Introduction
The Lamiaceae (Labiatae) family comprises approximately 236 genera and 7,173 species [1,2]. The genus Salvia is one of the most extensive groups in this family, representing around 900 species worldwide [3,4]. The term Salvia comes from the Latin "salvare," meaning "to heal or be safe and unharmed," referring to the healing properties of these species [2,5-9], which are recognized in worldwide traditional medicine. In the Americas, around 500 species are registered in Mexico, Central America, and South America, representing the second most diverse territory, with approximately 312 species, of which 75 to 88% are endemic [5,10-12]. In Mexico, the most significant number of Salvia species is concentrated in the western and southeastern, along the Occidental Sierra Madre, the Trans-Mexican Volcanic Belt, and the Sierra Madre del Sur.
Salvia species are typically shrubs or climbing shrubs from 30 to 150 cm tall that can be annual or perennial [12]. Their stems are angular, characteristic of the Lamiaceae family, with leaves that are usually velvety or hairy, and they can often be rugose, entire, toothed, lobed, or pinnate. The flower stalks produce small bracts different from the basal leaves. Inflorescences are borne in clusters or panicles that produce brightly colored flowers, depending on the species [7,9,12]. The calyx is tubular or bell-shaped without a bearded throat, divided into two lips (that is why the name of labiates): the upper whole or tridentate and the lower cleft. The corolla is usually bilabiate. The stamens are two short structures with bicellular anthers. Many species have trichomes (hairs) on the surface of the leaves, stems, and flowers [7,9,12].
Several Salvia species have great economic importance due to their edible, aromatic, and medicinal properties. Many of these species contain high amounts of essential oils, phenolic compounds, antioxidants, and other valuable chemical constituents [5]. The main compounds described in the Salvia species are terpenoids and flavonoids. Aerial parts, especially flowers and leaves, contain flavonoids, triterpenoids, and monoterpenes, while the roots contain primarily diterpenoids [7,9,10]. Salvia species have been used since ancient times for different ailments, ranging from aches to epilepsy, and the primary uses are for treating colds, bronchitis, tuberculosis, hemorrhages, and menstrual disorders, among others [7,9]. The Mexican Salvia species are highly valued for their medicinal, nutritional, and ritualistic uses and are often used as part of vernacular medicine or in mystical/religious rituals. Prominent examples are Salvia divinorum (“planta de la pastora”), which is a hallucinogen plant used in rituals by the Mazatecas, an endemic population in the northeastern of Oaxaca [13], and Salvia hispanica (chia), which is widely used as a food source since pre-Hispanic times [14].
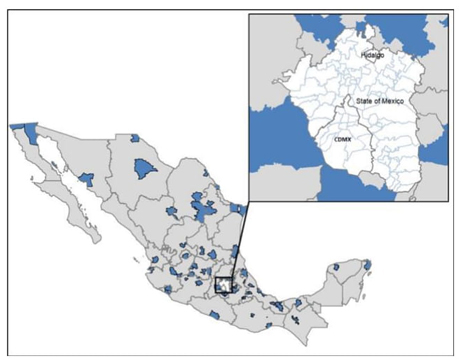
Ramamoorthy (2001) botanically identified 33 Salvia species in the Valle de México [9] (Table 1). The Valle de México has an altitude of 2,240 meters (7,350 ft), covering around 7,866 km2, and includes 16 town halls in Mexico City, 59 municipalities of the State of Mexico, and one municipality in the State of Hidalgo [15]. Geographically, it is located between the Anahuac Lake and Volcano Region of the physiographic province of the Neo-volcanic Axis and is surrounded by the mountains of Monte Alto, Monte Bajo, and Las Cruces, as well as the Sierra Nevada and Chichinauhtzin mountain range (Fig. 1). This surface presents intermountain, valleys, plateaus, and ravines, as well as semi-deep land, in which are located the lakes of Texcoco, Xochimilco, and Chalco. There are also isolated topographic prominences, such as the "Cerro de la Estrella," the "Cerro del Peñón," and the "Cerro de Chapultepec." The Valle de México also represents the most populated region of Mexico, with more than 20 million inhabitants, who often agree with these species despite their lack of knowledge about their medical uses and properties. In this region, 33 species of Salvia had been recorded [9]. Although several researchers worldwide have contributed ethnobotanical, phytochemical, and pharmacological information for some of these species [5,16-19], it is still necessary to continue working on the supplementation and organization of this information. In certain instances, these species exhibit a broad range of botanical synonyms or variations in their nomenclature, which can result in some confusion, like S. polystachya, that have 12 botanical synonymies and 11 common names. Therefore, their taxonomic identification often represents a problem. This review aims to organize and synthesize the ethnobotanical, pharmacological, and phytochemical knowledge of the 33 Salvia species described by Ramamoorthy in the Valle de México [9]. These species have been extensively documented by diverse research groups in Mexico and other regions, including Europe and Asia [20-25]. Our primary objective is to critically analyze and compare these data, advancing their study at the ethnopharmacological, phytochemical, and therapeutic levels. By doing so, we seek to validate the traditional uses attributed to these remarkable plant species.
Table 1 Scientific name of the 33 Salvia species described by Ramamoorthy in the Valle de México [9].
| 1. | S. axillaris Moc & Sessé ex Benth. | 2. | S. carnea Kunth. | 3. | S. chamaedryoides Cav. |
| 4. | S. circinata Cav. | 5. | S. concolor Lamb. ex Benth | 6. | S. elegans Vahl. |
| 7. | S. filifolia Ramamoorthy | 8. | S. fulgens Cav. | 9. | S. gesneriiflora Lindl & Paxton |
| 10. | S. helianthemifolia Benth. | 11. | S. hirsuta Jacq. | 12. | S. hispanica L. |
| 13. | S. keerlii Benth. | 14. | S. laevis Benth. | 15. | S. lavanduloides Kunth. |
| 16. | S. leucantha Cav. | 17. | S. melissodora Lag. Me Vaugh. | 18. | S. mexicana L. |
| 19. | S. microphylla H.B.&H. | 20. | S. misella Kunth. | 21. | S. mocinoi Benth. |
| 22. | S. moniliformis Fern. | 23. | S. oreopola Fern. | 24. | S. patens Cav. |
| 25. | S. polystachya Cav. | 26. | S. prunelloides Kunth. | 27. | S. pulchea DC. |
| 28. | S. reflexa Hornem. | 29. | S. reptans Jacq. | 30. | S. stachyoides Kunth. |
| 31. | S. tiliifolia Vahl. | 32. | S. tubifera Cav. | 33. | S. verbenacea L. |
Methodology
Information from the 33 species of Salvias recorded by Ramamoorthy in the Valle de México [9] was obtained from diverse databases, such as Web of Science, Google Scholar, Google Books, Scopus, ScienceDirect, SpringerLink, Wiley Online, PubMed, textbooks, taxonomic reviews, university theses, and SciFinder. With the obtained data, such as botanical characteristics, botanical synonymy, empirical uses, and biological activities, a meta-analysis was performed, and the compounds isolated were documented.
Results and discussion
Botanical synonymy, popular names, and distribution
Plant nomenclature is ruled by the International Code of Botanical Nomenclature, which aims to provide a correct and accepted name for a taxon based on publication priority. The application of the norms of the code and the taxonomic studies that imply some change in the circumscription of the taxon result in changes in nomenclature and botanic synonymy, such that in the study of medicinal plants, the synonymies can be a problem by creating confusion in any investigation [26,27]. Therefore, the first step was identifying which species had synonyms or some variation (Table 2), highlighting that many of the Salvia species studied (84 %) presented some of these conditions. The plant with a significant number of synonyms was S. polystachya, with 12 synonymies, seven variations, and three subspecies, followed by S. carnea, with 13 synonymies and two variations: S. fulgens, with 11 synonymies and three variations, and S. mexicana with nine synonymies and three variations. This situation illustrates how easy it is to make mistakes when working with species Salvia, so taxonomic identification is a priority before any study. Another frequent problem for species identification focuses on popular or common names with ethnobotanical relevance. However, In Mexico, the popular names vary depending on the region where they are found. Of the included species in the present study, 57.6% had more than one popular name, where "mirto," "chia," and "salvia" are the most used. S. microphylla is recognized with 18 popular names, followed by S. lavanduloides with 15 names. The consulted bibliography recorded a single popular name for five species; no popular name for nine species was documented. The importance of the correct name of the plant species consists in being able to avoid confusion or even a duplicate work for incorrect use of the names; in the case of S. circinata (S. amarissima), it is possible to observe publications with both names; it is essential to corroborate the correct and accepted scientific name of the plant. [28,29].
