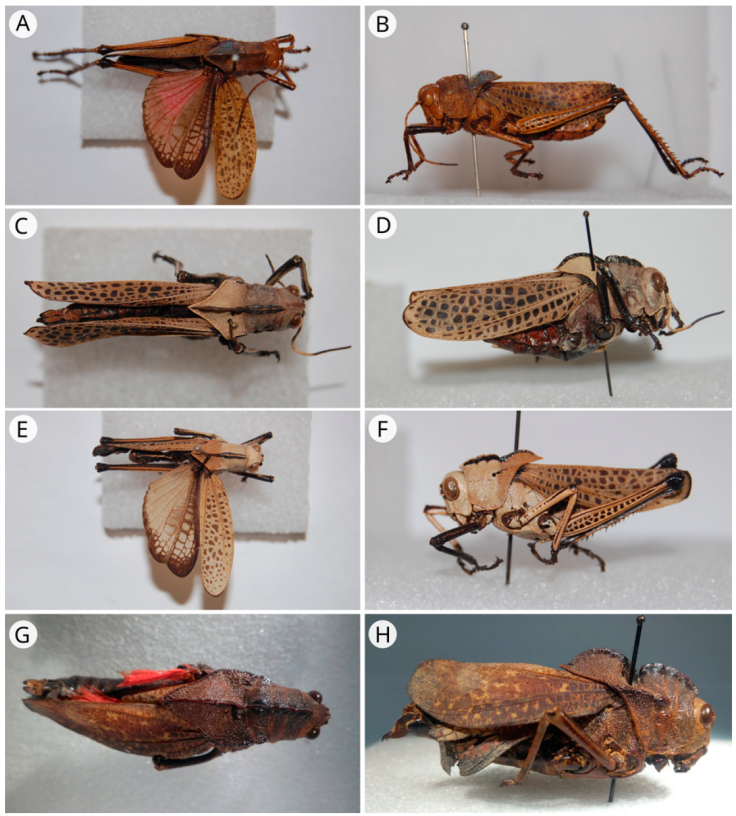
Figure 5 A) Taeniopoda pulchella (holotype, male, dorsal view); B) T. pulchella (holotype, male, lateral view); C) T. gutturosa (syntype, male, dorsal view); D) T. gutturosa (syntype, male, lateral view); E) T. gutturosa (syntype, female, dorsal view); F) T. gutturosa (syntype, female, lateral view); G) T. bicristata (syntype, female, dorsal view); H) T. bicristata (syntype, female, lateral view).
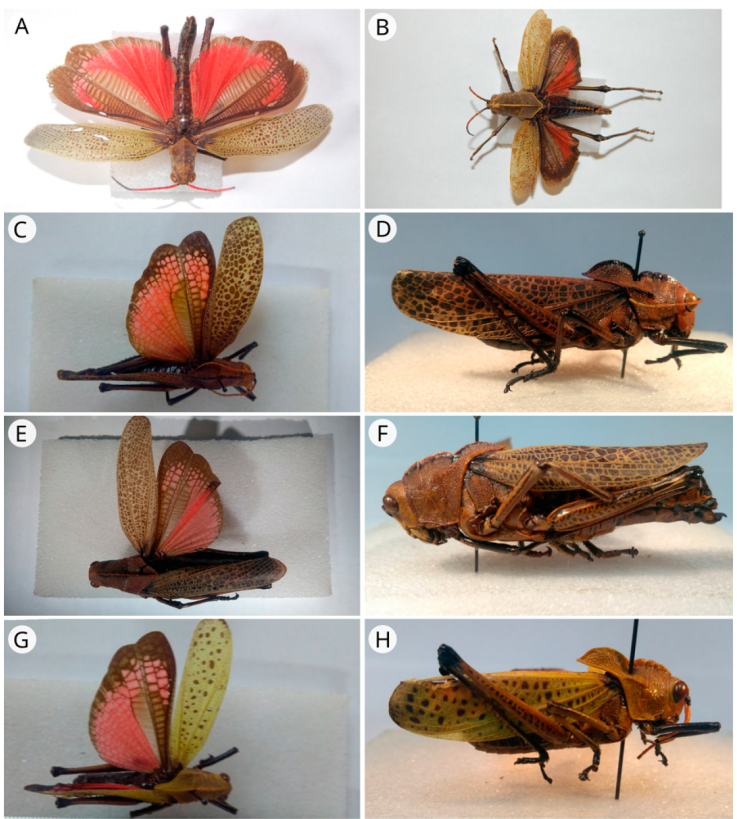
Figure 6 A) Rhomalea puncticornis (syntype, male, dorsal view); B) R. puncticornis (syntype, female, dorsal view); C) Taeniopoda maxima (syntype, male, dorsal view); D) T. maxima (syntype, male, lateral view); E) T. tamaulipensis (holotype, female, dorsal view); F) T. tamaulipensis (holotype, female, lateral view); G) T. varipennis (syntype, male, dorsal view); H) T. varipennis (syntype, male, lateral view).
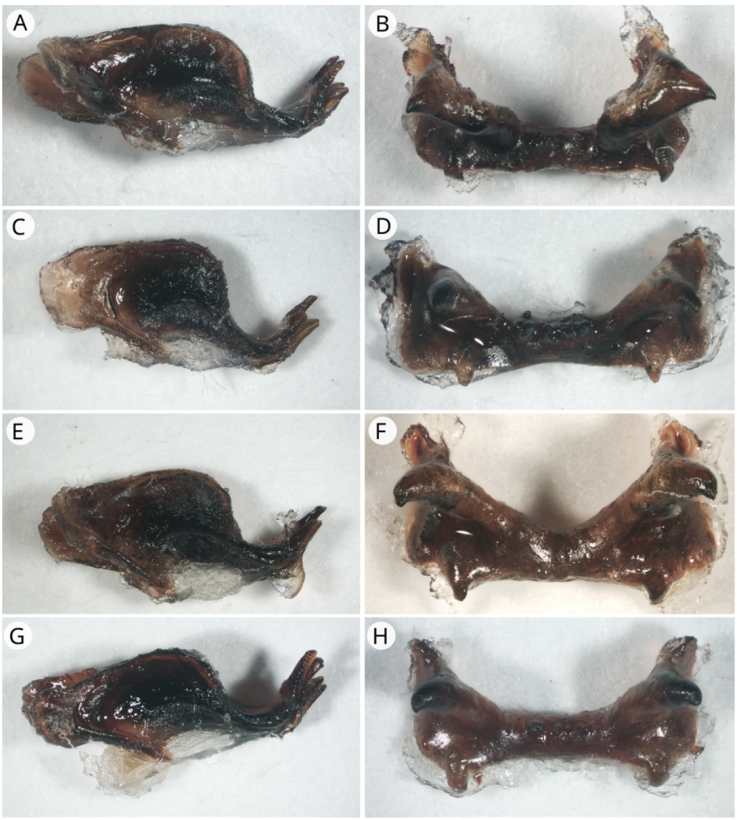
Figure 7 Endophallus (lateral view) and epiphallus (dorsal view). A) Endophallus of R. microptera; B) epiphallus of R. microptera; C) endophallus of R. auricornis; D) epiphallus of R. auricornis; E) endophallus of R. centurio; F) epiphallus of R. centurio; G) endophallus of R. citricornis; H) epiphallus of R. citricornis.
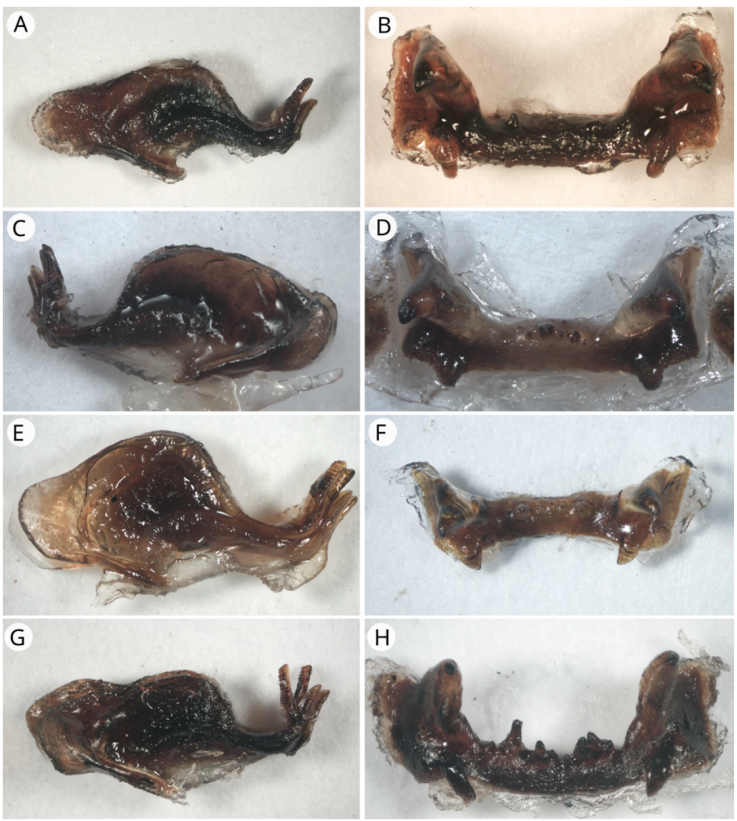
Figure 8 Endophallus (lateral view) and epiphallus (dorsal view). A) Endophallus of R. eques; B) epiphallus of R. eques; C) endophallus of R. guatemalensis; D) epiphallus of R. guatemalensis; E) endophallus of R. gutturosa; F) epiphallus of R. gutturosa; G) endophallus of R. obscura; H) epiphallus of R. obscura.
