Introduction
The functionalization of the magnetic nanoparticles (the F-MNPs) is crucial for chemical processes, and they have many applications in medical and biological research. Meanwhile, the amine-functionalized magnetite nanoparticles (the AF-MNPs) have received more attention because of their properties such as excellent magnetism, tunable sizes, good crystallinity, and facile dispersion in water [1-13].
The AF-MNPs and their derivatives have been used for different fields in organic chemistry such as bio-conjugated to biological molecules (proteins, nucleic acids, and peptides) [1], for cellulase immobilization to prevent wastage of cellulosic resources [2], to prepare cis-pinane [3], catalyst for Heck reaction [4], synthesis of xanthenes [5], selective separation of catecholamines in urine [6], hydrogenation and Heck reactions by Pd immobilized on the AF-MNPs [7], for attachment of polyacrylic acid [8], for the treatment of gastric, colon, and pancreatic cancers in near future [9], for the synthesis of carbamates [10], for degradation of the methyl orange and p-nitrophenol reduction in aqueous media [11], enhanced photocytotoxicity [12], and removal of carmoisine dye from aqueous solutions [13].
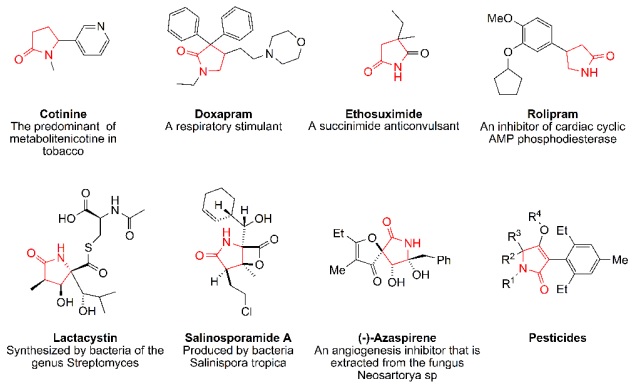
On the other hand, pyrrolin-2-one skeleton has biological activity in some synthetic drugs and natural products, for example: Cotinine [14], Doxapram hydrochloride [15], Ethosuximide [16], Rolipram [17], Lactacystin [18], Salinosporamide [19], (−)-Azaspirene [20] and Pesticides [21] (Scheme 1).
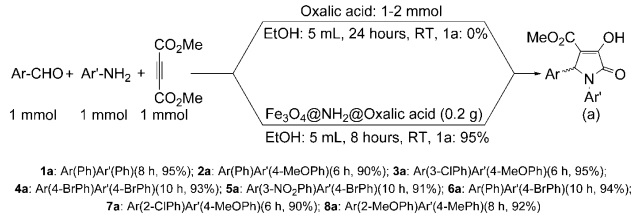
Some procedures have been reported for the synthesis of pyrrolin-2-ones [22-27]. However, a coupling of arylamines and aromatic aldehydes with acetylenedicarboxylates is an important synthetic method for the synthesis of this class of compounds. This method has been studied by several protocols such as using of nano-TiO2 [28], in water as a green solvent [29], by p-toluensulfonic acid [30], under ultrasound irradiation [31], or microwave irradiation [32], by ionic liquids [33], in the presence of nano-magnetic particles [34], and AmberChrom as a polymer catalyst [35]. For improving the obtained result as a part of the synthetic project we turned attention to the synthesis of a more stable and convenient catalyst for this procedure. Thus, we decided to link oxalic acid on magnetite nanoparticles by a covalent bond. Our survey showed using of amine-functionalized magnetite nanoparticles (the AF-MNPs) is a good approach for this subject. Thus, pyrrolin-2-ones were synthesized by a new methodology from aldehydes, anilines and dimethyl acetylenedicarboxylate by Fe3O4@NH2@oxalic acid as a reusable nano-magnetic catalyst at room temperature (Scheme 2).