The geographical distribution of these 33 species is not exclusive to the Valle de México. Most of them are distributed in several states of Mexican territory (Table 2). The data indicate that in the state of Michoacan, there are around 27 species, followed by the State of Mexico with 18, and the State of Hidalgo with 17. The best-distributed species in Mexico are S. polystachya and S. hispanica (Table 2). These data are essential if we consider that the same common name can be used to name different species of the same or other genera, or a single species can receive several names, which vary from one region to another, and because some species share the same distribution in the Valle de México, including Ciudad de México, Estado de México, and Hidalgo. We agree with [2] that research focused on medicinal plants requires essential botanical assistance, especially in taxonomy and nomenclature.
The distribution of the plants in the different regions also affects the kind and concentration of secondary metabolites in the plant. In S. hispanica, the weather, altitude, humidity, and nutrients of the region of Veracruz, which is in the East of Mexico, with significant humidity, being a jungle area, are not the same conditions that the State of Durango, in the north of the country, with a desert climate. The different territorial, geographic, and climatic conditions provoke changes in the metabolites, and it may affect all the Salvia species that have a wide distribution in the country, even in the same species with different geographical distribution. These changes are a significant area of study to determine the impact of the different conditions in synthesizing metabolites of pharmacological interest [28,30].
Table 2 Scientific name, botanical synonymy, popular names, and distribution of Salvia species from the Valle de México.
NS = Not specified
Botanical characteristics
The different species of the Salvia genus have similar morphological characteristics [31]. Table 3 enlists some botanical characteristics reported by Ramamoorthy in 2001 [9], complemented by Lara-Cabrera [32]. Most of these species (75.5 %) are "perennial herbaceous" of 0.15 m (S. helianthemifolia Benth.) to 4 m (S. fulgens Cav.) and can be found at different altitudes ranging from 650 to 2400 meters. In the different species, the leaves vary in size from 5-8 mm to 50-140 mm long and have various shapes, from elliptical to ovate. The flowering time in plants is of great importance; it involves essential changes in metabolism and the translocation of nutrients, ensuring the production of seeds and, therefore, the survival of the species [33,34]. In the salvias studied, it was possible to document data on flowering times for 14 species, less than half (42.2 %) of the studied plants, and no pattern was observed in these data, so it is possible to find different species of Salvia in bloom throughout the year. The colours of the bilabiate calyx and the corolla are also diverse (red, pink, blue, lilac, and white), although the blue corolla is predominant (69.7 %). However, in at least nine species (27.3 %), the colour of the corolla can be variable. Habitat and altitude, among other abiotic and biotic environmental factors, can modify their physical or chemical characteristics, impacting the secondary metabolism's evolution and phenotypic plasticity [35].
Considering the similarity observed in the distinct Salvia species, it is essential and necessary to take special care in the taxonomic identification to avoid correlation errors and extrapolation [26], which could put in risk the reproducibility and continuation of pharmacological and chemical studies with these species [2,30]. The chemical composition varies between species, seasons, and habitats, as well as the stage of development or the plant organ (ontogeny of leaves, flowers, and fruits), factors that lead to significant qualitative differentiations where the composition can undergo significant changes. Some components can vary from traces (10 %) in the initial stages up to 50-70 % in the full bloom stage [36], which should be considered in phytochemical studies.
Table 3 Botanical characteristics of Salvia species from Valle de México. [10]
| Plant name | Habitat | Leaves | Flowers | Flowering | Altitude range (meters) | Vegetation |
|---|---|---|---|---|---|---|
| S. axillaris |
|
|
|
NS | 2400-2800 |
|
| S. carnea |
|
|
|
Sep - May | 2800-3500 |
|
| S. chamaedryoides |
|
|
|
NS | 2300-2800 |
|
| S. circinata |
|
|
|
Aug - Nov | 1650-2800 |
|
| S. concolor |
|
|
|
Sep | 2650-3300 |
|
| S. elegans |
|
|
|
NS | 2550-3100 |
|
| S. filifolia |
|
|
|
Jul - Nov | 2390-2800 |
|
| S. fulgens |
|
|
|
NS | 2650-3400 |
|
| S. gesneriiflora |
|
|
|
Oct - May | 1950-3200 |
|
| S. helianthemifolia |
|
|
|
Aug - Apr | 2000-3200 |
|
| S. hirsuta |
|
|
|
Jun - Oct | 2250 - 2600 |
|
| S. hispanica |
|
|
|
Sep - Nov | 2050-2500 |
|
| S. keerlii |
|
|
|
Jul - Dec | 2170-3100 |
|
| S. laevis |
|
|
|
Jun - Nov | 1520 -3200 |
|
| S. lavanduloides |
|
|
|
Oct - May | 1650 -3300 |
|
| S. leucantha |
|
|
|
Sep - Dec | 1000-2800 |
|
| S. melissodora |
|
|
|
Jul - Mar | 1550 - 2600 |
|
| S. mexicana |
|
|
|
NS | 2250 - 3000 |
|
| S. microphylla |
|
|
|
NS | NS |
|
| S. misella |
|
|
|
NS | 650-2250 |
|
| S. mocinoi |
|
|
|
NS | 2400-2650 |
|
| S. moniliformis |
|
|
|
NS | 2300-2800 |
|
| S. oreopola |
|
|
|
NS | 2600 |
|
| S. patens |
|
|
|
NS | 2500-2800 |
|
| S. polystachya |
|
|
|
Jun.-Nov. | 2250-2900 |
|
| S. prunelloides |
|
|
|
NS | 2400-3600 |
|
| S. pulchea |
|
|
|
NS | 2350-2400 |
|
| S. reflexa |
|
|
|
NS | 2250-2600 |
|
| S. reptans |
|
|
|
NS | 2300-2700 |
|
| S. stachyoides |
|
|
|
NS | 2800-3100 |
|
| S. tiliifolia |
|
|
|
NS | 2300-2600 |
|
| S. tubifera |
|
|
|
NS | 2300 |
|
| S. verbenacea |
|
|
|
NS | 2300 | NS |
NS: Not specified
Traditional uses and pharmacology
Regarding Traditional Medicine, Mexico is recognized as the second most important country in the world that uses that kind of therapy, with a tremendous ancestral tradition and richness in the use of medicinal plants to treat different diseases and for ritual, only right after China [37]. The different ethnic groups living in Mexico maintain deep and ancestral knowledge of medicinal plants as traditional practices and beliefs about diseases and cures [37]. This cultural legacy dates back to published works written in the 16th century and still survives in modern Mexico [38]. The use and knowledge of medicinal plants by the Mexican population is a common practice for three main reasons: 1) the need to treat diseases, 2) an extensive flora, and 3) the existence of many indigenous groups that preserve their traditions [39]. Unsurprisingly, the population turns to various species of Salvia to treat diverse ailments, given the botanical abundance and diversity these plants represent in Mexico.
Table 4 provides a detailed account of the ethnobotanical uses we have documented for the 33 Salvia species included in this study. Based on our data, we can infer that leaves are the most frequently employed part of various Salvia species. This preference arises due to the ease of leaf collection and the minimal impact on plant viability. In some cases, the complete plant, or other parts of the plant (roots and steam) used are specifically described. Comparing the metabolites expressed in different plant parts is essential to comprehensively understand metabolite synthesis. Investigating whether specific compounds are localized to certain plant regions or distributed uniformly across the entire plant represents a critical avenue for further research.