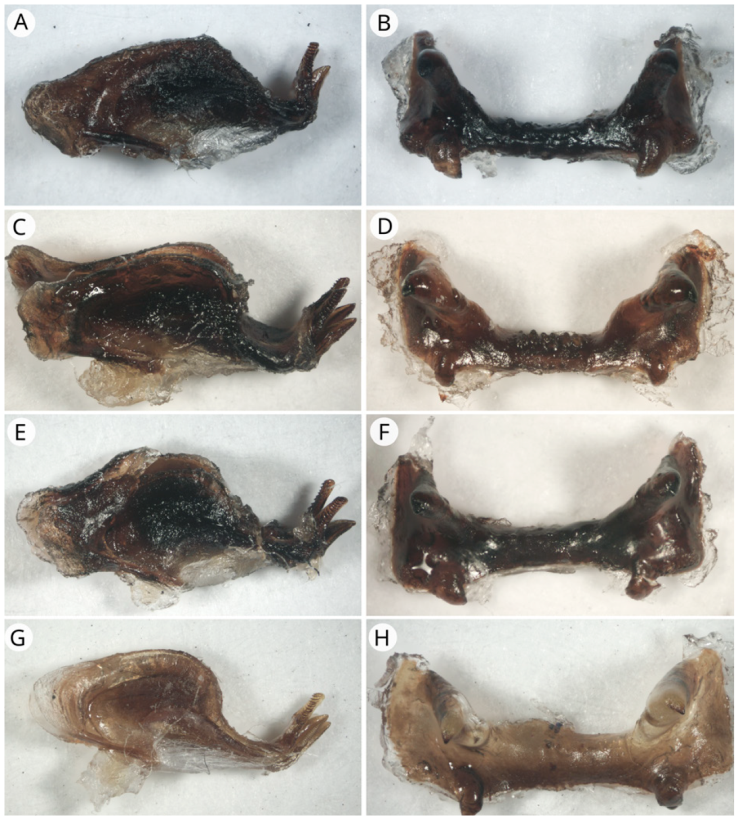
Figure 9 Endophallus (lateral view) and epiphallus (dorsal view). A) Endophallus of R. picticornis; B) epiphallus of R. picticornis; C) endophallus of R. reticulata; D) epiphallus of R. reticulata; E) endophallus of R. tamaulipensis; F) epiphallus of R. tamaulipensis; G) endophallus of R. varipennis; H) epiphallus of R. varipennis.
Diagnosis. Members of Romalea can be distinguished from the remaining Romaleini genera by having the following morphological features: 1) distinct pronotum, tectiform with a medial carina forming a pronotal crest and lateral carinae with distinct lateral angles, 2) anterior margin of pronotum obtuse-angulate, posterior margin rectangular to acute-angulate, 3) prosternal process developed, spiniform and directed ventro-caudal, 4) head without marked rostral development, and 5) first anal vein transversally dividing the tegmina into 2 sections. See Table 1 for comparison of Romalea with related genera.
Table 1 Comparative diagnostic characters of Romaleini genera that occur along the distribution area of RomaleaServille, 1831.
| Genus | Geographic distribution | Length of pronotum (mm) | Crest of pronotum | Fastigium of vertex | Body length (mm, from fastigium to end of hind femora) | Length of tegmina (mm) | References |
|---|---|---|---|---|---|---|---|
| Brachystola Scudder, 1876 | Northern America: USA and Mexico |
|
Absent or diffuse, never cut by a sulcus | Moderately prominent |
|
|
Bruner, 1904; Cigliano et al., 2022 |
| ChromacrisWalker, 1870 | Northern America: Mexico. Central America: Honduras. Southern America: Ecuador, Perú, Brazil, Costa Rica |
|
Well-defined keel in hind part of pronotum, but not elevate; transverse impressed lines very strongly marked | Conical, depressed, with 2 converging keels |
|
|
Walker, 1870; Pictet & Saussure, 1887; Cigliano et al., 2022 |
| DracotettixBruner, 1889 | Northern America: USA and Mexico |
|
Tectiform, cristate, strongly quadrilobed | The vertex of the head is broad and heavily projecting. Fastigium with rounded apex and longitudinally carinate |
|
|
Bruner, 1889; Hebard, 1931; Cigliano et al., 2022 |
| LitoscirtusBruner, 1907 | Northern America: Mexico |
|
The median carina more or less prominent, subcristate, slightly arcuate both in front and behind the principal sulcus, on the anterior portion gently trilobed | The vertex about as wide as the shortest diameter of one of the eyes in the male, in the female a little wider. The fastigium only gently depressed rather profoundly sulcate and furnished with a median longitudinal carina |
|
|
Bruner, 1904; Hebard, 1931; Lightfoot & Weissman, 1991; Cigliano et al., 2022 |
| Phrynotettix Glover, 1872 | Northern America: USA and Mexico |
|
Median carina more or less distinct but most developed on the etazoan, transverse sulci arcuate, particularly the second and third | Vertex about equally long as broad; fastigium with median carina distinct |
|
|
Rehn, 1902; Bruner, 1904; Cigliano et al., 2022 |
| RomaleaServille, 1831 | Northern America: USA; Mexico and Central America |
|
Median carina well marked along the pronotum, not very raised to well developed | Vertex globose; fastigium declivent, lateral ridges obtuse-angulate, frontal costa marked, narrow and sulcate from apical frons to medial ocellus |
|
|
This work; Serville, 1831; Stål, 1876; Cigliano et al., 2022 |
| SpaniacrisHebard, 1937 | Northern America: USA |
|
Medial carina slightly visible, never elevated, cut by 3 transversal sulcus | Vertex rounded, fastigium weakly or subobsoletely carinate, emarginated |
|
|
Hebard, 1937; Rehn & Grant, 1960; Cigliano et al., 2022 |
| Tytthotyle Scudder, 1897 | Northern America: USA |
|
Pronotum disk nearly flat, without medial carina; the 3 transverse impressed lines nearly equally plain, continuous, the last a little in advance of the middle | Vertex moderately wide, shallowly sulcate, with a rather prominent median longitudinal carina that extends from the apex across occiput to front edge of pronotum |
|
|
Strohecker et al., 1968; Bruner, 1889; Cigliano et al., 2022 |
Distribution. Species of this genus occur from southern and eastern USA to Panama in Central America (Figs. 10, 11).
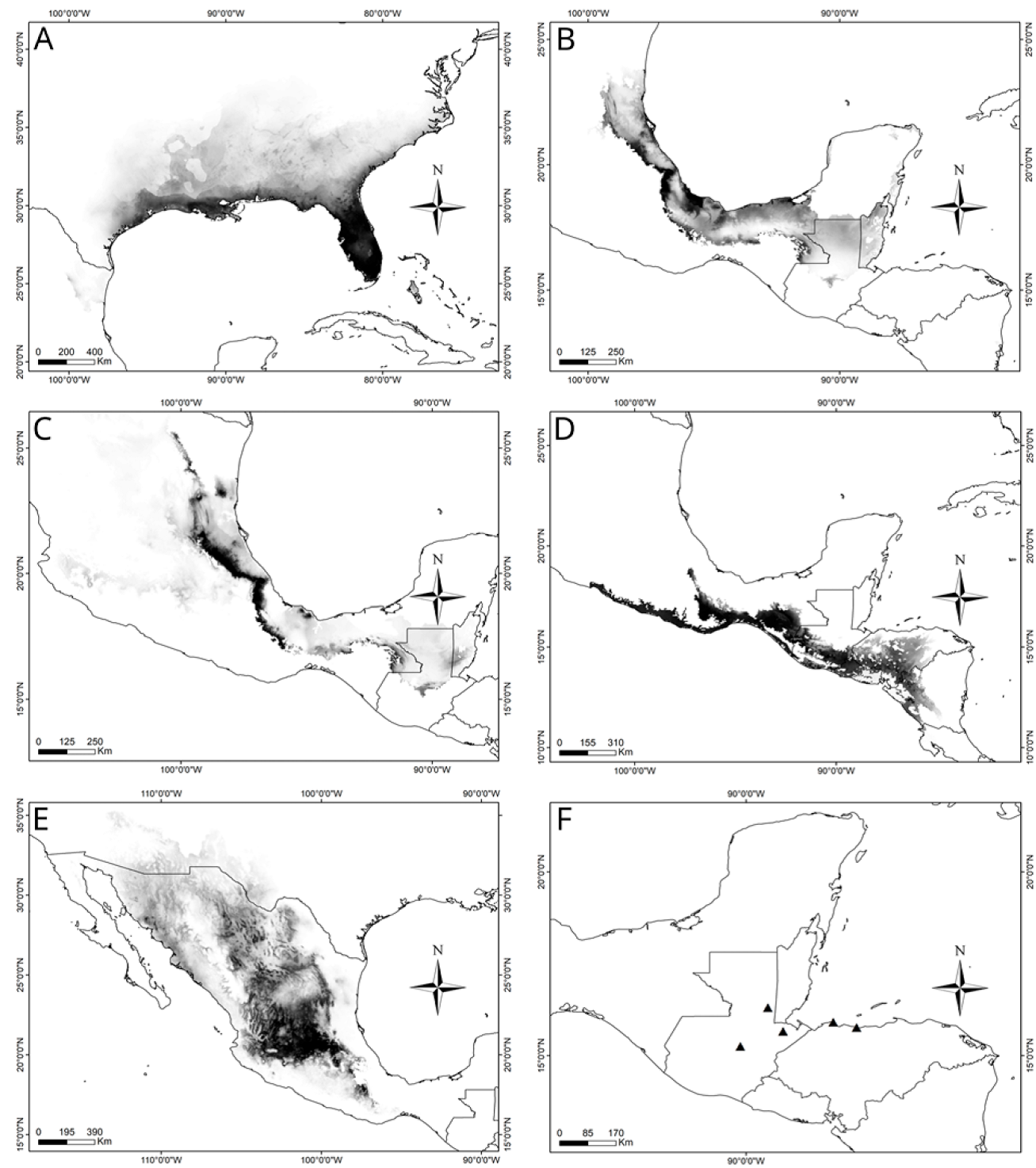
Figure 10 Potential geographic distribution maps of A) R. microptera, B) R. auricornis, C) R. centurio, D) R. citricornis, E) R. eques, and F) R. guatemalensis.
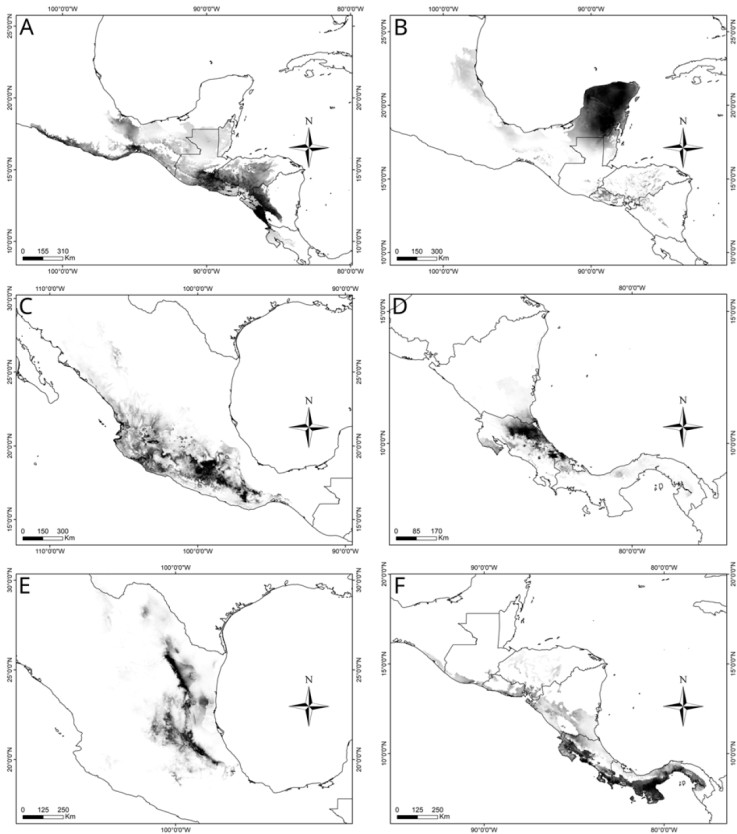
Figure 11 Potential geographic distribution maps of A) R. gutturosa, B) R. obscura, C) R. picticornis, D) R. reticulata, E) R. tamaulipensis, and F) R. varipennis.
