Introduction
Lipids are large and diverse groups of naturally occurring organic compounds which also perform different functions in the system of living organisms [1]. They have been found to be involved in some biological activities that can help the body to run smoothly. Some of these activities include helping the brain to work well, joint mobilization, energy production, lubrication of the cells which help to protect the delicate organs of the body and also help in internal cellular communication [1]. In addition, they help to transport some vital fat-soluble components (vitamins, sterols) within the body system. Some examples of lipids components include fatty acids, phospholipids, and waxes.
Fatty acids are important constituents of the membrane cell. They are part of lipids which are widely spread in nature and are of important biological, structural and functional roles [2]. Furthermore, fatty acid produces a huge quantity of adenosine triphosphate (ATP) thus, helping to provide energy by storing used calories [3,4]. Apart from this, some lipids also help to improve the health of the human heart which made the American heart Association to recommend a daily triacylglycerol level of 100 mg for a proper functioning of the heart [4].
Bioactive lipids are the group of lipids that can exhibit biological activity and provide health benefits. Some examples of these bioactive lipids are fat soluble vitamins, phytosterols, carotenoids, phenolic lipids, Sphingomyelins [5]. Fat soluble vitamins are vitamins that are soluble in fats and are absorbed and transported in the body like fats. They are also stored in the liver and fat tissue [6].
These fat-soluble vitamins are vitamins A, D, E and K, and they are important for a wide range of biological processes in the body. Vitamins A and E are considered essential vitamins because, the body cannot synthesize them and so, they are obtained from diets while Vitamin K is synthesized in the colon and vitamin D can be synthesized by the body from exposure to sunlight. Although, these vitamins are important, their storage can lead to their excessive accumulation in human body which can also become toxic. This often occur in people that takes single supplements of fat-soluble vitamins rather than from foods rich in vitamins [7].
Phytosterols and phospholipids have been found to have numerous health benefits. This include helping in the development and proper cognition of the brain. They also help to prevent development of certain diseases thus helping the body to be in a condition of total well- being [8-10].
In view of the envisaged vast health benefits and importance of the availability of these lipids to the body system, there is a continuous search for naturally occurring sources of these lipids in some underutilized plants and their plant oils.
Hura crepitans plant is commonly known as Sandbox tree. It belongs to the family of Euphorbiaceae and has a height of about 9 meters on the average [11]. It houses the masculine and feminine flowers on the same tree and the back of the tree is covered with spines, the fruits are green when not ripe but turns brown on ripening (as shown in Fig. 1) and the pod explodes violently to liberate their seeds to the surrounding. The seed oil is used as purgative [11] and has also been claimed to be poisonous when ingested in excess amount while it could also cause cornea damage or partial blindness when in contact with the eye [11]. Notwithstanding these shortcomings, some researchers have found that the seeds contain high percentage of poly unsaturated fatty acids, good mineral composition and nutritional composition [11-14], but there is paucity of information to the best of our knowledge on the phospholipids and some bioactive lipids of the seed oil. Therefore, the aim of this research is to investigate the lipids and bioactive lipid profiles, phytochemical composition, nutritional and antinutritional properties and industrial properties` potentials of this underutilized African plant.
Materials and methods
Collection of samples
Matured fruits were collected from Hura crepitans trees within Ekiti State University Ado- Ekiti premises and was identified at the Department of Plant Science of the University. The fruits were dehulled manually to obtain the seeds. The seeds were washed with distilled water to remove the contaminants and were air dried at room temperature. The dried seeds were then ground to powder using electric grinder. The powdered seeds were kept in airtight container until further use.
Extraction of oil
100 g of the powdered sample was loaded into Soxhlet extractor with n hexane as the solvent and the oil was extracted for 8 hours at 60 -70 °C. Extracted oil was then concentrated using a rotary evaporator and was refrigerated until further analysis.