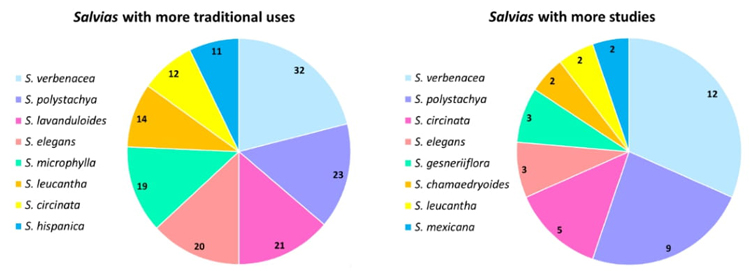
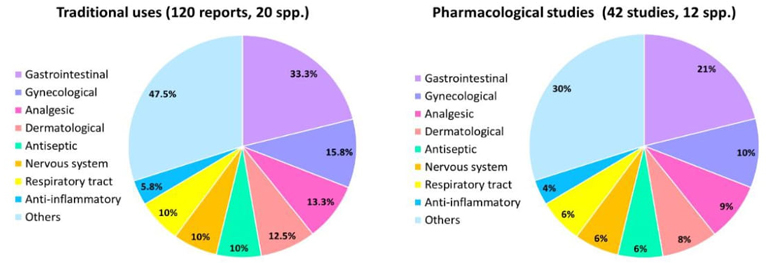
Of the 33 species registered in the Valle de México, 20 are used for everyday purposes, mainly S. verbenacea, S. polystachya, S. lavanduloides, and S. elegans (Fig. 2). These species' most frequently reported uses were gastrointestinal diseases, such as stomach pain and diarrhea. Notably, diarrhea remains a significant health problem in Mexico, ranking as the second most common ailment across all age groups [40]. Additionally, these Salvia species find application in promoting childbirth, managing gynecological issues (such as menstrual colic), and serving as antipyretic agents. Furthermore, they are utilized for wound treatment, diabetes management, and respiratory conditions (Table 4).
Pharmacological studies play an essential role in unraveling the therapeutic potential of medicinal plants. In the case of Salvia species, approximately 13 out of the 33 species (representing 39 %) have undergone pharmacological scrutiny involving investigations into extracts, fractions, and isolated compounds. A total of 28 distinct pharmacological effects have been documented, with notable prominence given to antioxidant, anti-bacterial, and anti-hyperglycemic properties. Among the studied species (Fig. 2), S. verbenacea stands out with 11 reported pharmacological activities, followed by S. polystachya (9 activities) and S. circinata (5 activities). The predominant mode of preparation for these species involves herbal infusions or tisanes, in which the bioactive compounds are extracted using water and heat [41].
Table 4 provides a comprehensive overview of pharmacological studies across diverse Salvia species. Notably, cytotoxic and anticancer activities emerge as promising avenues, offering new prospects for cancer treatment. Some species exhibit anti-bacterial, anti-fungal, and anti-parasitic effects. Other species are also used for treating fever, rheum, and edema, while their anti-inflammatory, antinociceptive, and antipyretic actions are similar to non-steroidal anti-inflammatory drugs (AINEs). The actions at the level of the nervous system, derived by their traditional uses of cultural connotation ("susto," "mal de ojo," "aire"), were recorded as anti-depressants, anxiolytics, and neuroprotective in different experimental conditions.
Interestingly, our pharmacological investigations align with the effects observed in traditional medicine. Specifically, many studies have focused on medicinal plant species' gastrointestinal and gynecological effects. However, it is crucial to emphasize that the number of research validating these plants' traditional uses is limited. For example, while 120 traditional uses have been documented for 20 species, only 42 specific studies have been conducted on 12 Salvia species (Fig. 3). Even more pertinent is that only a handful of these studies have developed into identifying the pure compounds responsible for those effects. Some species have yet to be studied; for example, based on this work, species such as S. filifolia and S. laevis lack pharmacological studies that support the attributed medicinal uses; furthermore, no specific compounds have been identified in these species.
Our comprehensive review underscores the imperative to validate the diverse traditional uses attributed to Salvia species. Certain species, such as S. polystachya and S. circinata, have been associated with hypoglycemic effects through the inhibition of α-glucosidases and sodium-dependent glucose cotransporter-1 (SGLT-1) [28,42]. Furthermore, Salvia species find application in hypertension management, with emerging evidence at the vascular level. However, studies supporting these effects in other Salvia species remain scarce and underscore the need for multidisciplinary research, including bioassay-guided studies, to validate all traditional uses.
Table 4 Medicinal uses and pharmacological effects of identified Salvia species from the Valle de México.
| Plant name | Traditional use | Part used | Pharmacological effect | Extract | Ref. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cp | Ap | L | B | S | F | R | Sd | Fr | NS | Ext | Frt | IC | ||||
| S. axillaris | Expectorant | X | [70] | |||||||||||||
| S. chamaedryoides | “Espanto” | X | Anti-bacterial | X | X | X | [71-72] | |||||||||
| Abortive | X | Hypoglycemic | X | X | X | |||||||||||
| “Aire” | X | |||||||||||||||
| S. circinata | “Espanto” | X | Anti-conceptive | X | X | X | [16,29, 71-75] | |||||||||
| “Aire” | X | Anti-hyperglycemic | X | X | ||||||||||||
| Analgesic | X | Anti-inflammatory | X | X | ||||||||||||
| Anti-diabetic | X | Anti-MDR | X | |||||||||||||
| Diarrhea | X | X | Cytotoxic | X | ||||||||||||
| Helminthiases | X | |||||||||||||||
| Lack of appetite | X | |||||||||||||||
| Menstrual colic | X | |||||||||||||||
| Rheumatism | X | |||||||||||||||
| Stomachache | X | |||||||||||||||
| Ulcers | X | |||||||||||||||
| Vomit | X | |||||||||||||||
| S. elegans | “Espanto” | X | X | Anti-hypertensive | X | X | [8,39, 71,72, 76-82] | |||||||||
| “Mal de ojo” | X | Anti-depressant | X | |||||||||||||
| “Aire” | X | Anxiolytic | X | |||||||||||||
| “Aire” (in babies) | X | X | X | |||||||||||||
| Anxiety | X | |||||||||||||||
| Cooling | X | |||||||||||||||
| Cough | X | |||||||||||||||
| Fever | X | |||||||||||||||
| Injured feet | X | |||||||||||||||
| Insomnia | X | X | ||||||||||||||
| Knocking / edema | X | X | X | X | ||||||||||||
| Measles | X | X | X | |||||||||||||
| Pain in the knees | X | |||||||||||||||
| Postpartum | X | X | X | X | ||||||||||||
| Relapse of Ladies | X | |||||||||||||||
| Sick shower | X | X | X | |||||||||||||
| Skin rashes | X | |||||||||||||||
| S. elegans | Stimulate saliva | X | [8,39, 71,72, 76-82] | |||||||||||||
| Stomachache | X | X | X | |||||||||||||
| Vomit | X | |||||||||||||||
| S. filifolia | Deposition | X | [83] | |||||||||||||
| S. fulgens | “Fuegos” induced by fever | X | [39,72] | |||||||||||||
| Sleeping draught | X | X | ||||||||||||||
| Sleeping draught (infants) | X | X | X | |||||||||||||
| S. gesneriiflora | Diarrhea | X | Antioxidant | X | [40,76, 84,85] | |||||||||||
| Stomachache | X | Spasmolytic | X | |||||||||||||
| Anti-inflammatory | X | |||||||||||||||
| S. hispanica | Bile | X | Antioxidant | X | [4,39, 84] | |||||||||||
| Cathartic | X | |||||||||||||||
| Cough | X | |||||||||||||||
| Diarrhea | X | X | ||||||||||||||
| Expulsion of larvae / foreign bodies from the eyes | X | |||||||||||||||
| Eye burns | X | |||||||||||||||
| Labor pain | X | |||||||||||||||
| S. hispanica | Laxative | X | [4,39, 84] | |||||||||||||
| Muscle pain | X | |||||||||||||||
| Nutritional supplement | X | |||||||||||||||
| Spit blood | X | |||||||||||||||
| S. laevis | Kidney diseases | X | [72,76, 86] | |||||||||||||
| Promote conception | X | |||||||||||||||
| S. lavanduloides | “Torzón” | X | [39,72,79,81, 87] | |||||||||||||
| “Aire” | X | |||||||||||||||