Key to recognized species of RomaleaServille, 1831
1 With a medial carina 0.25 times or less maximum height of pronotum (Fig. 2C), forming a low elevated pronotal crest……………………………………………………………….2
- With a medial carina elevated at least 0.3 times total height of pronotum (Fig. 2D), forming an elevated pronotal crest, without black ring at distal margin of antennal flagellar segments (Fig. 2A) ……………………………………………………………….5
2 Tegmina reduced – length of pronotum……………………………………………………………….R. microptera (Palisot de Beauvois, 1817)
- Tegmina not reduced, with black ring at distal margin of antennal flagellar segments (Fig. 2B) ……………………………………………………………….3
3 Head and pronotum background color, green……………………………………………………………….4
Head and pronotum background color, black (Fig. 3E) ……………………………………………………………….R. equesBurmeister, 1838
4 Sides of medial carina and pronotal sulci black, with a slightly excavated black furrow in the dorsal part of pronotum between 3rd and 4th sulci (Fig. 4E) ……………………………………………………………….R. tamaulipensis (Rehn, 1904)
- Sides of medial carina, pronotal sulci and pronotum with slightly excavated furrow in the dorsal part of pronotum between 3rd and 4th sulci, with the same color of pronotum (Fig. 4C) ……………………………………………………………….R. picticornisWalker, 1870
5 Pronotum considerably robust, lateral carinae considerably distinct. Pronotal disc flattened, table shaped (Fig. 4B) ……………………………………………………………….R. obscura (Bruner, 1907)
- Pronotum robust, lateral carinae distinct. Pronotal disc not flattened, roof shaped……………………………………………………………….6
6 Posterior margin of pronotum slightly acute-angulate to rectangular……………………………………………………………….7
- Posterior margin of pronotum very acute-angulate, produced caudally……………………………………………………………….9
7 Pronotum and head ground color, green. Tegmina veins and veinlets green to brown-yellowish……………………………………………………………….8
- Pronotum and head ground color, dark brown to black. Tegmina veins and veinlets brown to light brown (Fig. 3C) ………………………………………………………………. R. centurio (Drury, 1773)
8 Robust. Large size (55.75-49.67 mm). With broadly apical black margin in tegmina (Fig. 3F) ………………………………………………………………. R. guatemalensis De Jesús-Bonilla, Barrientos-Lozano & Zaldívar-Riverón sp. n.
Slender. Medium size (47.57-32.07 mm). Without apical or narrow black margin in tegmina (Fig. 3B).....................................................................................................................................R. auricornisWalker, 1870
9 Medial and lateral carinae black .................................................................................................................................... 10
- Medial and lateral carinae lighter than pronotum (Fig. 4F)...................................................R. varipennis (Rehn, 1905)
10 Pronotum and head ground color purple to black (Fig. 4D)........................................... R. reticulata (Fabricius, 1781)
- Pronotum and head ground color, green or red.............................................................................................................. 11
11 Pronotum and head ground color red. Pale portion of the flagellum is red color (Fig. 4A) .................................................................................................................................. R. gutturosa (Bolívar, 1901)
- Pronotum and head ground color green. Pale portion of the flagellum is yellow to lemon-yellow (Fig. 3D) ..................................................................................................................................R. citricornis (Bruner, 1907)
Romalea microptera (Palisot de Beauvois, 1817) (Fig. 3A)
Gryllus (Locusta) guttataStoll, 1813: 23.
Dictyophorus reticulatusThunberg, 1815: 259.
Acridium micropterumPalisot de Beauvois, 1817: 146; Scudder, 1901: 9.
Romalea microptera; Serville 1831: 280; Rehn & Hebard, 1914: 256; Hebard, 1916: 19; Roberts, 1941: 239 Rehn & Grant, 1959a: 249; Rehn & Grant, 1961: 233; Dirsh, 1961: 394; Kevan, 1980: 139; Jones, 1981; Helms et al., 2003: 135-140; Capinera et al., 2004: 149; Mefferd et al., 2005: 31-32; Stauffer & Whitman, 2007: 103-114; Capinera, 2008: 1268-1270; Schowalter, 2018: 1-17.
Rhomalea giganteaBurmeister, 1838: 619.
Romalea marci M. A. Serville, 1838: 623.
Rhomalea microptera;Charpentier, 1845: Tab 49: 49 (misspelling); Kirby, 1910: 369.
Dictyophorus micropterus;Pictet & Saussure, 1887: 347; Bruner, 1907: 230.
Romalea gloveriKirby, 1910: 370. Size (head-tegmina): females 70-50 mm, males 55-43 mm. Head: scape and pedicel pale yellow to orange; first 7-9 antennal flagellar segments orange to yellow with black dorsal coloration, remaining flagellar segments black. Fastigium slightly declivent (Fig. 3A). Vertex and fastigium orange or green-yellowish, orange or black; coronal suture yellow to orange. Frons green-olivaceous to orange or black; facial carina and epistomal margin yellow to orange, lighter than frons; vertex and gena usually with black spots. Pronotum: pronotum with a medial carina 0.25 times or less its maximum height. Medial carina transversely furrowed in prozona, slightly rugose in metazona. Sides of carina and transverse sulci broadly black. Pronotal disc and lateral lobes green-olivaceous, orange-yellowish, orange or black. Medial carina and posterior margins of pronotum yellow to orange, lighter than pronotum. Dorsum of prozona with 1 to 5 slightly excavated black dots between the 3rd and 4th sulci. Posterior margin rectangulate. Tegmina: tegmina reduced to 3/4 of the abdomen, with red to pink coloration on medial portion. Veins and veinlets yellowish green, yellow, orange or black. Abdomen: color of tergites variable in combinations green, grey, yellow or black, with yellow to orange dorsomedial line. Sternites color variable as tergites, with anterior black margin, and yellow to orange caudal margin. Hind leg: medial area of femur yellow to orange, in melanistic specimens black. Dorsal, ventral and external lateral carinae yellow to orange, ventral carinae paler than dorsal carinae. External dorsolateral face black, with the same color of dorsal carina or discontinuously black; external ventrolateral face with a series of black dots. Semilunar process black, cover plate yellow to orange, insertion of extensor tibia muscle paler than rest of femur. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus lobiform shaped with right or interior orientation. Epiphallus bridge sculpture developed. Margin of lateral plate of epiphallus sub-angular (Fig. 7A, B).
Diagnosis. This species has a wide variation in color, but generally has yellow to orange ground color forms. Melanistic variations are almost entirely black. It can be distinguished from the remaining species of Romalea by the following combination of characters: (1) low pronotal crest, (2) tegmina and wing reduced to 3/4 of abdomen length, (3) tegmina with a red to pink central area, (4) dorsum of prozona with 1 to 5 dorsal slightly excavated black dots between the 3rd and 4th sulci, and (5) vertex slightly declivent.
Distribution. This species is distributed along the southeast part of the USA, from Texas to Florida and northwards to Tennessee (Fig. 10A).
Material examined: USA. 1 ♀; Florida: Collier Co, Big Cypress Bend; 25°56’32.6” N, 81°28’10.9” W; 5 Aug 2009; leg. Jensen, Mugleston 1 ♂; Florida, Collier Co, Port of the Islands Marina; 25°57’25.6” N, 81°30’47.5” W; 7 Aug 2009; leg. Jensen, Mugleston.
Remarks
This is the best-known species of the genus, with numerous published studies on its morphology, physiology and ecology. This species has a considerable color variation, and it is usual that the western melanistic specimens are confused with R. eques, though they can be easily distinguished from each other by the reduced wings of R. microptera.
Romalea auricornis (Walker, 1870), comb. rev. (Fig. 3B)
Rhomalea auricornisWalker, 1870: 538 (misspelling).
Taeniopoda pulchellaBolívar, 1901: 269; Bruner, 1907: 237; Kirby, 1910: 72; Hebard, 1924: 263; Descamps, 1975: 51.
Taeniopoda auricornis;Rehn, 1904: 532; Bruner, 1907: 237; Kirby, 1910: 372; Hebard, 1924: 261, 1932: 270; COPR, 1982: 114; Descamps, 1975: 51; Fontana et al. ,2008: 224-225; Barrientos-Lozano et al., 2013: 321-322; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Size (head-tegmina): females 47.57-31.83 mm, males 38.96-32.07 mm. Head: scape and pedicel grey to pale yellow; first 13-17 antennal flagellar segments yellow, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex, fastigium and frons dark green to yellowish green; coronal suture, facial carina and epistomal margin green to yellowish green. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes green to dark green. Medial carina rugose or slightly rugose. Lateral carinae slightly prominent and rugose. Pronotal crest and lateral carinae same color of pronotum or slightly darker. Sides of pronotal crest and lateral carinae with the same color of pronotum or slightly darker. Pronotal disc and lateral lobes green to dark green. Posterior margin slightly acute-angulate. Tegmina: veins and veinlets green to yellowish green. Abdomen: tergites grey and black, with green yellowish dorsomedial line. Sternites grey or with the same color of tergites. Hind leg: medial area of femur green to yellowish green. Dorsal, ventral and external lateral carinae with the same color of medial area. External dorsolateral face with same color of dorsal carina or finely black; external ventrolateral face with the same color of external ventrolateral carina and with a series of black dots. Semilunar process black, cover plate and insertion of extensor tibia muscle black or paler green. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus angular or lobiform shaped with right or interior orientation. Epiphallus bridge sculpture strongly developed. Margin of lateral plate of epiphallus sub-angular (Fig. 7C, D).
Diagnosis. This species is similar to R. centurio, but it can be recognized by having: (1) elevated pronotal crest, (2) green to dark green ground color, and (3) veins and veinlets green to yellowish green.
Distribution. Mexican states of Hidalgo, Veracruz, and the southern portion of Tamaulipas. (Fig. 10B).
Remarks
Romalea centurio and R. auricornis are morphologically similar; however, they can be distinguished from the latter species by the above diagnosis features and its disjunct distribution.