Determination of phospholipid profile of the sample
The phospholipid profile of the extracted oil was determined using the method of Raheja et al [15], with slight modification. 0.01 g of the extracted oil was added to test tubes, and nitrogen was passed over the oil to completely remove the solvent. Chloroform (0.40 mL) was then added to the tubes, followed by 0.10 mL of a chromo-genic solution. The content of the tube was heated to 100 °C in a water bath for 80 seconds and cooled to room temperature; 5 mL of hexane was then added, and the tube was gently shaken several times. The solvent and the aqueous layer were allowed to separate; the hexane layer was recovered and concentrated to 1.0 mL for GC analysis using a gas chromatography instrument (HP 6890 Powered with HP ChemStation Rev. A 09.01 [1206] software, Agilent Technologies, Inc.) equipped with pulse flame photometric detector. The conditions were as follows: stainless steel column 30 m × 0.25 mm × 0.25 (m (HP-INNOWax, Agilent Technologies, Inc.); column temperature of 250 °C; carrier gas N2, 35 mL/minutes and H2, 30 mL/minutes. Oven temperature program: initial temperature of 50 °C; first ramping at 10 °C/minutes for 20 minutes and maintained for 4 minutes; second ramping at 15 °C/minutes for 4 minutes and maintained for 5 minutes.
Determination of the bioactive lipids
Phytosterol
The phytosterol profile was determined according to the method of the International Organization for Standardization [16]. After the addition of 1.0 mL of internal standard solution, approximately 250 mg of the oil was saponified with an ethanolic potassium hydroxide solution; the unsaponifiable fraction was isolated by solid-phase extraction on an aluminum oxide column, and the steroid fraction was obtained after thin layer chromatography. Bands were visualized using n-hexane/ethyl acetate (80:20, v/v) as the developing solvent. The sterol profile was analyzed using GC analysis carried out with a HP 6890 Powered with HP ChemStation Rev. A 09.01 (1206) software (Agilent Technologies, Inc.) fitted with a HP-INNOWax column (30 m × 0.25 mm × 0.25 (m, Agilent Technologies, Inc.) equipped with flame ionization detector. Nitrogen carrier gas was used at a flow rate of 35 mL/minute and a pressure of 22 psi, 1 mL/minute. The injector and detector temperatures were 250 °C and 320 °C, respectively, and the oven was programmed to decrease in temperature from 60 to 15 °C at 4 °C/minute. The injection volume was 1 L, with a split ratio of 20:1. The total sterol content was determined by considering all peaks of sterols eluted between cholesterol and Δ7 -avenasterol. Peaks were identified by comparing the relative retention times of the samples with those obtained from standards.
Fatty acids
The fatty acid composition was determined according to Cocks and Rede [17] with slight modification. 0.5 g of the extracted oil was mixed with 3 mL of dimethyl ether and 0.2 mL of sodium methoxide to form a colloidal solution. The solution was allowed to settle and was centrifuged to precipitate. The solid was filtered and the filtrate was kept for GC analysis. 1 (L of the filtrate was injected into the gas chromatography instrument (HP 6890 Powered with HP ChemStation Rev. A 09.01 [1206] software, Agilent Technologies, Inc.) equipped with a flame ionization detector. The conditions were the same as described for determination of phospholipid composition above. Individual fatty acids were identified by comparing their retention times with a certified fatty acid methyl ester. The relative percentage of each fatty acid was quantified as the percentage of total fatty acids.
Fat soluble vitamins
Vitamin A
Quantity of vitamin A present in the sample was determined according to the methods of Sebrell and Harris [18] with slight modification. 1 mL of oil sample was measured into a 250 mL volumetric flask and dissolved in 20 mL of petroleum ether. Acetone (2:1 v/v) mixture was added to the extract. The flask containing the mixture was placed on a shaker to shake at 200 rpm for 20 minutes to ensure uniform mixing at room temperature. The mixture was later centrifuged at 4000 rpm for 10 minutes and the supernatant collected and made up to 50 mL with the solvent mixture. The supernatant was transferred to a 250 mL separatory funnel to separate the organic layer (upper layer). The aqueous layer was discarded, and the organic layer was transferred into the 50 mL volumetric flask and made up with solvent mixture for determination of absorbance. Working standard of ß-carotene of range 0 - 50 ppm were prepared from stock solution. The absorbance of samples as well as working standard solutions was read on a Cecil CE2041 UV Spectrophotometer at a wavelength of 450 nm against blank and the amount of vitamin A present in the sample was determined.