| Alopecia | X | X | ||||||||||||||
| Anti-dysentery | X | X | ||||||||||||||
| Antipyretic | X | X | ||||||||||||||
| Bronchitis | X | X | X | |||||||||||||
| Coldness (children) | X | |||||||||||||||
| Controlling vaginal bleeding | X | |||||||||||||||
| Cough | X | X | X | |||||||||||||
| Diarrhea | X | |||||||||||||||
| Fever | X | |||||||||||||||
| S. lavanduloides | Gallbladder condition | X | X | X | [39,72,79,81, 87] | |||||||||||
| Gynecological diseases | X | |||||||||||||||
| Hemostatic | X | X | ||||||||||||||
| Oxytocic | X | X | ||||||||||||||
| Paralysis | X | |||||||||||||||
| Stomachache | X | |||||||||||||||
| Toothache | X | |||||||||||||||
| Vomit | X | |||||||||||||||
| Wash wounds | X | |||||||||||||||
| Whooping cough | X | X | ||||||||||||||
| S. leucantha | “Espanto” | X | Anti-bacterial | X | [4,39, 71,72, 76,87-89] | |||||||||||
| Abortive | X | X | Cytotoxic | X | ||||||||||||
| “Aire” | X | X | ||||||||||||||
| Bile (courage) | X | |||||||||||||||
| Chest/lung pain | X | X | ||||||||||||||
| Cough | X | |||||||||||||||
| Kidney Diseases | X | X | ||||||||||||||
| S. leucantha | Liver disease | X | X | [4,39, 71,72, 76,87-89] | ||||||||||||
| Matrix fall | X | |||||||||||||||
| Menstrual colic | X | |||||||||||||||
| Postpartum | X | |||||||||||||||
| Relapse of ladies | X | |||||||||||||||
| Stomachache | X | X | ||||||||||||||
| Stops menstruation | X | |||||||||||||||
| S. melissodora | Diarrhea | X | [79,90] | |||||||||||||
| Pain | X | |||||||||||||||
| S. mexicana | Bile | X | Anti-inflammatory | X | [72,79,91,92]. | |||||||||||
| Diarrhea | X | Antioxidant | X | |||||||||||||
| Menstrual colic | X | |||||||||||||||
| Promote conception | X | |||||||||||||||
| Stomachache | X | |||||||||||||||
| S. microphylla | “Empacho” | X | X | X | Anti-microbial | X | [39-40,72, 76,83, 84,89, 93,94] | |||||||||
| “Espanto” | X | X | X | X | ||||||||||||
| “Mal de ojo” | X | |||||||||||||||
| S. microphylla | “Aire” | [39-40,72, 76,83, 84,89, 93,94] | ||||||||||||||
| Anti-dysentery | X | X | ||||||||||||||
| Bile | X | |||||||||||||||
| Bone strengthening | ||||||||||||||||
| Diarrhea | X | X | ||||||||||||||
| Earache | X | |||||||||||||||
| Gynecological diseases | X | |||||||||||||||
| Headache | X | |||||||||||||||
| Insomnia | X | |||||||||||||||
| Leg scald | X | |||||||||||||||
| Menstrual colic | X | X | ||||||||||||||
| Nerves | X | |||||||||||||||
| Postpartum baths | X | X | ||||||||||||||
| Promote conception | X | X | ||||||||||||||
| Stomachache | X | X | ||||||||||||||
| Waist pain | X | |||||||||||||||
| S. misella | Bruising | X | X | Antioxidant | X | [39,95,96] | ||||||||||
| Erysipelas | X | X | ||||||||||||||
| Skin rashes | X | |||||||||||||||
| Warts | X | X | ||||||||||||||
| Wash wounds | X | X | ||||||||||||||
| S. patens | Children's restroom (3 months) | X | [77] | |||||||||||||
| Infected wounds | X | |||||||||||||||
| Joint heating | X | |||||||||||||||
| S. polystachya | Anti-abortion | X | Anti-protozoal | X | [39-40,72, 76,83, 84,89, 93,94] | |||||||||||
| Anti-diuretic | X | Anti-amoebic | X | |||||||||||||
| Anti-dysentery | X | Anti-giardial | X | |||||||||||||
| Anti-gastric | X | Anti-hyperglycemic | X | X | X | |||||||||||
| Anti-hemorrhagic | X | Antioxidant | X | |||||||||||||
| Anti-malarial | X | Acts over dermal fibroblast expression | X | |||||||||||||
| Antipyretic | X | Protective (Cerebral ischemia) | X | |||||||||||||
| Scabies | X | α-Glucosidase Inhibitor | X | |||||||||||||
| S. polystachya | Diarrhea | X | SGLT1 Inhibitor | X | [39-40,72, 76,83, 84,89, 93,94] | |||||||||||
| Diuretic | X | |||||||||||||||
| Emollient | X | |||||||||||||||
| Flu | X | |||||||||||||||
| Gastritis | X | |||||||||||||||
| Hair growth | X | |||||||||||||||
| Headache | X | |||||||||||||||
| Menstrual colic | X | |||||||||||||||
| Nosebleed | X | X | ||||||||||||||
| Parasites | X | |||||||||||||||
| Promote conception | X | |||||||||||||||
| Purgative | X | X | ||||||||||||||
| Stomachache | X | |||||||||||||||
| Wounds disinfect | X | |||||||||||||||
| Wound healing | X | |||||||||||||||
| S. reflexa | Stomach affections | X | [72] | |||||||||||||
| S. reptans | Diarrhea | X | X | X | Anti-bacterial | X | X | X | [72,94] | |||||||
| Fever | X | |||||||||||||||
| Stomachache | X | X | X | |||||||||||||
| Swelling | X | X | ||||||||||||||
| Twists | X | |||||||||||||||
| Wound healing | X | |||||||||||||||
| S. tiliifolia | Abscesses | X | Neuroprotective | X | [39,91,92,95, 96,101] | |||||||||||
| Mumps | X | |||||||||||||||
| Snake bite | X | |||||||||||||||
| Vomit | X | |||||||||||||||
| S. verbenacea | Abscesses | X | X | Anti-bacterial | [20] | |||||||||||
| “Aire” | X | Anticancer | ||||||||||||||
| Anti-hypertensive | X | Anti-fungal | ||||||||||||||
| Antipyretic | X | Anti-hemolytic | ||||||||||||||
| Anti-rheumatic | X | Anti-hyperglycemic | ||||||||||||||
| Antiseptic | X | Anti-hypertensive | ||||||||||||||
| Anti-spasmodic | X | X | Anti-leishmanial | |||||||||||||
| S. verbenacea | Anti-sweat | X | X | Antioxidant | [20] | |||||||||||
| Anxiety | X | Anti-parasitic | ||||||||||||||
| Astringent | X | X | Immunomodulatory | |||||||||||||
| Carminative | X | X | Inhibitory effect of xanthine oxidase | |||||||||||||
| Wound healing | X | X | X | X | X | Skin effect | ||||||||||
| Cooling | X | |||||||||||||||
| Contusion | X | |||||||||||||||
| Cough | X | |||||||||||||||
| Dermatological | X | |||||||||||||||
| Diabetes | X | |||||||||||||||
| Digestive problems | X | X | X | |||||||||||||
| Disinfectant | X | X | ||||||||||||||
| Diuretic | X | |||||||||||||||
| Fever | X | |||||||||||||||
| Genitourinary | X | |||||||||||||||
| Healing | X | |||||||||||||||
| Healing of burns | X | X | ||||||||||||||
| S. verbenacea | Insomnia | X | [20] | |||||||||||||
| Laryngitis | X | |||||||||||||||
| Menstrual colic | X | |||||||||||||||
| Respiratory problems | X | |||||||||||||||
| Stomachache | X | |||||||||||||||
| Vulnerary | X | X | ||||||||||||||
| Wound treatment | X | |||||||||||||||
| Wound eyes | X | |||||||||||||||
Cp = Complete plant; Ap = Aerial parts, L = leaf; B = Branch; S = Steam; F = Flower; R = Root; Sd = Seed; Fr = Fruit; NS = Not specified; Ext = Extract; Fr = Fraction; IC = Isolated compound
Phytochemical studies
During the 1980s and 1990s, several research groups in Mexico, led by Alfredo Ortega, Lydia Rodriguez-Hahn, and Baldomero Esquivel, initiated innovative research focused on identifying compounds from extracts of Mexican sages. These first studies laid the foundation for subsequent research due to the rich content of secondary metabolites, including terpenoids and flavonoids. [43-47]. The aerial parts of these Salvia species, especially the flowers and leaves, harbor phenolic compounds, including flavonoids and terpenoids (such as monoterpenoids, diterpenoids, and triterpenoids); interestingly, diterpenoids were predominantly localized in the roots [46].