Material examined: holotype: Mexico. ♂; “Taeniopoda pulchella”: Valcárcel; MNCN_ent 147350 Tipos 7479 (possible holotype) (Fig. 5A, B). Other material: Mexico. 2 ♀♀, 2 ♂♂; Veracruz, Fortín, Hotel; 18.907188°N, 97.011801°W; alt.1,057 m; leg. M. García-París, N. Percino, 2 ♀♀, 1 ♂; Veracruz, Barranca de San Miguel; 18°53’05.0” N, 97°00’07.0” W; 10 Nov 2011; leg. M. García-París, N. Percino, 3 ♀♀, 3 ♂♂; Veracruz, Ixhuatlan del Café; 19°4’56” N, 97°1’47” W; alt. 1,507 m; 6 Oct 2013; leg. A. Zaldívar-Riverón, 1 ♀; Veracruz, Santiago Tuxtla; 18°27’48.5” N 95°17’21.9” W; alt. 312 m; 3 Oct 2014, 1 ♀, 1 ♂; Veracruz, Teocelo; 19°23’04.3” N, 96°58’51.6” W; alt. 1,176 m; 26 Oct 2014; leg. E. Recuero, A. Soto, 1 ♀, 1 ♂; Veracruz, Xico, Cascada de Texolo; 19°24’22.7” N, 96°59’30.8” W; alt. 1,182 m; 13 Sep 2013, 3 ♀♀; Veracruz, Santa Lucrecia; Oct 1922.
Romalea centurio Drury 1773, comb. rev. (Fig. 3C)
Romalea centurio Drury, 1773, 78, comb. rev.
Monachidium superbum (Stål, 1855: 352).
Rhomalea centurio;Saussure, 1859: 392.
Taeniopoda superba (Stål, 1873: 50).
Taeniopoda centurio;Pictet & Saussure, 1887: 348; Bolívar, 1901; 268; Rehn, 1903: 12; Bruner, 1907: 236; Kirby, 1910: 372; Hebard, 1924: 265; Barrientos-Lozano et al., 2013: 322-323; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Taeniopoda reticularisBolívar, 1901: 269
Size (head-tegmina): females 52.00-37.70, mm, males 44.56-33.17 mm. Head: scape and pedicel grey to dark yellow; first 14-15 antennal flagellar segments pale yellow, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex, fastigium, and frons brown to dark brown; coronal suture, facial carina, and epistomal margin same color of frons or slightly lighter. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes dark brown to black. Medial carina sulcate-rugose at the prozona, rugose at metazona. Lateral carinae rugose. Pronotal crest and lateral carinae dark brown to black, darker than pronotum. Sides of pronotal crest, and lateral carinae dark brown to black. Posterior margin slightly acute-angulate. Tegmina: veins and veinlets brown to light brown. Apical margin narrowly dark brown to black. Abdomen: tergites brown and black, with light brown dorsomedial line. Sternites dark brown to black. Hind leg: medial area of femur brown to light brown. Dorsal, ventral, and external lateral carinae with the same color of medial area. External dorsolateral face the same color of dorsal carina; external ventrolateral face with the same color of ventral carina or with a series of black dots. Semilunar process black, cover plate and insertion of extensor tibia muscle same color of upper marginal area or black. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus lobiform shaped with interior, right or exterior orientation. Epiphallus bridge sculpture strongly or slightly developed. Margin of lateral plate of epiphallus sub-angular or rounded (Fig. 7E, F).
Diagnosis. This species is similar to R. auricornis, but it can be distinguished by having: 1) elevated pronotal crest, 2) head and pronotum with a dark brown to black ground color, and 3) the tegmina black and yellowish brown with an apical black margin.
Distribution. Southern Sierra Madre Oriental in the states of San Luis Potosí, Querétaro, Hidalgo and Puebla, Mexico, to Nicaragua in Central America (Fig. 10C).
Material examined: Mexico. 1 ♀1 ♂; Hidalgo, S. de Molango; 20°44’10.9” N, 98°43’02.3” W; alt. 1,426 masl; 11 Sep 2012; leg. M. García-París, N. Percino, 1 ♀, 2 ♂♂; Puebla, Cuetzalan; 20°01’00.6” N, 97°31’37.6” W; alt. 983 m; 11 Oct 2013, 5 ♀♀, 2 ♂♂; Querétaro, Jalpan de Serra, Valle Verde, el Pilón; 21°30’10.8” N, 99°10’13.8” W; alt.1,152 m; 16 Aug 2014; leg. D. Dubovikoff, A. Zaldívar-Riverón, 2 ♀♀, 3 ♂♂; San Luis Potosí, Xilitla, Afuera de cueva de potrerillos; 21°18’26.0” N, 99°03’58.4” W; alt. 1,176 m; 25 Oct 2013; leg. O. Franke, C. Santibañes, J. Crúz, A. Guzmán, 1 ♀; San Luis Potosí, Xilitla, Las pozas de Edward James; 21° 23’45.6” N, 98°59’47.8” W; alt. 621 m; 26 Oct 2013; leg. L. Barrientos-Lozano.
Remarks
Some populations assigned to this species have a ground color violet, almost like R. reticulata. However, R. centurio can be distinguished from the latter species by having the veins and veinlets brown to light brown (red brown to purple in R. reticulata).
Romalea citricornis (Bruner 1907), comb. n. (Fig. 3D)
Taeniopoda citricornisBruner, 1907: 234, Kirby, 1910: 371; Hebard, 1924: 270, 1932: 270; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Size (head-tegmina): females 47.90-39.07 mm, males 47.04-36.68 mm Head: scape and pedicel black; first 13-15 antennal flagellar segments yellow to lemon-yellow, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex, fastigium, and frons green or lemon green; coronal suture, facial carina, and epistomal margin same color of frons or slightly lighter. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes olivaceous. Medial carina rugose or slightly rugose. Lateral carinae rugose. Pronotal crest, sides of pronotal crest, and lateral carinae black. Posterior margin acute-angulate. Tegmina: veins and veinlets brown or green. Abdomen: tergites brown and green, with brown yellowish dorsomedial line. Sternites brown to light green. Hind leg: medial area of femur brownish to light green. Dorsal, ventral and external lateral carinae with the same color of medial area. External dorsolateral face with the same color of dorsal carina or black; external ventrolateral face black, or with the same color of ventral carina and with a series of black dots. Semilunar process black, cover plate and insertion of extensor tibia muscle black or with the same color of external dorsolateral face. Male genitalia: posterior margin of the endophallus plate rounded. Ancora of epiphallus angular or lobiform shaped with right or exterior orientation. Epiphallus bridge sculpture strongly or slightly developed. Margin of lateral plate of epiphallus rounded (Fig. 7G, H).
Diagnosis. Romalea citricornis can be distinguished from the remaining species of the genus by having the following combination of characters: (1) elevated pronotal crest, (2) green ground color, (3) pronotal crest and lateral carinae black, (4) yellow or lemon-yellow pale portion of antenna, and (5) posterior margin acute-angulate.
Distribution. Southern Mexico to Guatemala (Fig. 10D).
Material examined: Mexico. 8 ♀♀, 7 ♂♂; Oaxaca, Municipio Asunción Ixtaltepec, Santiago Ixtaltepec; 16°41’18” N, 94°54’17” W, alt. 213 m; Oct 2014; leg. A. Villaluz, 1 ♂; Oaxaca, Municipio Asunción Ixtaltepec, Santiago Ixtaltepec; 16°41’18” N, 94°54’17” W; alt. 213 m; 22 Dic 2012; leg. A. Zaldívar Riverón, V. Salinas Ramos, 3 ♀♀, 4 ♂♂; Chiapas, Tuxtla Gutierrez, Copoya; 16°42’21.9” N, 93°07’02.3” W; alt. 1,273 m; 13 Sep 2014; leg. A. Zadívar-Riverón, D. Dubovikoff, V. S. De Jesús-Bonilla et al., 1 ♀, 1 ♂; Chiapas, Tierra y Libertad; 16°22’08.1” N, 93°52’06.5” W; alt. 687 m; Sep 2014. Guatemala. 1 ♂; Jutiapa; Asunción Mita; 8 Oct 1993; leg. X. Leiva, 1 ♂; Guatemala; Guatemala; 12 Oct 1985; leg. M. Zepeda, 2 ♀♀; Santa Rosa, Estanzuela de Ixhuatán; 23 Sep 2000; leg. Carlos Avila Ramos, 1 ♂; Santa Rosa, Barberena, El Naranjito; 30 Oct 1990; leg. C. MacVean, 1 ♀; Quetzaltenango, Finca Lozano; 1 Jul 1991; leg. E. Lozano, 1 ♂; Jalapa, Mataquescuintla, Finca Buena Vista; 4 Dic 1996; leg. D. Rodríguez, 1 ♂; Jalapa, Mataquescuintla; 15 Sep 1991; leg. Claudia Maza, 1 ♂; Guatemala, Puerta parada; 19 Jul 1984; leg. J. Schuster, 1 ♂; Escuintla, Palín; 16 Oct 1999; leg. R. Juárez, 1 ♂; Coatepeque, Finca Santa Gertrudis; 21 May 1983; leg. Hazard.
Remarks
The validity of this species was previously uncertain. The original figure of Bruner (1907) was of poor quality and probably was based on a specimen with a low pronotal crest. Hebard (1932) records that the pronotal crest in this species is high, resembling R. varipennis. Examination of specimens from southern Mexico and Central America shows that it has a high pronotal ridge.
Romalea eques (Burmeister, 1838), comb. rev. (Fig. 3E)
Rhomalea equesBurmeister 1838: 620; Saussure 1859: 392.
Taeniopoda burmeisteriBolívar, 1901: 266.
Taeniopoda eques;Kirby, 1910: 372; Hebard, 1924: 256; Hebard, 1925: 7; Hebard ,1932: 270; Rehn & Grant, 1959a: 253; Rehn & Grant, 1961: 243; Whitman & Loher,1984: 1-12; Richman et al., 1993: 95; Eades, 2000: 204; Capinera et al., 2004: 150; Rivera-García, 2006: 135; Stauffer & Whitman, 2007: 103-114; Fontana et al., 2008: 225-226; Whitman & Richardson, 2010: 377-380; Barrientos-Lozano et al., 2013: 323-324; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Size (head-tegmina): females 50.26-41.78 mm, males 50.97-38.24 mm. Head: scape black to green; pedicel black; first 9-11 antennal flagellar segments yellow with black ring at distal margin (sometimes segments 1-3 black), remaining antennal flagellar segments black. Fastigium strongly declivent. Vertex and fastigium black; coronal suture yellow. Frons black or occasionally yellow, facial carina and epistomal margin yellow. Gena with a distinct vertical yellow band. Pronotum: pronotum with a medial carina 0.25 times or less its maximum height. Medial carina of pronotum rugose. Pronotal disc black; lateral lobes black. Medial carina and posterior margins of pronotum yellow. Posterior margin rectangulate. Tegmina: veins and veinlets green yellowish, fenestrated areas black. Apical margin usually broadly black. Hind leg: medial area of femur yellow to green yellowish. Dorsal, ventral, and external carinae yellow to green yellowish. External lateral faces black. Semilunar process, cover plate, and insertion of extensor tibia muscle black. Abdomen: tergites black, with yellow dorsomedial line. Sternites black with yellow caudal margin. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus lobiform shaped with interior, right or exterior orientation. Epiphallus bridge strongly sculptured, slightly or absent. Margin of lateral plate of epiphallus sub-angular or rounded (Fig. 8A, B).