Experimental
Materials
All reagents were purchased from Sigma-Aldrich Company. FT-IR spectra were recorded on a Bruker spectrometer. XRD patterns were recorded using a Holland Philips (model: PW1730) (radiation, λ= 0.154056 nm, CuK), Step size 0.05°, time per step 1 second (2θ=5°-80°). SEM images were performed on a Model FESEM: TESCAN company (model: MIRA III) manufactured by Czech. EDX (Energy Dispersive X-Ray Analysis) was taken by FESEM: model: MIRA II from TESCAN company manufactured by Czech (Detector: SAMX, France). VSM analysis was taken by model MDKB manufactured by Kavir Kashan Magnet Company (Iran). TGA analysis was recorded by Model Q600; TA company manufactured by the USA. IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. Pyrrolinone derivatives were characterized by their 1H-NMR, FT-IR spectra. All yields referred to isolated pure products. TLC was applied for reaction monitoring over silica gel 60 F254 aluminum sheet.
Synthesis of Fe3O4@NH2 nanoparticles
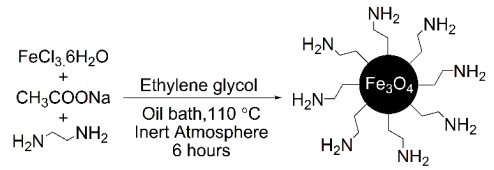
The magnetic amine-functionalized nanoparticles (Fe3O4@NH2) were prepared via solvothermal reaction with major modification [1]. FeCl3.6H2O (1.5 gr), 1, 2-ethylenediamine (3 g), and sodium acetate anhydrous (12 g) were dissolved in ethylene glycol (45 mL) via vigorous mechanical stirring. Then, the reaction was heated at 110°C for 6 hours under the Ar atmosphere. The obtained magnetic nanoparticles were isolated by a powerful magnet, and washed with distilled H2O, and EtOH several times. Then, the product was dried at 60 °C for 2 hours.
Preparation of Fe3O4@NH2@Oxalic acid nanoparticles
Fe3O4@NH2 nanoparticles (0.25 g) were dispersed in ethanol (15 mL) by ultrasonic bath for 30 minutes. Then, a solution of oxalic acid (0.4 g) in ethanol-water (20mL/10mL) was prepared and was charged in the above solution. The obtained mixture was irradiated by ultrasonic irradiation for 4 hours at 60 °C under the Ar atmosphere. The obtained magnetic nanoparticles (i.e Fe3O4@NH2@Oxalic acid) were isolated by a powerful magnet, washed with ethanol, and distilled water several times to remove all organic impurities. Then, the product was dried at 60 °C for 2 hours.
The synthesis of 1,5-diphenyl-3-hydroxy-4-methoxycarbonyl-3-pyrrolin-2-one (1a) by Fe3O4@NH2@oxalic acid; A typical procedure
A mixture of benzaldehyde (0.106 g, 1 mmol), aniline (0.093 g, 1 mmol), and Fe3O4@NH2@oxalic acid (0.2 g) in 5 mL of ethanol was prepared in a round-bottomed flask (25 mL) equipped with a magnetic stirrer and was strongly stirred at room temperature for 40 min. Then to the mixture dimethyl acetylenedicarboxylate (0.14 gr, 1 mmol) was added. The reaction was completed within 8 hours. The reaction was monitored over silica gel 60 F254 aluminum sheet (eluent: ethyl acetate/n-hexane: 1/1). The catalyst was separated by using a strong magnet. The separated catalyst was washed with acetone and ethanol two times then dried in an oven (50 °C) and reused for further reactions. The solvent has been evaporated by a rotary instrument and further residue was recrystallized from methanol. The white solid achieved an excellent yield of 0.295 g (95 %) with a melting point of 182-183 °C [30]. The product was characterized by 1H-NMR, 13C-NMR, FT-IR and C, H, N elemental analysis.
