Special Issue
Fabrication of a Reactive Functionalized Microfilm with Aromatic Amines Applied to the Growth of Langerhans Cells
Fabricación de un microfilm funcionalizado reactivo con aminas aromáticas aplicado al crecimiento de células de Langerhans
-
Publication dates-
March 24, 2025
Jan-Mar , 2024
- Article in PDF
- Article in XML
- Automatic translation
- Send this article by e-mail
- Share this article +
Abstract.
This study reports the synthesis of ultrathin polymeric films through layer-by-layer deposition and covalent cross-linking of poly(2-vinyl-4,4'-dimethylazlactone) and branched poly (ethylene imine) (PEI) which were functionalized with aromatic amines that encompass anilines. To assess the effect of these aromatics molecules on the adhesion and proliferation of Langerhans β-cells, we prepared 35 bilayers of unfunctionalized and functionalized films with aromatic amines, which were characterized in terms of their physical, chemical, and biological properties by a battery of experimental techniques including 1H and 13C, NMR, mass spectrometry, attenuated total reflectance Fourier transform infrared spectroscopy, field emission scanning electron microscopy and cell adhesion and staining. The films were nanometric, transparent, resistant to manipulation, chemically reactive, and highly cytocompatible. We demonstrated that films functionalized with aromatic molecules support the attachment and growth of in vitro Langerhans β-cells. This study provides the basis for a general approach to designing and functionalizing ultrathin films that promote cell growth on surfaces of interest for investigation in cell biology studies and a broad range of other biomedical applications.
Keywords::
Ultrathin films, azlactone, β-cells, aromatic amine
Introduction
With much of our lives spent sedentarily and with food being so readily available, metabolic diseases are becoming a real problem.[1] Metabolic syndrome (MetS) is a collection of issues such as obesity, high blood pressure, high cholesterol, and difficulty in metabolizing carbohydrates, increasing the risk of type 2 diabetes.[2] Insulin resistance and hyperinsulinemia in the body are linked to MetS, but it's still unclear which of these comes first.[3-5] Both of these issues affect the pancreas, which produces, stores, and releases insulin to keep blood sugar levels in check. This connection between MetS and how the pancreas works shows the importance of understanding the problem with the pancreas in MetS. [6]
-
1J. Clin. Invest, 2019
-
2J. Clin. Invest, 2019
-
3Annu. Rev, 2003
-
5Obes. Rev, 2015
-
6Diabetes Care, 2003
A serious global health issue, diabetes mellitus is a non-communicable, multifactorial, complex metabolic condition.[7] It is characterized by abnormalities in the metabolism of carbohydrates, proteins, and lipids brought on by defects in insulin secretion, insulin synthesis, or both.[8,9] Obesity is a significant risk factor for the onset of type 2 diabetes (T2D), as is well known. Since 1975 to the present, the incidence of obesity has increased thrice globally, which has contributed to the sharp increase in T2D prevalence in recent years.[10,11] Due to increased adipose tissue bulk and decreased FFA clearance, obesity is linked to raised amounts of circulating free fatty acids (FFAs).[12] Higher amounts of free fatty acids being released from the esterification of fat in the body have been linked to this.