In conjunction with other phytochemical studies, we compiled information in Table 5 from 56 sources that report on compounds from 20 Salvia species, resulting in a total of 315 identified compounds (Fig. 4). Among these, S. leucantha stands out with an impressive 92 reported compounds, followed closely by S. verbenacea (81 compounds) and S. circinata (34 compounds). Notably, 43 of these compounds are described in more than one species, highlighting β-sitosterol, as well as ursolic and oleanolic acids that were reported in 8 and 7 different species of Salvia, respectively, compounds that have been identified as the most common terpenes in the Salvia genus [45,46], evidencing the phylogenetic relationships in these species.
Phenolic compounds and terpenoids are the main components in fruits, vegetables, and various spices used for nutritional purposes [48]. Interestingly, the therapeutic active principles in several plant-derived medicinal extracts are also flavonoids and terpenoids [49,50]. In plants, terpenoids exhibit the most remarkable structural diversity, which includes diverse subclassifications. For example, the diterpenoids could be classified as clerodanes, kauranes, abietanes, or casbanes, to name a few [51]. They provide a chemical defense against environmental stress and a mechanism to repair wounds and injuries. In addition, mainly monoterpenes are usually responsible for the characteristic fragrance of many plants (pollinator attraction). On the other hand, high concentrations of terpenoids can be toxic and, therefore, constitute an essential weapon against herbivores and pathogens, such as anti-food or insecticides [44,51-54].
In recent years, there has been growing pharmacological interest in these compounds due to their diverse biological activities that can focus on the prevention and therapy of various diseases, as documented in various studies. Our research data further support this trend, revealing that many of the 315 compounds documented (Table 5) are terpenoids (mainly diterpenes, sesquiterpenes, and monoterpenes). While the phytochemical studies on Salvia species do not explicitly focus on identifying biological effects, some working groups have determined that diterpenes stand out mainly for their anti-inflammatory, antitumor, anti-diabetic, and antiviral activities. The monoterpenes show anti-microbial activity against pathogens such as Mycobacterium tuberculosis [55] and inhibit the growth of fungi such as Rhizoctonia solani [56]. For their part, sesquiterpenes have been shown to have a broad spectrum of biological activities that include anti-microbial, cytotoxic, anti-inflammatory, anti-bacterial, anticancer, antiviral, and anti-fungal properties, in addition to exerting effects on the central nervous and cardiovascular systems [57].
As previously mentioned, among the most reported compounds in these Salvias species are the pentacyclic triterpenes: the ursolic acid, a triterpenoid, is extensively studied and boasts a multitude of biological effects: it acts as an insulin mimetic, insulin sensitizer, anti-inflammatory, antioxidant, anticancer, anti-obesity, anti-diabetic, antiangiogenic, anti-microbial, cardioprotective, neuroprotective, hepatoprotective, anti-skeletal muscle atrophy and thermogenic [31,58-60]. Likewise, oleanolic acid, an isomer of ursolic acid, has effects such as hepatoprotective, anti-inflammatory, anti-hyperglycemic, antioxidant, anticancer, and neuroprotective [42,60,61]. Another noteworthy compound reported in various Salvias species is β-sitosterol, a phytosterol whose chemical structure is similar to cholesterol, which has diverse biological actions described that include anxiolytic, sedative, analgesic, angiogenic, anthelmintic, antimutagenic, immunomodulatory, anti-bacterial, anticancer, anti-inflammatory, genotoxic, hypolipidemic, hypocholesterolemic, hepatoprotective, and respiratory diseases; furthermore, β-sitosterol promotes wound healing and exhibits antioxidant and anti-diabetic effects [62,63].
Another important group of compounds in the Salvia species are the flavonoids, a class of polyphenolic compounds that are naturally biosynthesized in plants. The subgroups of flavonoids include flavones, flavonols, flavanones, flavanonols, anthocyanidins, flavanols, and isoflavones [64,65]. Flavonoids have long been known to be synthesized at specific sites. They are responsible for the color and aroma of flowers and fruits to attract pollinators, protect plants from different biotic and abiotic stresses, and act as unique UV filters, detoxifying agents, and defensive anti-microbial compounds [64-67]. These natural products are well known for their beneficial effects on health, such as anti-diabetic, antiulcer, antiviral, antioxidant, anti-inflammatory, antimutagenic, cytotoxic, and anticarcinogenic [64,65,68].
The diverse compounds described from the Salvia species (Fig. 4) are evidence of structural variability, mainly from the terpenoid structures, where a minimum change in the position or the presence and absence of some functional groups changes the type of compound reported. This, in turn, could generate a different activity that can be observed in biological assays [28]. Besides, some of the same compounds in different species could not be at the same concentration [30,69] and might affect the expected effect.
Table 5 Isolated compounds of Salvia species from Valle de México.