FT- IR (KBr)(cm−1): 3260 (OH), 2958, 1702 (C=O), 1680 (C=O), 1498, 1382, 1232, 1002; 1H NMR (CDCl3): δ 3.60 (s, 3H, CH3), 6.10 (s, 1H, CH), 7.07-7.62 (m, 10 H, Ar and br s, 1H, OH); 13C NMR (CDCl3): δ 50.55, 61.05, 112.35, 122.96, 125.80, 128.13, 128.38, 128.73, 129.13, 136.74, 137.00, 153.14, 162.96 (C=O), 164.49 (C=O). Anal. Calcd for C18H15NO4: C, 69.89; H, 4.89; N, 4.53. Found: C, 69.92; H, 4.81; N, 4.48.
Results and discussion
A literature survey showed that the preparation of the AF-MNPs by direct coupling of amino agents to magnetite nanoparticles has been carried out by different chemical procedures. The chemical conditions for these methods have been summarized in table 1 for more consideration.
Table 1 Synthesis of the AF-MNPs by different protocols.
| Entry | Magnetite source | Amino agent | Steps | Size, nm |
| 11-3, 7-8, 40, 44-46, 48, 50, 52-53 | FeCl3•6H2O | 1,6-hexadiamine | 1 | 1.95-200 |
| 25 | FeCl3•6H2O & FeCl2•4H2O | 1,6-hexadiamine | 1 | 20 |
| 36, 38, 49 | FeCl3•6H2O & FeCl2•4H2O | H2N(CH2)3Si(OCH3)3 | 2 | 13-150 |
| 436a | FeCl3•6H2O | H2N(CH2)3Si(OCH3)3 | 2 | 250 |
| 536 | FeCl3•6H2O & FeSO4•7H2O | H2N(CH2)3Si(OCH3)3 | 2 | 10 |
| 642, 45 | Fe(NO3)3•9H2O | H2N(CH2)3Si(OCH3)3 | 2 | 20-40 |
| 74, 39, 41 | FeCl3•6H2O | triethylenetetramine | 1 | 70-100 |
| 837 | FeCl3•6H2O & FeCl2•4H2O | (CH3O)3Si(CH2)3NH(CH2)2NH(CH2)2NH2 | 2 | 20-100 |
| 943 | FeCl3.6H2O | poly(propyleneglycol)bis(2-aminopropyl ether) | 1 | 20 |
| 1047 | FeCl3•6H2O | 2,2-(ethylenedioxy)-bis-(ethylamine) | 1 | 6 |
| 1151 | FeCl3•6H2O | 2-aminoterephthalic acid | 2 | 4 |
| 126 | FeCl3•6H2O & FeSO4•7H2O | H2N(CH2)3Si(OEt)3 | 2 | none |
In continuous of these procedures (table 1), Fe3O4@NH2 was synthesized by a new protocol. The magnetite nanoparticles were prepared from FeCl3•6H2O. Also, 1, 2-ethylenediamine was used as an amino agent. As shown in table 2, some experiments were carried out for optimizing the best ratio of the reagents, temperature, and reaction time. All reactions were performed by a one-pot reaction of FeCl3•6H2O and 1, 2-ethylenediamine in the ratio (1:10) in ethylene glycol at different temperatures & times under an inert atmosphere.
Table 2 Optimization reaction conditions for the synthesis of Fe3O4@NH2 (product) from FeCl3•6H2O (A), 1, 2-ethylenediamine (B), and sodium acetate anhydrous (C) in ethylene glycol (45 mL).