[13] Rising evidence suggests that higher FFA levels may play a role in the etiology of T2D and hence serve as a mechanistic link between obesity and diabetes. According to a large body of evidence, the two main abnormalities that underlie the pathophysiology of T2D are insulin resistance and pancreatic β-cell dysfunction.[14,15] Lipotoxicity is a frequent term for these harmful effects of FFAs on glucose homeostasis.[16] The ability of islet cells to make, store, and insulin release in response to nutrients like glucose, lipids[17], and a subset of amino acids distinguishes them from other types of cells. The β-cells are incredibly sensitive to the nutrient’s environment and can change significantly the amount of insulin produced within minutes due to a complex process called stimulus-secretion coupling in response to changes in blood glucose levels (between 4.5 and 8 mM).[18-21]
-
7DNA Cell Biol, 2014
-
8Acta Biomed, 2005
-
9Diabetes Care, 2004
-
10J. Mol. Med. (Berl), 1996
-
11Apoptosis, 2009
-
12Diabetes, 2008
-
13Lancet, 1963
-
14Clin. Sci. (Lond), 2007
-
15Cells, 2019
-
16Endocr. Rev, 2002
-
17Nutrients, 2018
-
18EMBO J., 1993
-
21Nat. Rev. Immunol, 2011
Surface-microfabrication techniques have been widely used to control cells in cultures. Different surface properties like charge, hydrophilicity, and topology have been used to influence how cells attach. Also, recently, different types of molecules have been attached to regulate growth, differentiation, and apoptosis.[22]
-
22Biomaterials, 1999
This research builds on other studies that have discussed creating materials that can either help or prevent mammalian cells and bacterial cells from sticking on a surface.[23-25] In this study, we report a step toward these broad goals through the creation of covalently crosslinked and chemically reactive ultrathin films that are easily modifiable to encourage cell adhesion. Our method is based on techniques for the reactive layer-by-layer fabrication of thin polymer films to create surface coatings that can be functionalized and patterned post-fabrication to present a wide range of chemical or biological functionality.[26] These films will allow us to seed β-cells to analyze their adhesion and growth behaviour with aromatic amines; their study is vital because of the important role β-cells play in glucose homeostasis.[27]
-
23J. Polym. Sci. A Polym. Chem, 2017
-
25Science, 1994
-
26ACS Omega, 2020
-
27Mol. Cell Endocrinol, 2009
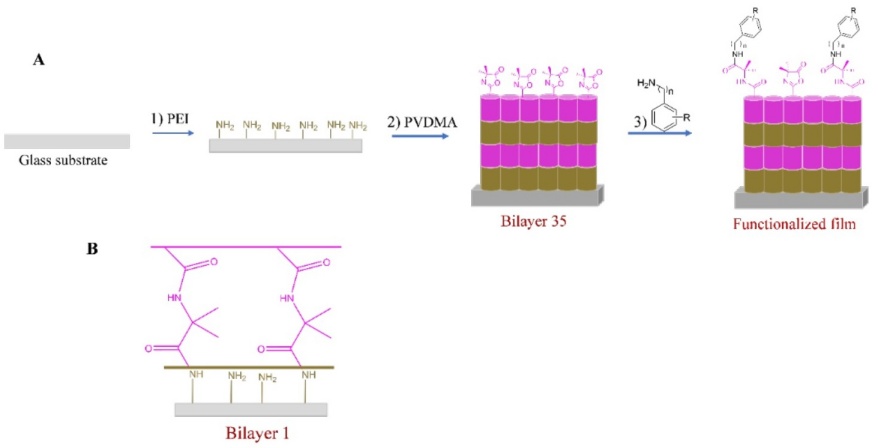
The method described here is based on techniques for fabricating thin polymer films on surfaces one layer at a time.[28] Given that layer-by-layer assembly allows for precise control over the thicknesses and compositions of multicomponent polymer films and can be used to deposit conformal coatings on the surfaces of topographically or topologically complex substrates, it is a particularly useful technique for fabricating surface coatings.[29] Our prior research has shown that the chemically reactive polymer poly(2-vinyl-4,4'-dimethylazlactone) (PVDMA) can be used to produce crosslinked thin films via reactive layer-by-layer assembly. The PVDMA azlactone activity quickly forms a 'click'-type reaction with primary amines, laying the groundwork for reactive layer-by-layer assembly with polymers containing primary amine groups.[30,31] The structure of this strategy is shown schematically in Fig. 1.
-
28Science (1979), 1997
-
29Adv.Mater, 2006
-
30Adv. Mater, 2007
-
31Drug Discov. Today, 2003
Thumbnail
Fig. 1
(A) Schematic drawing of the prepared LBL assembly, (B) LBL film with PVDMA at the uppermost layer (35 bilayers).
(A) Schematic drawing of the prepared LBL assembly, (B) LBL film with PVDMA at the uppermost layer (35 bilayers).