Diagnosis. This species can be recognized by the following combination of characters: (1) low pronotal crest, (2) general ground color black, (3) antennal flagellar segments with a black ring at the distal margin, and (4) lateral lobes and disc of the pronotum entirely black.
Distribution. Romalea eques is widely distributed along the semi-arid highlands from southern USA to the Mexican Plateau, Sierra Madre Oriental, Trans-Mexican Volcanic Belt, and the Pacific Coast of México (Fig. 10E).
Material examined: Mexico. 1 ♀,1 ♂; Aguascalientes, San José de García-Paredes; 20°08’34.70” N, 102°22’53.94” W; alt. 2,157 m; 23 Sep 2010; leg. M. García-París, N. Percino, 4 ♀♀; Coahuila, Cuatro Ciénegas, Rancho Casita, Ojo de agua; 27°08’03.9” N, 102°18’57.5” W; alt. 935 m; leg. U.O. García-Vázquez, M. Trujano-Ortega, 1 ♂; Coahuila, La angostura Saltillo; 25°21’38.48” N, 101° 1’21.44” W; alt. 1,566 m; 22 Sep 2010; leg. M. García-París, N. Percino, 1 ♂; Coahuila, Saltillo 3 km SE de La Angostura; 25.32748°N, 101.026°W; alt. 1,931 m; 21 Oct 2013; leg. U.O. García-Vázquez, M. Trujano-Ortega, 3 ♀♀, 1 ♂; Durango, Carretera Torreón; 25°31’48.5” N, 103°39’59.8”; alt. 1,355 m; 11 Aug 2015; leg. M. Trujano-Ortega, 1 ♀, 2 ♂♂; Guanajuato, Carretera Juventino Rosas, rumbo a Celaya al NE de Potrerillos; alt. 2,292 m; 19 Jun 2013; leg. E. O. Martínez-Luque, 2 ♀♀; Guanajuato, El Moro de Barajas; 20°19’03.9” N, 101°41’45.2” W; alt. 1,825 m; 17 Jul 2014; leg. E.O. Martínez-Luque, M. Canchola, J.J. Castro-Sánchez, 1 ♀; Guerrero, Tierra Colorada; 17°09’24.8” N, 99°32’27.0” W; alt. 620 m; Jul 2012; leg. E.O. Martínez-Luque, 1 ♀; Hidalgo, RMO Xhita; 20°38’04.1” N, 99°19’35.2” W; alt. 2,048 m; 10 Oct 2009; leg. M. García-París, N. Percino, 1 ♀, 1 ♂; Hidalgo, Tula de Allende, carretera Tula-Sn. Fco. Bojay; 20°04’43.08” N, 99°21’9.8” W; alt. 2,196 m; 24 Sep 2014; leg. A. Ramírez-Ponce, 1 ♀; Hidalgo, El Tablón; 11 Oct 2009; leg. M. García-París, N. Percino, 1 ♀, 2 ♂♂; Michoacán, Emiliano Zapata, Cerro grande cara sur; 20°00’25.5” N, 102°35’48.8” W; alt. 1,669 m; 7 Aug 2014, 1 ♀; Querétaro, Cadereyta, Bellavista del Río; 20°41’06.1” N, 99°34’37.3” W; alt.1,945 m; 11 Oct 2009; leg. M. García-París, N. Percino, 1 ♀; Querétaro, Cadereyta; 20°42’09.6” N, 99°47’00.8” W; alt. 2,072 m; 11 Oct 2009; leg. M. García-París, N. Percino”, 1 ♂; San Luis Potosí, Xilitla, Las pozas de Edward James; 21°23’44.5” N, 98°59’31.6” W; 17 Jul 2015; leg. O. Pérez-Flores, 3 ♀♀; Zacatecas, Sierra Vieja; 23°29’40.78” N, 102°7’42.69” W; alt. 2,085 m; 22 Sep 2010; leg. M. García-París, N. Percino.
Remarks
This species is morphologically similar to R. tamaulipensis, but it could be distinguished from the latter species by its black general coloration of pronotum and head.
Romalea guatemalensis De Jesús-Bonilla, Barrientos-Lozano & Zaldívar-Riverón, sp. n. (Fig. 3F)
http://zoobank.org/urn:lsid:zoobank.org:act:88C3363D-CC99-4563-BAF2-9A5B71C5BF1E
Size (head-tegmina): females 55.75-53.33 mm, males 52.80-49.67 mm Head: scape and pedicel black; first 15-16 antennal flagellar segments yellow, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex, fastigium, and frons yellowish green to dark green; coronal suture, facial carina, and epistomal margin yellow or yellowish orange. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Medial carina rugose or slightly rugose. Lateral carinae slightly prominent and rugose. Pronotal crest, lateral carinae, and lateral border of pronotum yellowish orange. Pronotal disc and lateral lobes yellowish green to green. Posterior margin acute-angulate. Tegmina: veins and veinlets brown yellowish. Apical margin broadly black. Abdomen: tergites grey and black, with yellowish dorsomedial line. Sternites grey or with the same color of tergites. Hind leg: medial area of femur green to pale yellow. Dorsal, ventral, and external lateral carinae with the same color of medial area. External dorsolateral face with the same color of dorsal carina or finely black; external ventrolateral face with the same color of ventral carina and with a series of black dots. Semilunar process, cover plate and insertion of extensor tibia muscle black. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus angular or lobiform shaped with right or interior orientation. Epiphallus bridge sculpture strongly developed. Margin of lateral plate of epiphallus sub-angular (Fig. 8C, D).
Diagnosis. R. guatemalensis morphologically resembles R. auricornis, but it can be distinguished by having: (1) pronotum green with yellowish lateral and medial carinae (lateral and medial carinae with the same color of pronotum in R. auricornis), (2) apical margin broadly black (narrowly black in R. auricornis), and (3) metazona strongly produced caudal (slightly produced in T. auricornis).
Distribution. This species was collected in localities of Guatemala; however, a broader sampling is pertinent (Fig. 10F).
Material examined: holotype: Guatemala. ♀; Baja Verapaz, Purulha, Orejuela; 15°14’58.7” N 90°09’21.2” W; alt. 1,359 m; 26 Sep 2015; leg. V.S. De Jesús-Bonilla, O. Pérez-Flores, Carmen L. Yurritia. CNIN (Fig. 3F). Paratypes: Guatemala. 2 ♀♀, 4 ♂♂; same data as for holotype, 1 ♂; El Petén, Poptún, Finca la Jarrilla; 16°18’18.5” N, 89°24’34.6” W; 4 Jul 2015; leg. Carmen L. Yurritia. CNIN
Etymology. The species name refers to Guatemala, the country where the type specimens were collected.
Remarks
Previously published molecular phylogenetic studies showed that this species is closely related to R. microptera, R. auricornis, R. centurio, and R. obscura, with these 5 species being nested in a single clade (De Jesús-Bonilla et al., 2017, 2019).
Romalea gutturosa (Bolívar, 1901), comb. n. (Fig. 4A)
Taeniopoda gutturosaBolívar, 1901: 268; Kirby,, 1910: 372; Hebard 1924: 269; COPR, 1982: 115; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Taeniopoda aurantiaeBruner, 1907: 235.
Size (head-tegmina): females 53.75-42.77 mm, males 46.19-42.86 mm Head: scape and pedicel black; first 11-15 antennal flagellar segments scarlet red, the rest of antennal flagellar segments black. Fastigium strong declivent. Vertex, fastigium, and frons scarlet red or dark orange; coronal suture, facial carina, and epistomal margin with the same color of frons or slightly lighter. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes scarlet red to dark orange. Medial carina rugose or slightly rugose. Lateral carinae rugose. Pronotal crest and lateral carinae black. Sides of pronotal crest, and lateral carinae black. Posterior margin acute-angulate. Tegmina: veins and veinlets brown or yellowish brown. Abdomen: tergites black and grey, with brown yellowish dorsomedial line. Sternites brown to red. Hind leg: medial area of femur red, orange or brownish. Dorsal, ventral and external lateral carinae with the same color of medial area. External dorosalteral face with the same color of dorsal carina or black; external ventrolateral face black, or with the same color of ventral carina. Semilunar process black, cover plate, insertion of extensor tibia black. Male genitalia: posterior margin of the endophallus plate rounded. Ancora of epiphallus angular shaped with interior orientation. Epiphallus bridge sculpture strongly developed. Margin of lateral plate of epiphallus rounded (Fig. 8E, F).
Diagnosis. This species can be recognized by the following combination of morphological features: (1) elevated pronotal crest, (2) scarlet red to dark orange ground color, (3) pronotal crest and lateral carinae black, (4) scarlet red to orange red pale portion of antenna, and (5) posterior margin acute-angulate.
Material examined: syntypes: Guatemala. 1 ♀; “Taenipoda gutturosa Allolectotype C.S. Carbonell 1966”; Escuintla; MNCN Cat. Tipos No 7244 MNCN_Ent 147347 (syntype) (Fig. 5C, D), 1 ♂; “Taenipoda gutturosa Allolectotype C.S. Carbonell 1966”; Escuintla; MNCN Cat. Tipos No 7244 | MNCN_Ent 147346 (syntype) (Fig. 5E, F). Other material: Guatemala. 2 ♀♀; Santa Rosa, Estanzuela de Ixhuatán, 24 Sep 2000; leg. Carlos Ávila Ramos, 1 ♂; Santa Rosa, El Naranjito; 8 Nov 1989; leg. R. Pérez, 1 ♂; Santa Rosa, El Cerinal; enero 1993; leg. A. C. Bailey, J. Monzon, 1 ♂; Santa Rosa, Barberena, El Naranjito; 2 Aug 1989; leg. R. Pérez, 3 ♀♀; Santa Rosa, Barberena, El Cerinal; enero 1993; leg. A.C. Bailey, J. Monzon, 1 ♀, 1 ♂; Retalhuleu, Finca Los Brillantes; 14°33’27.2” N, 91°37’06.5” W; 14 May 2016; leg. V.S. De Jesús-Bonilla, Eliam Percha, 1 ♀; Quetzaltenango, Hda. Batzá; 6 Jul 1985; leg. M. R. Sáenz, 1 ♂; Peten, Sayaxché; 7 Jul 1975; leg. José M., 1 ♂; Guatemala, San Miguel Petapa; 8 Oct 1988; leg. C. Taracena, 1 ♂; Guatemala, Amatitlán; Dic 78; leg. M. C. Vean, 1 ♂; Suchitepequez, Cuyotenango, La Ecantadora; 26 Aug 1978; leg. Martín Minundo. El Salvador. 2 ♀♀, 2 ♂♂; La Libertad; 13°40’59.68” N, 89°17’0.63” W; alt. 341 m; 14 Aug 2009; leg. L. Barrientos-Lozano.