| Entry | A/g | B/g | C/g | θ/° C | t/h | Product |
| 1 | 1.5 | 2 | 3 | 150-190 | 3 and 6 | impure |
| 2 | 1.5 | 3 | 6 | 200 | 3 and 6 | impure |
| 3 | 1.5 | 2 | 12 | 200 | 6 | impure |
| 4 | 1.5 | 3 | 6 | 110 | 3 | poor quality |
| 5 | 1.5 | 3 | 12 | 110 | 6 | good quality |
| 6 | 1.5 | 3 | 6 | 110 | 12 | poor quality |
The quality of the obtained AF-MNPs was determined by comparing FT-IR spectra as shown in Fig. 1(a-c). In Fig. 1(a), the band at 645 cm-1 assigned to the impurity some amount of maghemite [36b] when the reactions were carried out at 200 °C (table 2, entry 2-3). The same results were achieved at 150-190 °C (table 2, entry 1). Due to the absorbance bands at 3428 cm-1 (NH stretching vibration) and 2934 and 2862 cm-1 (asymmetric stretching vibration and symmetric stretching vibration of CH2), 1, 2-ethylenediamine was coated better at 110 °C within 6 hours (Fig. 1(c)) (Table 2, entry 5), while the coating was reduced in fewer time of reaction (3 hours, Fig. 1(b)) (Table 2, entry 4).
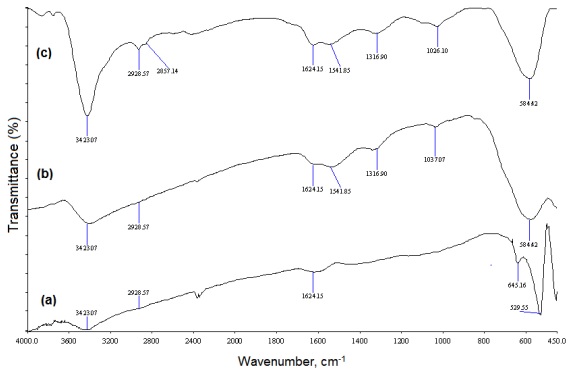
Fig. 1 (a) FT-IR spectrum of synthesized of Fe3O4-CH2CH2-NH2 at 200 °C within 6 hours (Table 2, entry 3). (b) FT-IR spectrum of synthesized of Fe3O4-CH2CH2-NH2 at 110 °C within 3 hours (Table 2, entry 4). (c) FT-IR spectrum of synthesized of Fe3O4-CH2CH2-NH2 at 110 °C within 6 hours (Table 2, entry 5).
Thus, the results (Table 2, entry 5) showed that a one-pot reaction of FeCl3•6H2O and 1, 2-ethylenediamine in the ratio (1:10) at 110 °C within 6 hours in ethylene glycol gave the best quality and quantity of Fe3O4-CH2CH2-NH2 (Fe3O4@NH2) as shown in Scheme 1. The chemical structure of Fe3O4@NH2 was characterized by FT-IR and VSM spectra.
A one-pot reaction of FeCl3.6H2O and 1, 2-ethylenediamine in the ratio (1:10) at 110 °C within 6 hours in ethylene glycol gave the best quality and quantity of Fe3O4-CH2CH2-NH2 (Fe3O4@NH2) as shown in Scheme 3.
Then, oxalic acid as an organic acid was linked to surface of Fe3O4@NH2 nanoparticles. The best reaction conditions were obtained by performing some reactions in different conditions as shown in Table 3.
Table 3 Optimization reaction conditions for the synthesis of Fe3O4@NH2@oxalic acid (product) from Fe3O4@NH2 (1 g) under the Ar atmosphere.
| Entry | moxalic acid / g | Conditions | θ / °C | t / h | Product1 |
| 1 | 0.4 | - | RT | 2 or 4 | no |
| 2 | 0.4 | ultrasonic | RT | 2 or 4 | no |
| 3 | 0.8 | ultrasonic | 40 | 2 or 4 | no |
| 4 | 1.2 | ultrasonic | 40 | 2 or 4 | no |
| 5 | 1.2 | ultrasonic | 60 | 2 | no |
| 6 | 1.2 | ultrasonic | 60 | 4 | yes |
| 7 | 1.2 | - | 60 | 4 | no |
| 82 | 1.6 | ultrasonic | 60 | 4 | yes |
| 92 | 1.2 | ultrasonic | 60 | 8 | yes |
1The formation of the product (Fe3O4@NH2@oxalic acid) was characterized by FT-IR spectrum.
2The obtained results from entries 8 and 9 are similar to entry 6. These results showed that more amounts of oxalic acid and more reaction time for the preparation of the product are not required.