| Scientific name | Parts used | Extract(s) used | No. | Classification | Compounds | Ref.. |
|---|---|---|---|---|---|---|
| S. axillaris |
|
Acetone | 1 | Terpenoid | 20-nor-abietane cryptotanshinone (cryptotanshinone) | [23, 102] |
| S. chamaedryoides | Aerial parts | Dichloromethane | - | - | Furano diterpenes | [22] |
| 2 | Terpenoid | 7α-hydroxybacchotricuneatin A | ||||
| 3 | Polyphenol | Galdosol | ||||
| 4 | Polyphenol | Rosmanol | ||||
| 5 | Terpenoid | Salvimicrophyllin B | ||||
| 6 | Terpenoid | Splendidin C | ||||
| 7 | Terpenoid | Tilifodiolide | ||||
| S. circinata |
|
Acetone: Methanol Ethyl acetate Hexane Methanol Aqueous | 8 | Terpenoid | (E)-pinocarvyl acetate | [18, 23, 29, 73, 103-105] |
| 9 | Flavonoid | 2-(3,4-dimethoxy phenyl)-5,6-dihydroxy-7-methoxy-4H-chromen-4-one | ||||
| 10 | Aromatic | 3-methoxy-p-cymene | ||||
| 11 | Flavonoid | 5,6,4´-trihydroxy-7,3´-dimethoxyflavone | ||||
| S. circinata | Aerial parts Flowers Leaves |
|
12 | Flavonoid | 5,7-O-diacetylacacetin | [18, 23, 29, 73, 103-105] |
| 13 | Flavonoid | 6-hydroxy luteolin | ||||
| 14 | Terpenoid | Acetylamarissinin B | ||||
| 15-21 | Terpenoid | Amarisolide A-G | ||||
| 22-25 | Terpenoid | Amarissinins A-D | ||||
| 26 | Flavonoid | Apigenin | ||||
| 27 | Flavonoid | Apigenin-7-O-β-D-glucoside | ||||
| 28 | Polyphenol | Caffeic acid | ||||
| 29 | Polyphenol | Chlorogenic acid | ||||
| 30 | Phenol | Ferulic acid | ||||
| 31 | Terpenoid | Germacrene D | ||||
| 32 | Flavonoid | Iso-quercitrin | ||||
| 33 | Terpenoid | Oleanolic acid | ||||
| 34 | Flavonoid | Pedalitin | ||||
| 35 | Flavonoid | Phloretin | ||||
| 36 | Flavonoid | Phlorizin | ||||
| 37 | Flavonoid | Quercetin | ||||
| 38 | Phenylpropanoid | Rosmarinic acid | ||||
| 39 | Flavonoid | Rutin | ||||
| 40 | Terpenoid | Spathulenol | ||||
| 41 | Terpenoid | Teotihuacanin | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 43 | Terpenoid | α-amyrin | ||||
| 44 | Terpenoid | α-bourbonene | ||||
| 45 | Terpenoid | α-caryophyllene | ||||
| 46 | Terpenoid | β-caryophyllene | ||||
| 47 | Terpenoid | β-selinene | ||||
| S. circinata |
|
|
48 | Terpenoid | β-sitosterol | [18, 23, 29, 73, 103-105] |
| 49 | Terpenoid | δ-elemene | ||||
| S. elegans |
|
Aqueous ethanol | 50 | Alcohol | 2-propanol | [8,80,82,106] |
| 51 | Flavonoid | 3-acetoxy-7-methoxyflavone | ||||
| 52 | Alcohol | 3-octanol | ||||
| 53 | Amino acid | Cystine | ||||
| 31 | Terpenoid | Germacrene D | ||||
| 54 | Terpenoid | Hederagenin (3β,23-dihydroxyolean12-en-28-oic) | ||||
| 55 | Terpenoid | Linalool | ||||
| 56 | Fatty acid | Linoleic acid | ||||
| 57 | Fatty acid | Linolenic acid | ||||
| 58 | Amino acid | Lysine | ||||
| 59 | Amino acid | Methione | ||||
| 33 | Terpenoid | Oleanolic acid | ||||
| 40 | Terpenoid | Spathulenol | ||||
| 60 | Aldehyde | trans-3-hexenal | ||||
| 61 | Terpenoid | trans-ocimene | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 46 | Terpenoid | β-caryophyllene | ||||
| S. fulgens | Aerial parts | Acetone | 62 | Terpenoid | 10β-hydroxybacchotricuneatin A (Bacchotricuneatin A) | [19, 23, 24, 107-110] |
| 63 | Terpenoid | nt-19-acetoxy-15,16-epoxy-6-hydroxy-3,13(16),14-clerodatrien-18-al | ||||
| 64 | Terpenoid | ent-19-O-acetoxy-15,16-epoxy-3,13(16),14-clerodatrien-6,18-diol | ||||
| 65 | Terpenoid | 7α-hydroxy-neoclerodane-3,13-diene-18,19:15,16-diolide | ||||
| S. fulgens | Aerial parts | Acetone | 66 | Terpenoid | Dehydrokerlin | [19, 23, 24, 107-110] |
| 67 | Terpenoid | Salvifulgenolide | ||||
| 68 | Terpenoid | Salvigenolide | ||||
| 69 | Terpenoid | Sandaracopimaric acid | ||||
| 70 | Terpenoid | trans-1,2-dihydrosalvifaricin | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. gesneriiflora | Aerial parts |
|
- | - | Alkaloids | [85, 111] |
| - | - | Anthraquinones | ||||
| - | - | Coumarins | ||||
| - | - | Saponins | ||||
| 28 | Polyphenol | Caffeic acid | ||||
| 29 | Polyphenol | Chlorogenic acid | ||||
| 38 | Phenylpropanoid | Rosmarinic acid | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 68 | Terpenoid | Salvigenolide | ||||
| S. hirsute | Roots | Acetone | 71 | Terpenoid | 14-deoxycoleon U | [112] |
| 72 | Terpenoid | 7α-acetoxy-royleanone | ||||
| 73 | Terpenoid | 8,11,13-abietatriene | ||||
| 74 | Terpenoid | 8,13-abietadiene | ||||
| 75 | Terpenoid | Cryptojaponol | ||||
| 76 | Terpenoid | Demethylcryptojaponol | ||||
| 77 | Terpenoid | Royleanone | ||||
| 78 | Terpenoid | Salviphlomone | ||||
| 79 | Terpenoid | Sugiol | ||||
| 80 | Terpenoid | Taxodione | ||||
| S. hispánica | Seeds |
|
28 | Polyphenol | Caffeic acid | [4, 113, 114] |
| 29 | Phenol | Chlorogenic acid | ||||
| 81 | Flavonoid | Daidzin | ||||
| 82 | Polyphenol | Gallic acid | ||||
| 83 | Flavonoid | Kaempferol | ||||
| 84 | Ethyl ester | Protocatechuic ethyl ester | ||||
| 37 | Flavonoid | Quercetin | ||||
| 38 | Phenylpropanoid | Rosmarinic acid | ||||
| 85 | Fatty acid | α-linolenic acid | ||||
| S. keerlii | Aerial parts | Acetone | 86 | Terpenoid | Kerlin | [23, 108, 115] |
| 87 | Terpenoid | Kerlinic acid | ||||
| 88 | Terpenoid | Kerlinolide | ||||
| S. lavanduloides |
|
|
72 | Terpenoid | 7α-acetoxy-royleanone | [19, 23, 108, 111, 116-118] |
| 89 | Terpenoid | Horminone | ||||
| 90-94 | Terpenoid | Salvianduline A-E | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. leucantha |
|
|
95 | Terpenoid | 1,10-di-epi-cubenol | [19, 21, 24, 39, 76, 88, 89, 107, 118-120] |
| 96 | Terpenoid | 1,8-cineole | ||||
| 97 | Alcohol | 1-octen-3-ol | ||||
| 98 | Terpenoid | 20-hydroxydugesin B | ||||
| 99 | Terpenoid | 2-epi-6,7-dihydrosalviandulin E | ||||
| 100 | Terpenoid | 3-epi-tilifodiolide | ||||
| 101 | Ketone | 3-octanone | ||||
| 102 | Terpenoid | 3β-methoxyisopuberulin | ||||
| 103 | Ketone | 4-methylene-isophorone | ||||
| 104 | Terpenoid | 6,7-dehydrodugesin A | ||||
| S. leucantha | Aerial parts Flowers | Acetone Chloroform Methanol Hexane | 105 | Terpenoid | 6,7-dehydrodugesin B | [19, 21, 24, 39, 76, 88, 89, 107, 118-120] |
| 106 | Terpenoid | 6,7-dihydrosalviandulin E | ||||
| 107 | Terpenoid | 7-epi-α-eudesmol | ||||
| 108 | Aromatic | Apiole | ||||
| 109 | Terpenoid | Aromadendrene | ||||
| 110 | Terpenoid | Bicyclogermacrene | ||||
| 111 | Terpenoid | Borneol | ||||
| 112 | Terpenoid | Bornyl acetate | ||||
| 113 | Terpenoid | Camphene | ||||
| 114 | Terpenoid | Cedrene | ||||
| 115 | Terpenoid | cis-cadin-4-en-7-ol | ||||