Vitamin E
The vitamin E was determined according to the method of Association of Official Analytical Chemists [19]. 1 mL of the sample was measured, macerated with 10 mL of n hexane in a test tube for 10 minutes and centrifuged for 10 minutes. The solution was filtered; 3 mL of the filtrate was transferred into a dry test tube in duplicates and evaporated to dryness in a boiling water bath. 2 mL of 0.5 N alcoholic potassium hydroxide was added and boiled for 30 minutes in a water bath. Then 3 mL of n-hexane was added and was shaken vigorously. The n-hexane was transferred into another set of test tubes and evaporated to dryness. 2 mL of ethanol was added to the residue and another 1mL of 0.2 % ferric chloride in ethanol was added. Then 1 mL of 0.5 %, 1, 1-dipyridyl in ethanol was added followed by the addition of 1 mL of ethanol to make it up to 5 mL The solution was mixed, and absorbance taken at 520 nm against the blank.
Vitamin K
3 mL of samples and blank was measured separately into test tubes. In each test tube, 2 mL of 0.2 % solution of 2, 4 - dinitrophenyl hydrazine was added and mixed well. It was heated in a water bath to almost dryness and was cooled at room temperature. 15 mL mixture of ammonia and alcohol in ratio 1:1 was added to each test tube and its absorbance was read at 635 nm against blank.
Vitamin D
2 mL of the oil was measured and 3 mL of hexane was added to it slowly at interval of 60 seconds. The phase was separated by centrifugation at 4000 rpm for 15 minutes. 4 mL of the upper organic phase was transferred to a small beaker and dried under liquid nitrogen gas. The dried extract was solubilized in methanol and the absorbance was taken at 275 nm and the reading was compared with that of standard at different concentrations.
Determination of phytochemicals and antinutrients
Saponin content
100 cm3 of 20 % aqueous ethanol was added to 3 g of the powdered sample in a 250 cm3 conical flask. The mixture was heated over a hot water bath for 4 hours with continuous stirring at a temperature of 55 °C. The residue of the mixture was re-extracted with another 100 cm3 of 20 % aqueous ethanol after filtration and heated for 4 hours at a constant temperature of 55 °C with constant stirring. The combined extract was evaporated to 40 cm3 over water bath at 90 °C. 20 cm3 of diethyl ether was added to the concentrate in a 250 cm3 separating funnel and vigorously agitated from which the aqueous layer was recovered while the ether layer was discarded. This purification process was repeated twice. 60 cm3 of n-butanol was added and extracted twice with 10 cm3 of 5 % sodium chloride. After discarding the sodium chloride layer, the remaining solution was heated in a water bath for 30 minutes, after which the solution was transferred into a crucible and was dried in an oven to a constant weight. The saponin content was calculated as:
Tannin content
One gram of the powdered sample in a conical flask was added to 100 cm3 of distilled water. This was boiled gently for 1 hour and then filtered. Addition of 5 cm3 Folin-Denis reagent and 10 cm3 saturated Na2CO3 solution into 50 cm3 of distilled water and 10 cm3 of diluted extract (aliquot volume) was carried out after being pipetted into a 100 cm3 conical flask for color development. The solution was allowed to stand for 30 minutes in a water bath at a temperature of 25 °C after thorough agitation. The optical density was measured at 700 nm using Spectrum Lab 23A spectrophotometer and compared on a standard tannic acid curve using the equation below:
Where C is the concentration of tannic acid read from the graph.