The results showed (Table 3, entry 6) that oxalic acid can be linked by using ultrasonic irradiation at 60 °C within 4 hours under the Ar atmosphere. Thus, the Fe3O4-CH2CH2NH-CO-CO2H (Fe3O4@NH2@oxalic acid) nanoparticles were successfully synthesized, as shown in Scheme 4. The chemical structure of the Fe3O4@NH2@oxalic acid was characterized by FT-IR, XRD, SEM, VSM, and EDAX spectra.
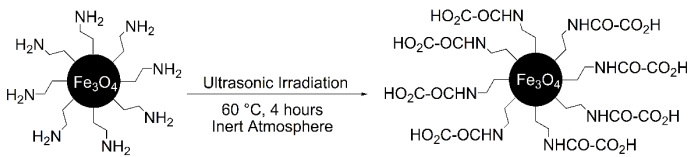
Scheme 4 The synthesis of Fe3O4@NH2@oxalic acid nanoparticles from Fe3O4@NH2 nanoparticles under ultrasonic irradiations.
The FT-IR spectrum of Fe3O4-CH2CH2-NH2 (Fe3O4@NH2) is shown in Fig. 2(a). The band at 3428 cm-1 was assigned to NH stretching vibration which was combined with OH stretching vibration of the absorbed water on Fe3O4. The bands at 2934 cm-1 and 2862 cm-1 were assigned to asymmetric stretching vibration and symmetric stretching vibration of CH2. The band at 1626 cm-1 was assigned to OH bending vibration of the absorbed water on Fe3O4. The band at 1547 cm-1 was assigned to NH bending vibration. The band at 1316 cm-1 was assigned to C-C stretching of vibration. The band at 1023 cm-1 was assigned to C-N stretching vibration and the band at 583 cm-1 was assigned to the Fe-O bending vibration [34b].
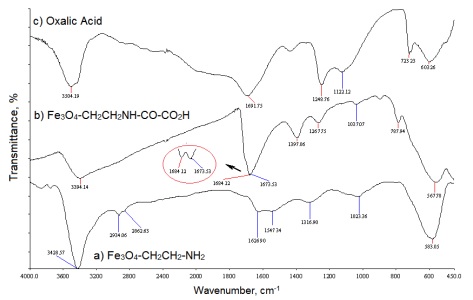
Fig. 2 The FT-IR spectra of (a) Fe3O4-CH2CH2-NH2 (Fe3O4@NH2), (b) Fe3O4-CH2CH2NH-CO-CO2H, (Fe3O4@NH2@oxalic acid) (c) oxalic acid.
The FT-IR spectrum of Fe3O4-CH2CH2NH-CO-CO2H (Fe3O4@NH2@oxalic acid) has been shown in Fig. 2(b). The band at 3394 cm-1 was assigned to OH stretching vibration of the COOH group. The band at 1684 cm-1 and 1673 cm-1 were assigned to C=O bond stretching vibrations of the COOH and CO-NH groups, respectively. The band at 1397 cm-1 was assigned to OH bending vibration of the COOH group. The band at 1267 cm-1 was assigned to C-O stretching vibration. The band at 1037 cm-1 was assigned to C-N stretching vibration. The band at 787 cm-1 was assigned to OH bending vibration of the COOH group, and 567 cm-1 was assigned to the Fe-O bending vibration.
A comparison of the FT-IR spectra of Fe3O4@NH2 in Fig. 2(a) and Fe3O4@NH2@oxalic acid in Fig. 2(b) shows that a band at 1626 cm-1 in Fig. 1(a) does not appear in the FT-IR spectrum of Fe3O4@NH2@oxalic acid Fig. 2(b). Because this band was replaced by a doublet band at 1684 cm-1 (acidic C=O) and 1673 cm-1 (amide C=O). This result that was indicated the successful attachment of oxalic acid to Fe3O4@NH2 nanoparticles.