Distribution. Central America, in Guatemala and El Salvador (Fig. 11A).
Remarks
Romalea gutturosa is closely related to R. citricornis, but they can be distinguished from each other by the external morphological features included in the key herein provided. Moreover, the geographic distribution of R. gutturosa is more restricted than the one of R. citricornis, (Figs. 10D, 11A).
Romalea obscura (Bruner, 1907), comb. n. (Fig. 4B)
Taeniopoda obscuraBruner, 1907: 235; Kirby, 1910: 372; Hebard, 1924, 273; Maes, 1998: 103; Barrientos-Lozano et al., 2013: 325; De Jesús-Bonilla et al., 2017: 600, 2019: 64, comb. n.
Taeniopoda bicristataBruner, 1907: 236; Kirby, 1910: 372; Hebard, 1924, 271, syn. n.
Size (head-tegmina): females 50.89-48.76 mm, males 46.14 mm. Head: scape and pedicel grey to dark yellow; first 15-17 antennal flagellar segments yellow, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex, fastigium, and frons brown to black mate; coronal suture, facial carina, and epistomal margin cinnamon, lighter than frons. Pronotum: pronotum with a medial carina elevated at least 0.3 times its total height, forming an elevated pronotal crest. Lateral carinae heavy rugose. Pronotal disc robust and flattened, table shaped in metazona. Pronotal disc and lateral lobes dark brown to matte black. Pronotal crest, lateral carinae, and posterior margins of pronotum light brown. Medial carina rugose. Sides of pronotal crest, and lower margin of lateral carinae black polished. Posterior margin rectangulate. Tegmina: finely reticulate. Veins and veinlets light brown; fenestrated areas black and light brown, mostly light brown in anal area. Abdomen: tergites brown and black, with light brown dorsomedial line. Sternites brown to black. Hind leg: medial area of femur brown or entirely black. Dorsal, ventral and external lateral carinae light brown to black. Ventral and external ventrolateral carinae paler than dorsal and external dorsolateral carinae. External dorsolateral face variable, same color of dorsal carina; external ventrolateral face with same color of ventral carina with black dots series. Semilunar process black, cover plate and insertion of extensor tibia muscle same color of upper marginal area. Male genitalia: posterior margin of the endophallus plate rounded. Ancora of epiphallus lobiform shaped with interior orientation. Epiphallus bridge heavily sculptured. Margin of lateral plate of epiphallus sub-angular (Fig. 8G, H).
Diagnosis. This is the most robust species within Romalea. It can be distinguished from the remaining species of the genus by the following combination of characters: (1) elevated pronotal crest, (2) dark brown to black ground color, (3) considerably robust pronotum, and (4) pronotal disc flattened, table shaped.
Distribution. Yucatán Peninsula, and a single, non-confirmed report from San Luis Potosí, Mexico (Fig. 11B).
Material examined: holotype: Mexico. ♀; “Taeniopoda bicristata Syntype Type H299 Mat”; [Puebla, Matamoros]; Type Bruner (holotype) (Fig. 5G, H). Paratypes: Mexico. 1 ♂; “T. obscura”; Temax, Yucatán; Bruner (syntype, etazoa type), 1 ♂; “T. obscura”; Mérida, Yucatán; leg. Gaumer; (paratype), 1 ♂; “T. obscura”; Temax, Yucatán; leg. Gaumer; (paratype), 1 ♀,1 ♂; “T. obscura”; Temax N. Yucatán; leg. Gaumer; (paratype). Other material: Mexico. 2 ♀♀; San Luis Potosí, Taninul; 21°57’19.9” N, 98°53’19.8” W; alt. 77 m; 09 Apr 2001; leg. L. Barrientos-Lozano, 3 ♀♀, 1 ♂; Yucatán, Kaxil kiuic; 20°05’34.4” N 89°33’49.7” W; alt. 85 m; 30 Jul 2014; leg. C.N. Ibarra-Cerdeña, 2 ♂♂; Yucatán, Mérida; leg. Gaumer, 1 ♂; Yucatán, Chichen Itza; leg. E. Thomposon; “F.M.N. Coll”, 1 ♀; Yucatán, Mérida; Gaumer, 1 ♀; Northern Yucatán. Guatemala. 1 ♀; Petén, Flores, Dos Lagunas; 17 Jun 1989; leg. Sergio Pérez, 1 ♀; Petén, Tikal; 2 Oct 1999; leg. Byron González.
Remarks
Romalea bicristata was described from a single female of uncertain locality (the label reads “Mat”, probably Matamoros, Puebla). We consider that the characters that separate R. bicristata from R. obscura, pronotal crest lower in the metazona in R. obscura and higher in R. bicristata, flattened portions on pronotal disc and sculpture of lateral carinae, actually represent individual variation of the only known specimen assigned to R. bicristata, and hence are not sufficient to consider the latter taxon as a separated species.
Romalea picticornis (Walker, 1870), comb. rev. (Fig. 4C)
Rhomalea picticornisWalker, 1870: 538 (misspelling); Thomas, 1873: 240
Taeniopoda pecticornisScudder & Cockerell, 1902: 39 (misspelling); Caudell, 1903: 795
Taeniopoda picticornis;Bruner, 1907: 234; Kirby, 1910: 371; Hebard, 1924: 256; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Taeniopoda staliBruner, 1907: 234(T. ståli, incorrect original spelling); Hebard, 1924: 261, 1932: 270; Fontana et al., 2008: 277; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Taenipoda stealiKirby, 1910: 371 (misspelling).
Size (head-tegmina): females 59.44-42.35 mm, males 51.21-43.51 mm. Head: scape green yellowish or black, pedicel black; first 11-12 antennal flagellar segments yellow, or orange to scarlet with black ring at the distal margin, the rest of antennal flagellar segments black. Fastigium strongly declivent. Vertex and fastigium Green to yellowish-olivaceous; coronal suture yellow. Frons green to yellowish-olivaceous, facial carina and epistomal margin yellow, lighter than frons. Pronotum: pronotum with a medial carina 0.25 times or less its maximum height. Medial and lateral carinae rugose. Pronotal disc and lateral lobes green to yellow-olivaceous. Sides of carinae and transverse sulci of the same color of pronotum. Posterior margin rectangulate. Tegmina: veins and veinlets green, fenestrated areas black. Abdomen: tergites olivaceous-yellowish, with yellow dorsomedial line. Sternites olivaceous-yellowish. Hind leg: medial area of femur yellow to olivaceous-yellowish. Dorsal, ventral and external lateral carinae yellow to olivaceous-yellowish. External dorsolateral face black, external ventrolateral face all black or with black dot series. Semilunar process black, cover plate black or olivaceous-yellowish, insertion of extensor tibia muscle grey to black. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus angular or lobiform shaped with interior orientation. Epiphallus bridge sculpture slightly or absent. Margin of lateral plate of epiphallus sub-angular or rounded (Fig. 9A, B).
Diagnosis. Romalea picticornis is morphologically similar to R. eques and R. tamaulipensis; however, R. picticornis can be distinguished from the latter 2 species by having the antennal flagellar segments with a black ring at the distal margin. It can also be distinguished from R. eques by its green to olivaceous-yellowish ground color (black in R. eques), and from R. tamaulipensis by its transverse sulci and sides of pronotal crest of the same color of pronotum, (black in R. tamaulipensis).
Distribution. This species has been reported for various localities along the Pacific Mountain Ranges and Pacific Coastal plains of Mexico, the Balsas Basin and highlands, Oaxaca and Puebla highlands, and the Trans-Mexican Volcanic Belt (Fig. 11C).
Material examined: syntypes: Mexico. 1 ♂; “Rhomalea puncticornis Walker”; (stali) (syntype) (Fig. 6A), 1 ♀, 1 ♂; “Rhomalea puncticornis”; Oajaca (= Oaxaca); (syntype) (Fig. 6B). Other material: Mexico. 4 ♀, 3 ♂; Colima, Coquimatlan, Arroyo El Tanque del General; 19°08’30.0” N, 104°01’27.3” W; alt. 504 m; 25-26 Aug 2013; leg. J.C. Arenas-Monroy, 1 ♂; Estado de México, Ixtapaluca; 19°18’52.24” N, 98°53’4.30” W; alt. 2,485 m; Nov 2012, 1 ♀; Estado de México, Ixtapan de la Sal; alt. 1,850 m; 5-6 Oct 2013; leg. E.O. Martínez-Luque, 1 ♂; Guerrero; Teloloapan; 18°22’1” N, 99°52’8” W; alt. 1,575 m; 03 Nov 2012, 1 ♀,1 ♂; Guerrero, Carretera Teloloapan-Iguala, ¿44 km E? de Xalostoc; 18°26’01.17” N, 99°45’15.99” W; alt. 1682 m, 2 ♀♀,1 ♂; Guerrero, Olinalá, Xixila; 18°00’07.0” N, 98°50’11.5” W; alt. 154 m; 11 Sep 2013, 2 ♂♂; Guerrero, Teloloapan, entrada al pueblo; 18°21’5.6” N, 99°50’37.2” W; alt. 1,562 m; 23 Nov 2013; leg. A. Zaldívar-Riverón, J.J. Martínez, M. García-París, 3 ♀♀, 2 ♂♂; Jalisco, La Huerta, Estación de Biología de Chamela; 19°29.620’ N, 105°02.749’W; alt. 87 m; 6 Sep 2009; leg. M. García-París, N. Percino, 1 ♀; Morelos, Tepalcingo, El Limón, Río de El Limón; 18°31’58.0” N 98°56’20.6” W; alt. 1,255 m; 25 Jul 2014; leg. E.O. Martínez-Luque, 1 ♂; Puebla, Totoltepec de Guerrero; 18°16’59.51” N, 97°48’25.80” W; alt. 1,508 m; 30 Nov 2013; leg. H. Álvarez-García, 1 ♀; Veracruz, Acultzingo, Acultzingo; 18°42’01.8” N, 97°18’48.6” W; alt. 1,840 m; 24 Jul 2014; leg. A.G. Clause, 1 ♀, 4 ♂♂; Veracruz, Acultzingo, Tecamalucan; 18°45’36.0” N, 97°12’36.0” W; alt. 1,384 m; 12 Sep 2014; leg. A. Zadívar-Riverón, D. Dubovikoff, V.S. De Jesús-Bonilla et al.
Remarks
Romalea picticornis was previously only distinguished from its synonym, R. stali, by its color of the pale portion of flagellum; however, we noticed that this antennal color is considerably variable, since even in fixed specimens the antennae can be fading from scarlet to yellow.