Alkaloids content
200 cm3 of 10 % acetic acid in ethanol was added to 3 g of the powdered sample in a 250 cm3 beaker and allowed to stand for 4 minutes. The extract was concentrated on a water bath to one-quarter of the original volume followed by addition of 15 drops of concentrated ammonium hydroxide drop wise to the extract until the precipitation was complete immediately after filtration. After 3 hours of mixture sedimentation, the supernatant was discarded and the precipitates were washed with 20 cm3 of 0.1 M of ammonium hydroxide and then filtered, the residue was dried in an oven and weighed. The percentage of alkaloid is expressed mathematically as:
Flavonoid
3 g of the powdered sample was mixed with 25 mL of 80 % aqueous methanol. The whole solution was filtered through the whatman filter paper. The filtrate was transferred to a crucible and evaporated into dryness over a water bath and weighed. The amount of flavonoid present in the sample was calculated using the equation below:
Oxalate content
Exactly 20 cm3 of 0.3 M HCl in each powdered sample (2.5 g) was extracted thrice by warming at a temperature of 50 oC for 1 hour with constant stirring using a magnetic stirrer. For oxalate estimation, 1 cm3 of 5 M ammonium hydroxide was added to 5 cm3 of extract to ensure alkalinity. 2 drops of phenolphthalein indicator, 3 drops of glacial acetic acid, and 1 cm3 of 5 % calcium chloride was added to make the mixture acidic before standing for 3 hours. This was followed by centrifugation at 3000 rpm for 15 minutes. After discarding the supernatant, the precipitate was washed three times using hot water by mixing thoroughly each time before centrifugation. 2 cm3 of 3 M tetraoxosulphate (VI) acid was added to each tube and the precipitate dissolved by warming in a water bath at 70 °C. Freshly prepared 0.01 M potassium permanganate (KMnO4) was titrated against the content of each tube at room temperature until the first pink colour appears throughout the solution. The solution was allowed to stand until it returned colorless after which it was heated and re-titrated again until a pink color appears and persists for at least 30 seconds. The amount of oxalate was calculated using the equation below:
Phytate content
Samples were extracted with 100 mL of 2 % HCl for 3 hours and then filter through a No 1 Whatman filter paper. 25 mL was taken out of the filtrate and place inside a conical flask and 5 mL of 0.3 % of ammonium thiocyanate solution was added as indicator. After which 10 mL of distill, water was added to give it the proper acidity and this was titrated against iron (III) chloride solution until a brownish yellow coloration persist for 5 minutes.
Determination of the physical, chemical, and nutritional properties of the sample
The physical and Chemical properties (acid, ionization, saponification, peroxide, FFA values) and the nutritional composition of the samples were determined using the method of the Association of Official Analytical Chemists [19].
Results and discussion
Phospholipid composition
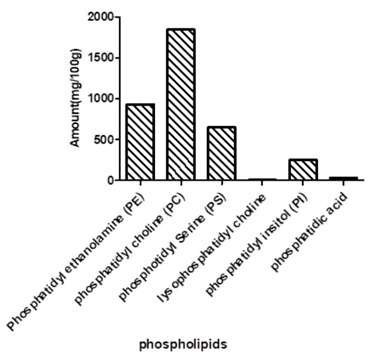
The result of the phospholipid composition of the plant is presented in Fig. 2. The result showed that the plant has a high amount of phosphatidyl choline (PC) (1847.27 mg/100 g). This was followed by phosphatidyl ethanolamine (PE) (933.31 mg/100 g) and phosphatidyl serine (PS) (647.79 mg/100 g). Phosphatic acid and lysophosphatidyl choline were detected in averagely low quantities in the plant and the total phospholipid composition of the investigated Hura crepitans plant was 3717.91 mg/100 g. Various classes of phospholipid have numerous health benefits because phospholipids are part of the lipid’s molecules found in cellular membranes; they are essential lipid molecules that make up the lipid bi-layers of cells.
The high amount of PC and PE in the plant is also of great importance because PC & PE are the most abundant phospholipid in mammalian cell membranes, comprising 30 - 50 % and 15 - 25 % respectively of total phospholipids in the cell [20,21] thus showing that the plant could be a source of these essential phospholipids.
PC has been found to be effective in ameliorating and curing liver disease [22]. It is effective in reducing the development of arthritis and helps to limit inflammatory process of joints [23,24] while PE could be helpful in suppressing cancerous cells, and in producing the energy that powers the whole cells thereby helping to support mitochondrial function [21].
The presence of PC, PE and PS in appreciable quantity coupled with the high amount of total phospholipid detected in the plants showed that the plant could be of great benefit as a potential source of these essential lipids.