The VSM spectrum was used to evaluate the magnetic measurement of magnetite nanoparticles at room temperature as shown in Fig. 3(a). Due to the lack of magnetic remanence and coercivity, the room-temperature magnetization curve of the Fe3O4@NH2 and Fe3O4@NH2@oxalic acid nanoparticles suggests a paramagnetic state of the nanoparticles. The magnetization curves indicate the saturation magnetization of Fe3O4@NH2@oxalic acid and Fe3O4@NH2 nanoparticles, which were diminished to 10.50 emu/g from 12.90 emu/g. These results are significantly smaller than reported values for bulk Fe3O4 (92 emu/g and 100 emu/g) [54]. In the Fe3O4@NH2 and Fe3O4@NH2@oxalic acid adsorbed water, amine agent, and oxalic acid reduce the measured magnetic strength because magnetic nanoparticles are diluted with diamagnetic physically.
The SEM images of nano-Fe3O4@NH2@oxalic acid (Fig. 3(b)) show the presence of spheric nanoparticles with 81 nm of average diameter.
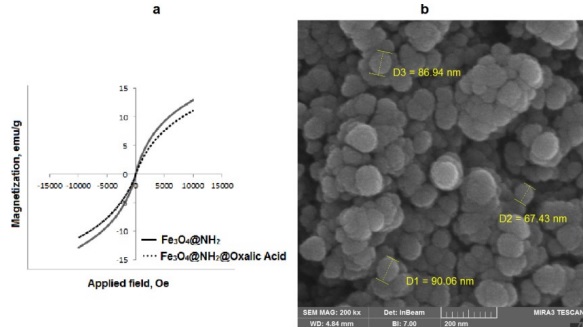
Fig 3 a) The room-temperature magnetization curve of the Fe3O4@NH2 and Fe3O4@NH2@oxalic acid nanoparticles. b) The SEM spectra of Fe3O4@NH2@oxalic acid.
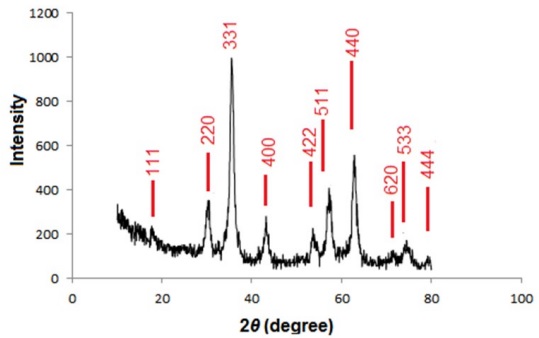
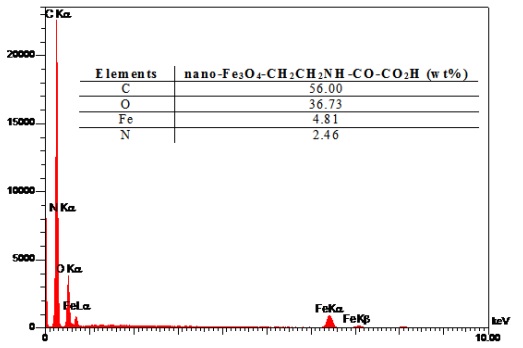
The XRD patterns of the Fe3O4@NH2@oxalic acid nanoparticles are presented in Fig. 4. The peak positions are indexed as (111), (220), (331), (400), (422), (511), (440), (620), (533) and (444) in the 2θ range of 20-80°. These results reveal the existence of a Fe3O4 core in the Fe3O4@NH2@oxalic acid which is in good agreement with the literature [54-55]. Fig. 5 shows EDAX analysis of Fe3O4@NH2@oxalic acid nanoparticles. The sample peaks are observed for carbon, oxygen, iron, and nitrogen. It reveals that carbon was from 1, 2-ethylenediamine and oxalic acid, oxygen from oxalic acid and Fe3O4, iron from Fe3O4, and nitrogen from 1, 2-ethylenediamine. These results shows that surface of Fe3O4 was coated more than 93 % by organic materials (1, 2-ethylenediamine, and oxalic acid).