| 116 | Terpenoid | cis-muurola-3,5-diene | ||||
| 117 | Terpenoid | Citral | ||||
| 118 | Terpenoid | Citronellal | ||||
| 119 | Terpenoid | Citronellol | ||||
| 120 | Ketone | Dehydrosabinaketone | ||||
| 121 | Terpenoid | De-O-acetylsalvigenolide | ||||
| 122 | Benzodioxol | Dillapiol | ||||
| 123 | Terpenoid | Dugesin B | ||||
| 100 | Terpenoid | 3-epi-tilifodiolide | ||||
| 124 | Terpenoid | Eremoligenol | ||||
| 125 | Terpenoid | Eudesma-4(15)7-dien-1β-ol | ||||
| 126 | Terpenoid | Geraniol | ||||
| 127 | Terpenoid | Geranyl acetate | ||||
| 128-129 | Terpenoid | Germacrene A, B | ||||
| 31 | Terpenoid | Germacrene D | ||||
| 130 | Terpenoid | Globulol | ||||
| S. leucantha |
|
|
131 | Terpenoid | Guaiol | [19, 21, 24, 39, 76, 88, 89, 107, 118-120] |
| 132 | Alcohol | Heptanol | ||||
| 133 | Terpenoid | Hinesol | ||||
| 134 | Terpenoid | Isocaryophyllene | ||||
| 135 | Flavonoid | Isosalipurpol | ||||
| 136 | Terpenoid | Isosalvipuberulin (Isopuberulin) | ||||
| 137 | Terpenoid | Isothujanol | ||||
| 139-142 | Terpenoid | Leucansalvialin F-J | ||||
| 55 | Terpenoid | Linalool | ||||
| 143 | Terpenoid | Linalyl acetate | ||||
| 144 | Terpenoid | Linalyl formate | ||||
| 145 | Terpenoid | neo-α-clovene | ||||
| 146 | Aldehyde | Nonanal | ||||
| 147 | Terpenoid | p-cymene | ||||
| 148 | Flavonoid | Quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside | ||||
| 90-94 | Terpenoid | Salvianduline A-E | ||||
| 149 | Terpenoid | Salvifaricin | ||||
| 150-153 | Terpenoid | Salvileucalin A-D | ||||
| 154 | Terpenoid | Salvileucantholide | ||||
| 155-158 | Terpenoid | Salvileucanthsin A-D | ||||
| 40 | Terpenoid | Spathulenol | ||||
| 159 | Terpenoid | Spiroleucantholide | ||||
| 160 | Terpenoid | Terpinen-4-ol | ||||
| 161 | Terpenoid | Terpinolene | ||||
| 7 | Terpenoid | Tilifodiolide | ||||
| 162 | Terpenoid | Tiliifolin C | ||||
| 163 | Terpenoid | t-muurolol | ||||
| S. leucantha |
|
|
164 | Terpenoid | trans-calamenen-10-ol | [19, 21, 24, 39, 76, 88, 89, 107, 118-120] |
| 165 | Terpenoid | trans-calamenene | ||||
| 166 | Terpenoid | trans-β-farnesene | ||||
| 167 | Terpenoid | Viridiflorol | ||||
| 168 | Terpenoid | α-bulnesene | ||||
| 169 | Terpenoid | α-cadinene | ||||
| 170 | Terpenoid | α-cadinol | ||||
| 171 | Terpenoid | α-copaene | ||||
| 172 | Terpenoid | α-guaiene | ||||
| 173 | Terpenoid | α-humulene | ||||
| 174 | Terpenoid | α-muurolol | ||||
| 175 | Terpenoid | α-pinene | ||||
| 176 | Terpenoid | α-terpineol | ||||
| 177 | Terpenoid | β-acoradiene | ||||
| 178 | Terpenoid | β-atlantol | ||||
| 179 | Terpenoid | β-bourbonene | ||||
| 46 | Terpenoid | β-caryophyllene | ||||
| 180 | Terpenoid | β-copaen-4α-ol | ||||
| 181 | Terpenoid | β-elemene | ||||
| 182 | Terpenoid | β-gurjunene | ||||
| 183 | Terpenoid | β-phellandrene | ||||
| 184 | Terpenoid | β-pinene | ||||
| 185 | Terpenoid | β-thujone | ||||
| 186 | Terpenoid | γ-cadinene | ||||
| 187 | Terpenoid | γ-terpinene | ||||
| 188 | Terpenoid | δ-cadinene | ||||
| 49 | Terpenoid | δ-elemene | ||||
| S. melissodora | Aerial parts |
|
189 | Terpenoid | 1-isopropyl-4b,8,8-trimethyl-9-oxo-4b,5,6,7,8,8a,9,10-octahydrophenanthrene-2,3,10-triyl triacetate | [19, 23, 108, 122, 123] |
| 190 | Terpenoid | 2α-hydroxy-7α-acetoxy-12-oxo-15:16-epoxy-neoclerodan-3,13(16),14-trien-18: 19-olide | ||||
| 191 | Terpenoid | 2β-7α-dihydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 192 | Terpenoid | 2β-acetoxy-7α-hydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 193 | Terpenoid | 2β-hydroxy-7-oxo-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 194 | Terpenoid | 2β-hydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 195 | Terpenoid | 7-oxo-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 196 | Terpenoid | 7α-acetoxy-2β-hydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 197 | Terpenoid | 7α-acetoxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 198 | Terpenoid | 7α-hydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 65 | Terpenoid | 7α-hydroxy-neoclerodane-3,13-diene-18,19:15,16-diolide | ||||
| 199 | Terpenoid | 7β-18,19-trihydroxy-ent-cleroda-3,13-dien-16,15-olide | ||||
| 200 | Terpenoid | 7β-hydroxy-ent-cleroda-3,13-diene-18,19:16,15-diolide | ||||
| 201 | Terpenoid | Brevifloralactone | ||||
| 202 | Terpenoid | Maytenoquinone | ||||
| 203 | Terpenoid | Melisodoric acid | ||||
| 33 | Terpenoid | Oleanolic acid | ||||
| 204 | Terpenoid | Portulide C | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. mexicana |
|
|
205 | Terpenoid | Arbutin | [92] |
| 206 | Terpenoid | Betulinic acid | ||||
| 207 | Terpenoid | Betulinol | ||||
| 208 | Terpenoid | Salvimexicanolide | ||||
| 209 | Terpenoid | Salviolide | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. microphylla |
|
Acetone | 210 | Terpenoid | 12-methoxycarnosic acid | [19, 25, 108, 124, 125] |
| 211 | Terpenoid | 14α-18-dihydroxyisopimaradiene | ||||
| 212 | Terpenoid | 14α-hydroxyisopimaric acid | ||||
| 213 | Phenolic ester | 2-(p-hydroxyphenyl) ethyl eicosaheptanoic acid ester | ||||
| 214 | Terpenoid | 7,15-isopimaradien14α, 18-diol | ||||
| 215 | Terpenoid | 7-oxo-sandaracopimarate | ||||
| 216 | Terpenoid | 7-oxo-sandaracopimaric acid | ||||
| 217 | Terpenoid | 7α-acetoxyisopimara-8(14),15-diene-18-oic acid | ||||
| 218 | Terpenoid | 7α-acetoxysandaracopimaric acid | ||||
| 65 | Terpenoid | 7α-hydroxy-neoclerodane-3,13-diene-18,19:15,16-diolide | ||||
| 219 | Terpenoid | 7α-hydroxysandaracopimaric acid | ||||
| 220 | Terpenoid | 8(14),15-sandaracopimaradien-7α,18-diol | ||||
| 221 | Carcocyclic | 8α-hydroxy-β-eudesmol | ||||
| 222 | Ester | Eicosaheptanoic acid 2-(p-hydroxyphenyl) ethyl ester | ||||
| 223 | Terpenoid | Erithrodiol 3-acetate | ||||
| 224 | Cumaric acid | Hexacosylferulate | ||||
| 225 | Terpenoid | Lupeol | ||||
| S. microphylla |
|
Acetone | 215 | Terpenoid | Methyl 7-oxosandaracopimarate | [19, 25, 108, 124, 125] |
| 226 | Terpenoid | Methyl 7α-hydroxysandaracopimarate | ||||
| 227 | Terpenoid | Microphyllandiolide | ||||
| 33 | Terpenoid | Oleanolic acid | ||||
| 5 | Terpenoid | Salvimicrophyllin B | ||||
| 228-230 | Terpenoid | Salvimicrophyllins A, C, D | ||||
| 220 | Terpenoid | Sandaracopimara-8(14),15-diene-7α,18-diol | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 231 | Terpenoid | β-eudesmol | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. patens | Flowers | Aqueous | 232 | Flavonoid | Protodelphin | [126] |