Phytosterol content
The result of the phytosterol content of the investigated plant is presented in Table 1. Based on the results obtained, Hura crepitans seed oil had a very high amount of β-Sitosterol which was followed by Campesterol and stigmasterol while the amount of total phytosterol in the plant oil was 881.55 mg/100g. Phytosterols are fatty compounds derived from plants and they represent the greatest portion of unsaponifiable matter in plant lipids. They are bioactive compounds that have been found to have numerous health benefits in the human body system [25]. β- sitosterol, Campesterol and stigmasterol are the most common phytosterols in the human diet and these three phytosterols are beneficial to human health. β sitosterol has been found to have the ability to control the formation of inflammation and inflammatory cytokines thus it helps to reduce pains and normalize the function of natural killer cells which play a central role in the pathogenetic mechanisms to prevent diseases [10]. Campesterol and other plant sterols have been found to lower the chance of cardiovascular problems such as heart failure, atherosclerosis, and stroke [26]. Considering the health benefits of these phytosterols, and their high availability in this African plant, Hura crepitans seed could be considered as an essential plant for potential industrial sources of these phytosterols and also as nutritional supplements which are essential human nutrients because the human body cannot synthesize these bioactive lipids [25]. A unique contribution of our study is the observation that prior to now, little is known about the phospholipid and phytosterol compositions of Hura crepitans seed oil. Thus, making the current study about the first to highlight the rich component of these essential compounds in the plant. Given the potential numerous health benefits of phytosterols and phospholipids, our study has raised the need for further research to explore more about the biochemical composition of Hura crepitans seeds.
Fatty acid composition of the investigated plant
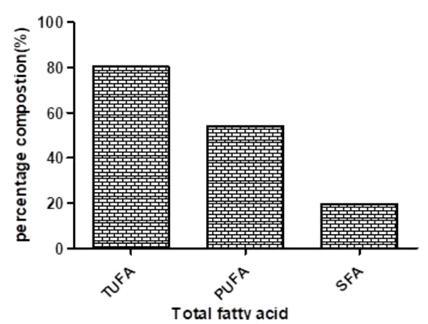
The result of the fatty acid composition of Hura crepitans plant is presented in Table 2 while the results of the total fatty acids is presented in Fig. 3. As shown in Table 2, the plant has high amount of linoleic and oleic acids (52.25 and 25.50 %) respectively. It also has a total saturated fatty acid composition of 19.69 %, total unsaturated fatty acid composition of 80.31 % and poly unsaturated fatty acid composition of 54.15 % (Fig. 3).
Table 2 Fatty acid composition of Hura crepitans plant.
| Fatty Acid | Percentage composition |
|---|---|
| Myristic acid | 0.43 |
| Palmitic acid | 12.09 |
| Palmitoleic acid | 0.60 |
| Margaric | 0.069 |
| Stearic acid | 5.32 |
| Oleic acid | 25.50 |
| Linoleic | 52.25 |
| Linolenic acid | 1.56 |
| Arachidic | 0.88 |
| Arachidonic | 0.105 |
| Behenic acid | 0.604 |
| Erucic acid | 0.230 |
| Lignoceric acid | 0.291 |
The fatty acid profile of the Hura crepitans plant presented in this present work is similar to the reports of Ezeh et.al. [27] and Alabi et al. [28] but has higher percentage composition of linoleic fatty acid when compared to the reports of Okolie et al. [29], Abdulkadir et al. [11] and Oyeleke et al. [12]. The discrepancies in these results may be due to the differences in geographical location, season of sampling and method of extraction and analysis. Linoleic fatty acids also known as Omega 6 fatty acids are precursor for other omega -6 fatty acids because the body cannot synthesize long chain fatty acids with odd numbers of carbon atoms [2] but these long chain fatty acids are important for the mammalian cells to perform various biological functions. Linoleic fatty acids are the major components of the adipose tissues like the brain where they take part in the development and maintenance of the central nervous system during both embryonic and adult stages [30]; they also help in sustaining the structural integrity of cellular membranes and serve as signaling molecules [30]. Another important fatty acid present in high amount is oleic acid which was the major monounsaturated fatty acid present in the sample. Oleic acid has antioxidant ability that prevents oxidative stress which can lead to various diseases [31], it is also beneficial in the treatment of type 2 diabetes and insulin sensitivity [32].