Romalea reticulata (Fabricius, 1781), comb. n. (Fig. 4D)
Gryllus reticulatusFabricius, 1781: 362; Donovan, 1800: Plate 12.
Acheta reticulata;Fabricius, 1787: 231.
Taeniopoda maximaBruner, 1907: 235.
Taeniopoda reticulata;Kirby, 1910: 372, Hebard, 1924: 103; Rowell, 2013: 106; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Size (head-tegmina): females 61.66-51.15 mm, males 57.90-51.18 mm. Head: scape and pedicel black; first 10-14 antennal flagellar segments yellow to orange, the rest of antennal flagellar segments black. Fastigium strong declivent. Vertex, fastigium and frons purple to black; coronal suture, facial carina, and epistomal margin crimson to purple. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes purple to black. Medial carina slightly rugose and polished. Lateral carinae heavy rugose. Pronotal crest and lateral carinae dark purple to black. Posterior margin acute-angulate. Tegmina: veins and veinlets red brown to purple. Abdomen: tergites and sternites purple to black. Hind leg: medial area of femur purple. Dorsal, ventral and external lateral carinae with the same color of medial area, black at apex. External dorsolateral face with the same color of dorsal carinae or black; external ventrolateral face with the same color of ventral carinae with black dots series. Semilunar process black and cover plate black; insertion of extensor tibia muscle black or grey. Male genitalia: posterior margin of the endophallus plate rounded. Ancora of epiphallus angular or lobiform shaped with exterior orientation. Epiphallus bridge sculpture strongly or slightly developed. Margin of lateral plate of epiphallus rounded (Fig. 9C, D).
Diagnosis. This species can be recognized by the following combination of characters: (1) elevated pronotal crest, (2) ground color purple to black, (3) pronotal crest and lateral carinae darker than rest of pronotum, and (4) posterior margin acute-angulate.
Distribution. Nicaragua, Costa Rica in the Caribbean slope, and Panama (Fig. 11D).
Material examined: syntype: Costa Rica. 1 ♂; “Taeniopoda maxima”; Limón; leg. M.A. Carriker, Jr; Type H297 (syntype) (Fig. 6C, D). Other material: Costa Rica. 1 ♂; Puntarenas, Cabo Blanco, Est. San Miguel; leg. M. Ramírez; 173174, 411412 INBio 1651349, 1 ♀; Limón, Talamanca, Sixaola; 9°37’57.3” N, 82°39’32.6” W; 7 Aug 1992; leg. K. Taylor; INBio 803105, 2 ♀♀, 3 ♂♂; Limón: Puerto Viejo de Talamanca; 9°39’10.4” N, 82°44’22.6” W; alt. 1 m; 9 Sep 2014; leg. V.S. De Jesús-Bonilla, 1 ♀; Limón: Pococi, Colorado; 10°38’38.6” N, 83°44’31.2” W; 16 Jul 1993; leg. F. Araya; INBio 1685036, 4 ♀♀, 4 ♂♂; Limón, Limón, La cieneguita; 9°58’58.5” N, 83°01’53.1” W; alt. 1 m; 9 Sep 2014; leg. V.S. De Jesús-Bonilla, 1 ♂; Limón, Est. Hitoy-Carere; “184200, 643300”; Nov 1990; leg. G. Carballo; INBio 269359, 5 ♀♀, 6 ♂♂; Limón: Cahuita; 9°44’03.4” N, 82°49’40.6” W; alt. 1 m; 9 Sep 2014; V.S. De Jesús-Bonilla, 1 ♂; Limón, 1 ♂; Heredia; Sarapiquí, La Virgen; 10°24’04.5” N, 84°02’57.5” W; 1 Oct1990; leg. R. Aguilar, INBio 526096, 1 ♂; Heredia, Sarapiquí, La Virgen; 10°24’04.5” N, 84°03’00.7” W; 1 July 1990; leg. A. Fernández; INBio 1377644, 1 ♀; Heredia, Braulio Carrillo, Est. Magsasay; “264600, 531100”; Jul 1990; leg. D. Acevedo; INBio 299017, 1 ♀, 2 ♂♂; Alajuela, Los Chiles, Caño Negro; 10°53’37.7” N, 84°47’20.0” W; 12 Aug 1993; leg. K. Martínez; INBio 1976539, 1976540, 1976542, 1 ♂; Alajuela, Los Chiles, Caño Negro; 10°53’37.7” N 84°47’20.0” W; 29 Jun 1992; K. Quesada; INBio 439523, 1 ♀; Alajuela, Los Chiles, Caño Negro; 10°24’04.5” N, 84°02’57.5” W; 1 Jun 1990; leg. G. Carballo; INBio 257197, 1 [without abdomen to determine sex]; Alajuela; Los Chiles, Caño Negro; 10°53’37.7” N, 84°47’20.0” W; 29 Jun 1992; leg. K. Quesada; INBio 439524, 1 ♀; Alajuela, Los Chiles, Caño Negro; 10°53’37.7” N, 84°47’20.0” W; 18 Aug 1992; leg. K. Martínez; INBio 696655, 1 ♂; Alajuela, Los Chiles, Caño Negro; 10°57’19.2” N, 84°44’58.6” W; 12 Sep 1993; leg. K. Quesada; INBio 1947371, 1 ♂; Carrillo; “vii-ix-03”, 1 ♀; Guapiles; 5 Jun 1909; leg. P. P. Calvert, 1 ♀; La Emilia; 19 Nov 1909; leg. P. P. Calvert, 1 ♂; San Carlos; leg. Schils & Burgdorf, 1 ♂; Santa Clara (Atl); leg. C.R. P. Biolley, 1 ♀, 4 ♂♂; Siquirres; 2-3 Jul 1903; leg. MA Carriker Jr. Nicaragua. 1 ♂; Chontales; Janson. Panama. 1 ♀; Darién 1914, leg. J. Zetek.
Remarks
This species has the southernmost geographic distribution for the genus Romalea.
Romalea tamaulipensis (Rehn, 1904), comb. n. (Fig. 4E)
Taeniopoda tamaulipensisRehn, 1904: 531; Bruner, 1907: 234; Hebard 1924: 261; Buzzetti & Barrientos-Lozano, 2011: 210; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Romalea tamaulipensis, comb. n.
Size (head-tegmina): females 57.32-43.31 mmm, males 48.79-41.82 mm. Head: scape yellow, green-yellowish or black; pedicel black; first 9-11 antennal flagellar segments yellow with a black ring at the distal margin, remaining antennal flagellar segments black. Fastigium strongly declivent. Vertex and fastigium green yellowish to dark green; coronal suture yellow. Frons green olivaceous to dark green; facial carina and epistomal margin yellow to green olivaceous, lighter than frons. Gena with a distinct vertical yellow band. Pronotum: pronotum with a medial carina 0.25 times or less its maximum height. Medial carina of pronotum rugose or slightly rugose. Sides of media carina black, transverse sulci narrowly black. Pronotal disc green olivaceous to almost dark green; lateral lobes green olivaceous to dark green, lighter than pronotal disc. Medial carina and posterior margins of pronotum yellow. Pronotum with dorsal slightly excavated black furrow between 3rd and 4th sulci. Posterior margin rectangular. Tegmina: veins and veinlets green yellowish, fenestrated areas black. Tip of elytron with a narrow black border Abdomen: tergites green to black, with yellow dorsomedial line. Sternites green with yellow to olivaceous green caudal margin. Hind leg: medial area of femur yellow to green yellowish. Dorsal, ventral, and external carinae yellow to green yellowish. External lateral faces black. Semilunar process, cover plate and insertion of extensor tibia muscle black. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus lobiform shaped with interior or right orientation. Epiphallus bridge sculpture strongly developed or absent. Margin of lateral plate of epiphallus sub-angular or rounded (Fig. 9E, F).
Diagnosis. This species can be distinguished from the remaining species of the genus by having the following combination of characters: (1) low pronotal crest, (2) general ground color green olivaceous, (3) antennal flagellar segments with a black ring at distal margin, (4) sides of medial carina black, color extends to lateral lobes of the pronotum, and (5) pronotum with dorsal slightly excavated black furrow between 3rd and 4th sulci.
Distribution. This species is mainly distributed along the Sierra Madre Oriental, but also has records in some localities in the Trans-Mexican Volcanic Belt and the southern Mexican Plateau (Fig. 11E).
Material examined: holotype: Mexico. ♀; “Taeniopoda tamaulipensis”; Altamira, Tamaulipas; 7 Jan 1903; leg. M.E. Hoag; Type 5113 (holotype) (Fig. 6E, F). Other material: Mexico. 3♀♀, 2 ♂♂; San Luis Potosí, Guadalcazar, Ábrego, Campamento Monternach; 22°39’23.2” N, 100°22’48.3” W; alt. 1,567 m; 22 Sep 2012, 1 ♂; Coahuila, 30 km antes de Santiago Aserradero; 25°23.347’ N, 100°15.180’ W; alt. 1,373 m; 10 Nov 2013; leg. L. Barrientos-Lozano, V.S. De Jesús-Bonilla, A.Y. Rocha-Sánchez, L. A. Hernández-Cortéz, 1 ♀; Hidalgo, El tablón; 11 Oct 2009; leg. M. García-París, N. Percino, 1 ♂; Hidalgo, Metznoxtla; 20°37’47.8” N, 98°51’28.0” W; alt. 1,612 m; 12 Sep 2012; leg. M. García-París, N. Percino, 1 ♀; Hidalgo, Pedregal; 20°39’05.7” N, 98°48’22.8” W; alt. 1,290 m; 12 Sep 2012; leg. M. García-París, N. Percino, 3 ♀♀; Hidalgo, RMO Xhita; 20°38’04.1” N, 99°19’35.2” W; alt. 2,048 m; 10 Oct 2009; leg. M. García-París, N. Percino, 1 ♀; Hidalgo, Venados; 20°28’31.4” N, -98°39’24.9” W; alt. 1,597 m; 11 Sep 2012; leg. M. García-París, N. Percino, 1 ♀,1 ♂; Nuevo León, Iturbide, Santa Rosa Km 4; 24°42.113’ N, 99°54.472’ W; alt. 1,513 m; 8 Nov 2013; leg. L. Barrientos-Lozano, V.S. De Jesús-Bonilla, A.Y. Rocha-Sánchez, L. A. Hernández-Cortéz, 1 ♀; Nuevo León, Linares-San Roberto, Carretera 58 Km 30; 24° 44.732’ N, 99°48.910’ W; alt. 852 m; 8 Nov 2013; leg. L. Barrientos-Lozano, V.S. De Jesús-Bonilla, A.Y. Rocha-Sánchez, L. A. Hernández-Cortéz, 2 ♀♀, 2 ♂♂; Nuevo León, Santiago, Arriba de la Cola de Caballo 6km antes de Santiago; 25°21.841’ N, 100°9.566’ W; alt. 455 m; 10 Nov 2013; leg. L. Barrientos-Lozano, V.S. De Jesús-Bonilla, A.Y. Rocha-Sánchez, L. A. Hernández-Cortéz, 1 ♀; Nuevo León, Santiago, Horse Tail Fall; 25°22.081’ N, 100°09.637’ W; alt. 701 m; 10 Jul 2013; leg. L. Barrientos-Lozano, 1 ♂; Nuevo León, Galeana, Puente de Dios; 15 Aug 2015; leg. M. Trujano-Ortega, 4 ♀♀, 2 ♂♂; Querétaro, Camino a Santa María Cocos; 18 Nov 2013; leg. C. Pedraza Lara, E. Recuero, 1 ♀; Querétaro, Sierra Gorda 3km N de Arroyo Seco; 21°34’15.0” N, 99°42’43.0” W; alt. 1,010 m; 14 Sep 2014; leg. U.O. García-Vázquez, M. Trujano-Ortega, 1 ♀; Querétaro, Sierra Gorda Carretera hacía Sótano de Barro; 24 Jul 2014; leg. A. Ramírez-Ponce, 1 ♀,1 ♂; Tamaulipas, Ocampo, Libramiento poniente Km 1.5; 22°50’15.25” N, 99°20’28.81” W; alt. 351 m; 12 Oct 2013; leg. L. Barrientos-Lozano, 2 ♀♀, 1 ♂; Tamaulipas, Rd. 66, Tula-Ocampo a 11 km de Tula; 22°56.867’ N, 99°36.267’ W, alt.1481 m; 12 Oct 2013; leg. L. Barrientos-Lozano.