| S. polystachya |
|
|
233 | Terpenoid | 15-epi-polystachyne G | [17, 23, 42, 98, 100, 107] |
| 234 | Flavonoid | 3',5,6,7-tetrahydroxy-4´-methoxyflavone | ||||
| 66 | Terpenoid | Dehydrokerlin | ||||
| 235 | Terpenoid | Linearolactone | ||||
| 33 | Terpenoid | Oleanolic acid | ||||
| 236-243 | Terpenoid | Polystachines A-H | ||||
| 149 | Terpenoid | Salvifaricin | ||||
| 244-247 | Terpenoid | Salvifilines A-E | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| S. reflexa | Leaves | Acetone | 248 | Terpenoid | 15,16-epoxy-8α-hydroxyneocleroda-2,13(16),14-triene-17,12R:18,19-diolide | [127] |
| 249 | Terpenoid | 6β-hydroxysalviarin | ||||
| 250 | Terpenoid | 8α-hydroxysalviarin | ||||
| S. reflexa | Leaves | Acetone | 33 | Oleanolic acid | [127] | |
| 251 | Terpenoid | Salviarin | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. reptans |
|
|
252 | Terpenoid | 1α,2α-epoxy-3,4α-dihydrolinearolactone | [23, 19, 93, 108, 128] |
| 253 | Terpenoid | 8α,9α-epoxy-7-ketoroyleanone | ||||
| 254 | Terpenoid | Diosmetin | ||||
| 89 | Terpenoid | Horminone | ||||
| 235 | Terpenoid | Linearolactone | ||||
| 33 | Flavonoid | Oleanolic acid | ||||
| 255 | Terpenoid | Salvireptanolide | ||||
| 42 | Terpenoid | Ursolic acid | ||||
| 48 | Terpenoid | β-sitosterol | ||||
| S. tiliifolia |
|
Acetone | 104 | Terpenoid | 6,7-dehydrodugesin A | [76, 101, 23, 108, 129, 130] |
| 256-257 | Terpenoid | Dugesins A, B | ||||
| 258 | Phenol | Ferruginol | ||||
| 136 | Terpenoid | Isosalvipuberulin (Isopuberulin) | ||||
| 259 | Terpenoid | Puberulin | ||||
| 94 | Terpenoid | Salvianduline E | ||||
| 149 | Terpenoid | Salvifaricin | ||||
| 260 | Terpenoid | Salvifolin | ||||
| 261 | Terpenoid | Salyunnanins I | ||||
| 262 | Terpenoid | Tilifolidione | ||||
| S. verbenacea |
|
|
263 | Aldehyde | (E)-2-hexenal | [23] |
| 264 | Terpenoid | (E)-caryophyllene | ||||
| 265 | Terpenoid | (E)-β-caryophyllene | ||||
| 266 | Terpenoid | (E)-β-farnesene | ||||
| 267 | Terpenoid | (E)-β-ionone | ||||
| S. verbenacea |
|
|
268 | Terpenoid | (E)-β-ocimene | [23] |
| 269 | Carboxylic acid | (Z)-9-octadecenoic acid | ||||
| 270 | Terpenoid | (Z)-β-ocimene | ||||
| 95 | Terpenoid | 1,10-di-epi-cubenol | ||||
| 96 | Terpenoid | 1,8-cineole | ||||
| 271 | Terpenoid | 13-epi-manool | ||||
| 272 | Terpenoid | 2,3-dihydro-1,4-cineol | ||||
| 273 | Terpenoid | 4-terpeniol | ||||
| 274 | Flavonoid | 5-hydroxy-3,4’,7-trimethoxyflavone | ||||
| 275 | Flavonoid | 5-hydroxy-7,4'-dimethoxyflavone | ||||
| 276 | Terpenoid | 6-13-hydroxy-7a- acetoxyroyleanone | ||||
| 277 | Aldehyde | 9,12,15-Octadecatrienal | ||||
| 26 | Flavonoid | Apigenin | ||||
| 278 | Aromatic | Benzaldehyde | ||||
| 110 | Terpenoid | Bicyclogermacrene | ||||
| 28 | Polyphenol | Caffeic acid | ||||
| 113 | Terpenoid | Camphene | ||||
| 279 | Terpenoid | Camphor | ||||
| 280 | Terpenoid | Carnosic acid | ||||
| 281 | Terpenoid | Caryophyllene oxide | ||||
| 282 | Flavonoid | Cirsilineol | ||||
| 283 | Flavonoid | Cirsiliol | ||||
| 116 | Terpenoid | cis-muurola-3,5-diene | ||||
| 184 | Terpenoid | cis-muurola-4(14),5-diene | ||||
| 164 | Terpenoid | E-Caryophyllene | ||||
| 181 | Terpenoid | epi-13-manool | ||||
| S. verbenacea |
|
|
185 | Terpenoid | epi-α-cadinol | [23] |
| 186 | Acetate | Ethyl hexadecanoate | ||||
| 30 | Terpenoid | Ferulic acid | ||||
| 31 | Flavonoid | Germacrene D | ||||
| 287 | Flavonoid | Hesperidin | ||||
| 288 | Fatty acid | Hexadecanoic acid | ||||
| 89 | Terpenoid | Horminone | ||||
| 289 | Terpenoid | Limonene | ||||
| 55 | Terpenoid | Linalool | ||||
| 56 | Fatty acid | Linoleic acid | ||||
| 290 | Flavonoid | Luteolin | ||||
| 291 | Terpenoid | Manool | ||||
| 292 | Terpenoid | Methyl carbonate | ||||
| 293 | Fatty acid | Methyl ester of 6-octadecenoic acid | ||||
| 294 | Terpenoid | Methyl eugenol | ||||
| 295 | Flavonoid | Naringenin | ||||
| 296 | Alkane | Nonane | ||||
| 297 | Alkane | Octane | ||||
| 298 | Fatty acid | Oleic acid | ||||
| 147 | Terpenoid | p-cymene | ||||
| 299 | Aromatic | Phenyl acetaldehyde | ||||
| 300 | Aromatic | p-hydroxybenzoic acid | ||||
| 301 | Terpenoid | Phytol | ||||
| 302 | Flavonoid | Retusin | ||||
| 38 | Phenylpropanoid | Rosmarinic acid | ||||
| 303 | Terpenoid | Sabinene | ||||
| 304 | Flavonoid | Salvigenin | ||||
| S. verbenacea |
|
|
305 | Terpenoid | Salvinine | [23] |
| 40 | Terpenoid | Spathulenol | ||||
| 80 | Terpenoid | Taxodione | ||||
| 161 | Terpenoid | Terpinolene | ||||
| 306 | Terpenoid | trans-sabinene hydrate | ||||
| 307 | Alkane | Tricosane | ||||
| 308 | Terpenoid | Tricyclene | ||||
| 309 | Terpenoid | Verbenacine | ||||
| 310 | Flavonoid | Verbenacoside | ||||
| 167 | Terpenoid | Viridiflorol | ||||
| 171 | Terpenoid | α-copaene | ||||
| 173 | Terpenoid | α-humulene | ||||
| 175 | Terpenoid | α-pinene | ||||
| 311 | Terpenoid | α-terpinyl acetate | ||||
| 312 | Terpenoid | α-thujene | ||||
| 46 | Terpenoid | β-caryophyllene | ||||
| 231 | Terpenoid | β-eudesmol | ||||
| 193 | Terpenoid | β-phellandrene | ||||
| 313 | Terpenoid | γ-amorphene | ||||
| 186 | Terpenoid | γ-cadinene | ||||
| 188 | Terpenoid | δ-cadinene | ||||
| 314 | Terpenoid | δ-selinene |
Conclusions
The several Salvia species in the Valle de México represent a vast plant resource with metabolites of pharmacological interest that play a significant role in Mexican Traditional Medicine. Salvia species represent a vast therapeutic use and have great potential for developing new bioactive compounds for treating diverse diseases due to the great variety of metabolites generated under diverse conditions, even in different populations of the same species. The data presented seek to promote research into these species through bio-assay-guided chemical studies that support their empirical use and the development of new herbal treatments. Enlarging the identification of new metabolites present in these plant species, taking into consideration that the variations of metabolites structures, the wide variety of Salvias and the poor study with some of them, could also generate new research opportunities in diverse areas of study. Finally, expanding the chemical, biological and pharmacological information might serve to develop methods of production of these plants, preserve them and improve their production and economic impact.











 nueva página del texto (beta)
nueva página del texto (beta)