TUFA- total unsaturated fatty acid; PUFA - poly unsaturated fatty acid, SFA- saturated fatty acid
Fat- soluble vitamins
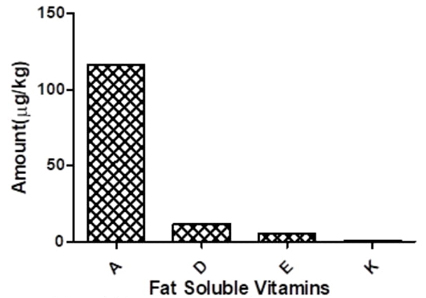
The result of the fat -soluble vitamin of the plant is presented in Fig. 4. Based on the result obtained, the plant contains majorly vitamin A (116.55 µg/kg) and little concentration of vitamins D, E and K. Vitamin A are important in multiple cellular processes like communication, recognition, adhesion and aggregation. These they do by helping in glycoproteins synthesis which are important in the above - mentioned cellular processes [6]. Vitamin A has also been found to be used in treatment of skin cancer, acne, and acne related diseases. It also has antioxidant activity, and it is required for the maintenance of normal mucous membrane and normal vision [6]. Therefore, the plant could be a source of vitamin A for various industrial processes and applications. The vitamin A profile of the Hura crepitans plant presented in this work is lower when compared to the reports of Udoh et al. [13] and Fowomola & Akindahunsi [14]; the variation in the results may be due to the differences in the processing techniques, season of harvest, method of determination of the parameter and geographical locations which is a reflection of the climatic conditions in which the plant is grown. Despite these differences, all the authors including the present study, have established that Hura crepitans contain high quantity of vitamin A thus confirming that the plant could be a good source of the vitamin.
Phytochemical and antinutritional composition
The results of phytochemical and antinutritional composition of the plant are presented in Table 3. The results showed that the plant contains appreciable amounts of saponin and alkaloid. Other investigated phytochemicals were detected in low quantity in the plant. For the antinutrients, phytate and oxalate were detected in minute quantities in the investigated African plants (Table 3). Saponins can control human cardiovascular disease and reduce blood cholesterol in human [33]. They also possess various pharmaceutical properties like anti- inflammatory [34], immunological adjuvant activities, hemolytic activities, antioxidant properties and anti - cancer effects thus attracting a lot of attention as potential targets for drug discovery and development [35].
Table 3 Phytochemical and Antinutritional composition of Hura crepitans plant.
| Phytochemical | Amount (mg/100 g) |
|---|---|
| Saponin | 1.633 |
| Alkaloid | 2.714 |
| Tannin | 0.399 |
| Flavonoid | 0.524 |
| Antinutrients | Amount (mg/100 g) |
| Phytate | 0.210 |
| Oxalate | 0.530 |
Pure isolated alkaloids and their synthetic derivatives are used as basic medicinal agents because of their analgesic, antispasmodic and antibacterial properties [36]. Therapeutically, alkaloids are particularly well known as anaesthetics, cardioprotective, anti-cancer and anti-inflammatory agents [37,38] and these further highlights the health and industrial benefits of this phytochemical. The fact that these phytochemicals were detected in the investigated plant showed that the plants could be used as a source of the phytochemicals; further affirming its usefulness in the industries to solving sundry human needs problems.
Phytate has been reported to be able to form a complex with protein by the actions of cations (Zn, Ca) which act as a bridge between the negatively charge protein carboxyl and the phytate thereby making the cation unavailable to the body system. Likewise, oxalate binds with another mineral such as calcium to form oxalate salt and complexes which have an inhibitory effect in peptic digestion and can lead to kidney stone disease [13,33,39]. Both antinutrients inhibit the bioavailability of minerals in the body system when ingested in high amount.
It has been suggested that dietary oxalate in the range of 50 - 200 mg is acceptable [40]. However, phytate amounts higher than 50 mg/ day could cause a significant reduction in zinc absorption [41]. The quantity detected in the investigated sample is far lesser than the toxicity level proposed by these researchers [40,41], thus suggesting that it is likely that Hura crepitans may be safe and less toxic. Nevertheless, there is need for further research on the antinutritional components of the plant to ascertain its safety.