The synthesized Fe3O4@NH2@oxalic acid nanoparticles were used for the synthesis of pyrrolin-2-one derivatives after optimum reaction conditions as shown in Table 4. It was best to combine benzaldehyde (1 mmol), aniline (1 mmol), and 0.2 g Fe3O4@NH2@oxalic acid nanoparticles as the catalyst in ethanol. The reaction was completed after 8 hours to give the corresponding pyrrolin-2-ones in 95 % yields as shown in Scheme 2.
Table 4 Optimization reaction conditions for the synthesis of 1,5-diphenyl-3-hydroxy-4-methoxycarbonyl-3-pyrrolin-2-one in the presence of Fe3O4@NH2@oxalic acid nanoparticles as catalyst at room temperature.a
| Entry | Catalyst | Amounts (g) | Solvent | t/h | Yields, %b |
| 1 | Fe3O4@NH2@oxalic acid | 0.1 | EtOH | 12 | 35 |
| 2 | Fe3O4@NH2@oxalic acid | 0.15 | EtOH | 12 | 60 |
| 3 | Fe3O4@NH2@oxalic acid | 0.2 | EtOH | 8 | 95 |
| 4 | Fe3O4@NH2@oxalic acid | 0.5 | EtOH | 6 | 93 |
| 5 | Fe3O4@NH2@oxalic acid | 1 | EtOH | 6 | 90 |
| 6 | Fe3O4@NH2@oxalic acid | 0.2 | MeOH | 2 | 90 |
| 7 | Fe3O4@NH2@oxalic acid | 0.2 | EtOAc | 24 | 75 |
| 8 | Fe3O4@NH2@oxalic acid | 0.2 | H2O | 24 | 0 |
| 9 | Fe3O4@NH2@oxalic acid | 0.2 | THF | 24 | 47 |
a benzaldehye (1 mmol), aniline (1 mmol), DAMD (1 mmol). b The yields were referred to as isolated products.
For more proofing, the reaction of a variety of anilines and aromatic aldehydes was performed under optimized reaction conditions. All the reactions were completed within 6-10 hours in the excellent yields of pure products (1-8a) (90-95 %) as shown in scheme 2. All products were characterized by FT-IR, 1H-NMR, 13C-NMR and C. H, N elemental analysis. In the 1H-NMR spectrum, the chemical shifts of the CH group in the heterocyclic ring appear around 5.62-6.41 ppm, as a single signal. In the FT-IR spectrum, the C=O stretching frequency of the products appears around 1675-1693 cm-1. The melting points of the products were measured and compared with the literature [30-35].
Due to the chemical structure of Fe3O4@NH2@oxalic acid, CO2H groups can provide an acidic surface with good polarity. All moieties in this reaction are polar. Thus, they can react and condensate more easily on Fe3O4@NH2@oxalic acid surface by dipolar-dipolar interactions. Fe3O4@NH2@oxalic acid nanoparticles activate the aldehyde functional group for reaction with aniline. Thus, an imine as an intermediate (step (I), Scheme 3) is produced. Then, the obtained H2O molecules from this condensation reaction, react with DMAD and produce a 1,3-dipolar intermediate (step (II), Scheme 3) [30], which would undergo the addition to the imine (step (III & IV), Scheme 3). Then, an intramolecular attack of the amino group on one of the esters results in the formation of the five-membered ring as pyrrolin-2-one skeleton (a, step (V), Scheme 3).
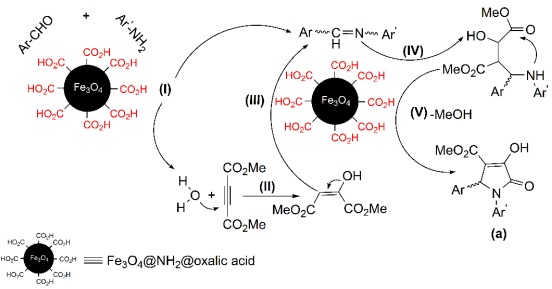
Scheme 3 The schematic and the tentatively proposed mechanism for the synthesis of pyrrolin-2-one derivatives by Fe3O4@NH2@oxalic acid as catalyst.