Remarks
Rehn’s original description (1904) of R. tamaulipensis was based on a single adult female. This redescription is therefore more informative, since it includes information of a number of specimens. The coloration described in the original description of the species, “general color burnt-sienna and ferruginous margin of medial carina and posterior margin of the pronotum”, actually corresponds to a discoloration acquired after fixation. Here we report the coloration of live specimens that were collected as part of this study.
Romalea varipennis (Rehn, 1905), comb. n. (Fig. 4F) Taeniopoda varipennisRehn, 1905: 410; Bruner, 1907: 237; Hebard, 1924: 271; Hebard, 1932: 271; COPR, 1982: 115; Maes, 1998: 115; Rowell, 2013: 108; De Jesús-Bonilla et al., 2017: 600, 2019: 64.
Taeniopoda flavidaBruner, 1907: 237.
Size (head-tegmina): females 59.54-43.67 mm, males 55.34-46.91 mm. Head: scape and pedicel black; first 12-15 antennal flagellar segments yellow to orange, the rest of antennal flagellar segments black. Fastigium strong declivent. Vertex, fastigium and frons green yellowish to orange; coronal suture, facial carina and epistomal margin yellowish to orange. Pronotum: with a medial carina elevated at least 0.3 times total height of pronotum, forming an elevated pronotal crest. Pronotal disc and lateral lobes orange yellowish to greenish-yellow. Medial carina slightly rugose and polished. Lateral carinae rugose. Pronotal crest, lateral carinae and posterior margin orange to orange-yellowish, lighter than rest of pronotum. Posterior margin acute-angulate. Tegmina: veins and veinlets green- yellowish. Abdomen: tergites brown and green, with brown yellowish dorsomedial line. Sternites green to orange. Hind leg: medial area of femur green-yellowish to orange-yellowish. Dorsal, ventral, and external lateral carinae with the same color of medial area. External dorsolateral face with same color of dorsal carinae; external ventrolateral face with same color of ventral carinae and with a series of black dots. Semilunar process black, cover plate and insertion of extensor tibia muscle black or with the same color of external dorsolateral face. Male genitalia: posterior margin of the endophallus plate sub-angular or rounded. Ancora of epiphallus angular or lobiform shaped with right or exterior orientation. Epiphallus bridge sculpture strongly or slightly developed. Margin of lateral plate of epiphallus sub-angular (Fig. 9G, H).
Diagnosis. Romalea varipennis can be distinguished from the remaining species of the genus by having the following combination of external morphological features: (1) elevated pronotal crest, (2) ground color orange yellowish to greenish-yellow, (3) pronotal crest and lateral carinae lighter than rest of pronotum, and (4) posterior margin acute-angulate.
Distribution. Nicaragua, Costa Rica, and Panama (Fig. 11F).
Material examined: syntype: 1 ♂; “Taeniopoda varipennis. Rehn Hebard Collection. Taeniopoda varipennis Rehn H300 Type”; Central America; (syntype) (Fig. 6G, H). Paratype: 1 ♂; “Taeniopoda varipennis”; Central America; (paratype). Homotype: 1 ♀; “Taeniopoda varipennis”; Gulf of Nicoya; (homotype). Other material: Costa Rica. 1 ♀; Guanacaste, Bagaces, Est. Palo Verde; 10°20’56.8” N, 85°21’08.3” W; alt. 10 m; Aug 1991; leg. D. Acevedo, 1 ♀,1 ♂; Guanacaste, Bagaces, Est. Palo Verde; 10°20’56.8” N, 85°21’08.3” W; 1 Jul 1991; leg. G. Dauphin, 2 ♂♂; Guanacaste, Bagaces, Est. Palo Verde; 10°20’56.8” N, 85°21’08.3” W; 12-24 Aug 1992; leg. U. Chavarría, 1 ♀; Guanacaste, La Cruz, Santa Elena Finca Jenny; 10°51’55.8” N, 85°34’24.6” W; 9 Jul 1993; leg. E. Araya, 1 ♀, 2 ♂♂; Guanacaste, Liberia, PN Sta. Rosa; 10°50’11.0” N 85°36’55.8” W; 6 Jul 1978; leg. D. Janzen, 1 ♂; Guanacaste, Liberia, Sector Las Pailas; 10°46’36.5” N 85°21’06.8” W; 24 Aug 1992; leg. C. Cano, 1 ♂; Guanacaste, Nicoya, Barra Honda, Los Mesones; 10°10’12.4” N, 85°21’02.9” W; 1 Jul 1995; leg. M. Reyes, 5 ♀♀, 10 ♂♂; Puntarenas, A 3km S de Curré; 8°58’31.4” N, 83°18’13.0” W; alt 93 m; 25 Jun 2015; leg. A. Zaldívar-Riverón, J.J. Martínez, V. Salinas-Ramos, V.S. De Jesús-Bonilla, 1 ♂; San José, San Antonio de Escazú; leg. WGE, 1 ♀; Puntarenas, Garabito, Estación Quebrada Bonita; 9°46’02.8” N, 84°36’29.2” W; 1 Jun 1992; leg. J. C. Saborio, 1 ♀, 3 ♂♂; Ciruelas; 15 Jul 1915; leg. A. Alfaro, 1 ♂; Ciruelas; Jun 1913; leg. A. Alfaro, 1 ♂♂; Ciruelas; leg. A. Alfaro, 5 ♀♀, 6 ♂♂; Gulf of Nicoya, 1 ♀; Orotina; 12 Oct 1915; leg. A. Alfaro; In Cementery #58, 1 ♀; Parismina; alt. 5m; 26-28 Jun; leg. M. Valerio, 1 ♂; Pozo Azul; (under wood), 1 ♀, 1 ♂; San José; (under wood), 2 ♀♀; San José; leg. A. Alfaro, 1 ♂; San José; alt. 1,161 m; leg. P. Biolley, 1 ♀; San José, 3 ♂♂; Ujurrás de Térraba; 10 Sep [19]07; leg. M. A. Carriker Jr. Nicaragua. 5 ♀♀, 2 ♂♂; Camoapa, 1 ♀; Nicaragua.
Remarks
Based on genetic and genomic information, R. varipennis is sister to R. reticulata (De Jesús-Bonilla et al., 2017, 2019). These 2 species occur in Costa Rica, though their geographic distribution is separated by the Tilarán, Guanacaste, Talamanca, and Central mountain ranges that cross the country.
Discussion
Recent studies with Sanger sequence (including both mitochondrial and nuclear gene markers) and genomic data showed that Romalea is deeply nested in a clade with the species of Taeniopoda, thus showing that the latter genus is paraphyletic (De Jesús-Bonilla et al., 2017, 2019). Although the synonymy of Taeniopoda and Romalea had not been formally established, a close relationship between their species had been previously suggested. In a review of the Romaleinae of North America, Rehn and Grant (1959a) emphasized the similarities of body, prosternal process, head and pronotum between R. microptera and R. eques. Moreover, they mentioned that the former species probably derived from the second or both shared a common ancestor. More recently, Mutun and Burst (2004) analyzed the genetic structure in R. microptera based on a fragment of the cytochrome b mitochondrial DNA gene. Though these authors found a close relationship between Romalea and Taeniopoda sensu lato, they did not make any taxonomic changes. The close relationship between Romalea and Taeniopoda had also been considered in previous comparative physiological and behavioral studies that included members of both genera (Staufer et al., 2004; Staufer & Whitman, 2007). Here we carried out an exhaustive morphological revision of the recognized species of Romalea and Taeniopoda, and integrated published information based on specific markers and genomic data to formally establish the synonymy of Taeniopoda with respect to Romalea (De Jesús-Bonilla et al., 2017, 2019). The proposed synonymy is based on robust phylogenetic information and contributes to a stable classification of the taxa involved.
Some taxonomic problems still need to be solved within Romalea. For instance, we took a conservative approach and maintained R. eques and R. tamaulipensis as separate species, though they could actually represent a ring species. This interesting evolutionary scenario needs to be studied in more detail, and thus it is necessary to carry out an intensive sampling along the geographic distribution of both species, particularly in areas where they appear to be in contact. Additional sampling will also allow a better understanding of the geographic distribution of R. obscura. Currently, this species appears to have a disjunct geographic distribution, with records in localities situated in the states of Yucatán, San Luis Potosí and Puebla, Mexico. The morphological feature examined in the study for this species led us to synonymize R. bicristata with R. obscura, though further collects and molecular phylogenetic studies will confirm the actual status of these 2 taxa.
Romalea reticulata and R. varipennis were found to represent sister species based on genomic-scale information (De Jesús-Bonilla et al., 2019). The former species is mainly distributed along the Atlantic slope of Nicaragua, Costa Rica and Panama, whereas T. varipennis occurs along the Pacific slope, with their populations apparently being separated by the Tilarán, Guanacaste, Talamanca and Central mountain ranges. However, some museum specimens identified as R. reticulata and R. varipennis were reported to be collected on the Pacific and Atlantic slopes, respectively. These localities refute the allopatric geographic distribution of these species, though this needs to be corroborated. Our discovery of a new species of Romalea from Guatemala also highlights the necessity to survey various regions in Central America that do not have records for this genus.











 nueva página del texto (beta)
nueva página del texto (beta)