Physicochemical properties and the nutritional composition of the plants
The physical and chemical properties of the investigated plant are presented in Table 4 while the nutritional parameters are presented in Table 5. The results of the physical and chemical properties of the investigated plant showed that the sample has percentage oil yield of 58.76 %. It has high iodine and saponification values (159.58 (Wijs) and 193.37 mgKOH/g respectively). It also has high quantity of unsaponifiable matters. The pH is 5.20 and the specific gravity is 0.87 g/mL. Regarding the nutritional composition of the plant, the amount of protein detected in the sample was 27.31 %, carbohydrate of 11.2 % and fat of 37.50 % (Table 5).
Table 4 The physical and chemical properties of Hura Crepitans seed.
| Parameters | Amount |
|---|---|
| Colour | Golden yellow |
| Percentage oil yield (%) | 58.76 |
| pH | 5.20 |
| Acid value (mgKOH/g) | 2.35 |
| Iodine value (Wijs) | 159.58 |
| Peroxide value (Meq/kg) | 5.26 |
| Saponification value (mgKOH/g) | 193.37 |
| Unsaponifiable matter (g/kg) | 14.85 |
| Specific gravity (g/mL) | 0.87 |
| Kinetic viscosity (cp) | 4.92 |
| Refractive index (nD40)0C | 1.45 |
| Free fatty acid (mgKOH/g) | 5.21 |
The percentage oil yield of the investigated plant is higher than 25.7 % reported for groundnut by Olatunya et al [42]. showing that the plant has higher oil yield than some conventional oil seeds. The low acid value, peroxide value, high iodine, and saponification values of the oil are indication of the oil’s potential industrial’s benefits. Acid values are indication of edibility of oil. The recommended codex acid value for edible virgin oils is 6.6 mg KOH/g according to Food and Agriculture Organisation [43] the acid value reported for the investigated oil is lower than the recommended value thus indicating that the oil could be safe. Iodine values are used to indicate the level of unsaturation in oil and are also used to classify the oil into drying or non-drying oil.
The high iodine value of the investigated oil is an indication that the plant oil has high level of unsaturation, and this has been further corroborated in the fatty acid composition of the oil. Oils with iodine values greater than 130 mgKOH/g are classified as drying oil; based on this Hura crepitans oil could be regarded as a drying oil and therefore, it could be used in the industries to manufacture cosmetics, oil paint and vanishes [44]. In addition, the saponification values are indication of the oil’s applicability in the production of soap [45]. The physical and chemical properties of Hura crepitans seed oil presented in this work is comparable with the reports of other authors who have previously worked on the plant [46-49]. Owing to the high oil yield of this plant and some of its chemical properties, the seed oil will be of great importance in various industries.
Table 5 Nutritional Composition of Hura crepitans flour
| Analysis | Amount (%) |
|---|---|
| Moisture content | 10.5 |
| Ash content | 7.55 |
| Fat | 37.5 |
| Crude fibre | 5.94 |
| Protein | 27.3 |
| Carbohydrate | 11.2 |
| Energy (kcal) | 321 |
The nutritional composition of the plant showed that it is rich in protein, fat, and carbohydrates and has averagely high amount of crude fiber which showed that the seed flour could be used in animal feed. The high ash content of the samples is an indication for presence of minerals. This observation is in agreement with the reports of Oyeleke et al. [12] and Okolie et al. [29] on Hura crepitans seed flour.
Conclusions
The samples are rich in phytosterols, phospholipids, vitamin A, polyunsaturated and monounsaturated fatty acids. All these bioactive lipids have been found to have numerous health benefits. They also help the body system to carry out some biological functions; thus, their availability in this plant showed that the plant could be a source of these essential ingredients. The plant also has averagely high quantity of alkaloids and saponin which are some of the major phytochemicals that have been found to be of great importance in pharmaceutical industries thus making this underutilized and readily available plant to be of great usefulness in the pharmaceutical industries as therapeutic agent. The amount of the antinutrients detected in the samples indicated that the plant may be safe and less toxic. The physical, chemical, and nutritional analysis of the plant showed that the plant is rich in protein, carbohydrates, and fat. It also has good chemical properties indicating its potential use in various industries. Therefore, this study showed that (Hura crepitans) an underutilized plant, has great industrial potentials and health benefits potentials rather than being considered as a waste. Nevertheless, more research is needed to confirm its safety profile.











 nueva página del texto (beta)
nueva página del texto (beta)