The reusability of Fe3O4@NH2@oxalic acid catalyst was examined. The recycled catalyst can be used three more times. For example, the second and third rounds yields of the product (Scheme 2, 1a) were 92 % and 93 % respectively. For more consideration, the efficiency of Fe3O4@NH2@oxalic acid nano-magnetic catalyst for the synthesis of 1,5-diphenyl-3-hydroxy-4-methoxycarbonyl-3-pyrrolin-2-one (Sheme 2, 1a) has been compared with other reported protocols as shown in Table 5. The comparison shows Fe3O4@NH2@oxalic acid catalyst has a good potential for the synthesis of pyrrolin-2-one derivatives.
Table 5 Comparison of synthesis of 1,5-diphenyl-3-hydroxy-4-methoxycarbonyl-3-pyrrolin-2-one by Fe3O4@NH2@oxalic acid with the different protocols.
| Entry | Protocol | Yield | t/h | Green Profile | Availability | Reusable Catalyst | Reference |
| 1 | Fe3O4@NH2@oxalic acid/EtOH/RT | 95 | 8 | yes | yes | yes | current |
| 2 | EtOH/H2O/RT | 87 | 15 | yes | yes | no | 29 |
| 3 | P-TsOH/EtOH/RT | 62 | 48 | no | yes | no | 30 |
| 4 | Citric Acid/US/ EtOH/RT | 90 | 0.3 | yes | no | no | 31 |
| 5 | P-TsOH/MW/EtOH/RT | 96 | 0.1 | no | no | no | 32 |
| 6 | TiO2 admicelle/H2O/RT | 75 | 18 | yes | no | no | 28 |
| 7 | [bmim]BF4/PEG-400/RT | 89 | 1 | yes | no | yes | 33a |
| 8 | MW/[BBSI]Cl/Ethylene glycol/RT | 90 | 0.1 | yes | no | yes | 33b |
| 9 | [BBSI]Cl/ball milling/RT | 90 | 0.5 | yes | no | yes | 33c |
| 10 | [BBSI]HSO4/ball milling/RT | 87 | 0.4 | yes | no | yes | 33c |
| 11 | Fe3O4@SiO2@Propyl–ANDSA/EtOH/RT | 87 | 8 | yes | no | yes | 34 |
| 12 | Fe3O4@PEG-400@oxalic acid/MeOH/RT | 90 | 24 | yes | no | yes | 35 |
| 13 | LEDs/Rose Benga /CH3CN-H2O | 94 | 0.8 | yes | no | yes | 56 |
| 14 | AmberChrom/brine/ EtOH/RT | 95 | 6 | yes | yes | yes | 35 |
Conclusions
In this research, the Fe3O4@NH2 nanoparticle was synthesized from FeCl3•6H2O and 1, 2-ethylenediamine. Then, Fe3O4@NH2@oxalic acid as an organoacid-magnetic nanoparticle was synthesized by coupling of Fe3O4@NH2 nanoparticle and oxalic acid under ultrasonic irradiations. Also, the nano-Fe3O4@NH2@oxalic acid was used for preparation of a variety of pyrrolin-2-ones. The reactions were performed in ethanol in the excellent of yields at room temperature. The recovered and regenerated catalyst can be useful for reaction with loss of activity. Thus, easy work-up, mild reaction conditions, recyclability, recovery, non-toxicity, economically affordable, and high efficiency are valuable characteristics that make this new method noteworthy.
Acknowledgements
The authors gratefully acknowledge the financial support of this work by the Research Council of the Islamic Azad University, Mahabad Branch, Mahabad, Iran. DOI:











 nueva página del texto (beta)
nueva página del texto (beta)