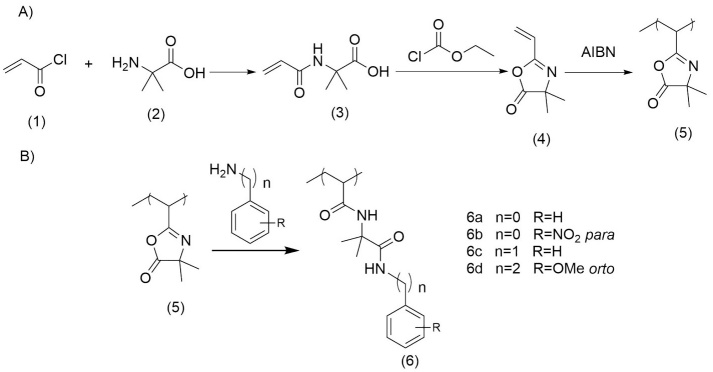
The synthesis of PVDMA was carried out by condensing acryloyl chloride (1) with 2-methylalanine (2) to produce the amide (3), which was reacted with ethyl chloroformate to obtain the vinyl-azlactone (VDMA) (4). This monomer was polymerized with the activation of AIBN to obtain PVDMA (5) (see Fig.2(A)). In particular, PVDMA reacts rapidly at room temperatures with primary amines, and aromatics amines in this study, making these materials attractive for the synthesis of a functionalized reactive thin film that can be easily modified post-fabrication with any chemical and/or biological motif that permits the interactions with cells. Due to its swiftness and precision, this ring-opening reaction has become a handy resource for many organic synthesis projects (Fig. 2(B)).
Thumbnail
Fig. 2
(A) Steps to obtain the PVDMA, (B) The ring-opening reaction of PVDMA with aromatics amines.
(A) Steps to obtain the PVDMA, (B) The ring-opening reaction of PVDMA with aromatics amines.
In this report, we describe the fabrication of a PVDMA/PEI ultrathin thin film functionalized with aromatic amines (aniline (6a), 4-nitroaniline (6b), benzylamine (6c), and 2-methoxyphenethylamine (6d)), to demonstrate that these films functionalized with HAAs can promote adhesion and growth of β-cells.[32] During food cooking, heterocyclic aromatic amines (HAAs) are produced. HAAs have been tested in mice, proving to be carcinogenic, inducing liver, gastrointestinal tract, pancreas, mammary gland, and prostate cancers. These results with HAAs in solution. In this report, the aromatic amines covalently bound to the films were studied, and the capacity of this modified surface on β-cell adhesion and proliferation was evaluated. Therefore, it is important to study the biocompatibility of films functionalized with aromatic amines. Inspection of the optical images reveals that β-cells have adhered and grown normally on these surfaces.
-
32Chem. Soc. Rev, 2000
Experimental
Materials and methods
Acryloyl chloride (97 % containing 400 ppm phenothiazine stabilizer), 2-methylalanine (98 %), ethyl chloroformate (97 %), triethylamine (99 %), NaOH (reagent grade), HCl (reagent grade, 37 %), 1, 4-dioxane (99.8 %), 2,2'-azobis (2-methylpropionitrile) (98 %), Branched poly (ethylene imine) (PEI, MW = 25000) reagent grade, DMSO, hexane, acetone, glass microscope slides, aniline, 4-nitroaniline, benzylamine and 2-methoxyphenethylamine were purchased from a commercial source (Aldrich) and use without further purification. Anhydrous solvents for organics synthesis were prepared by passing through a solvent purification tower. Compressed air used to dry films and coated substrates was purchased from Aldrich.
General procedures
Glass substrates (76 × 25 mm) used as a surface for the films, were cleaned with acetone, ethanol, methanol, and deionized water and dried with compressed air. The optical thicknesses of films deposited on glass substrates were determined using Field Emission Scanning Electron Microscopy (FESEM), thicknesses were determined at least five different standardized locations on each substrate. Laser scanning confocal microscopy images were acquired on a Leica TCS SP8 laser scanning confocal microscope (Mannheim, Germany). Thin-layer chromatography (TLC) was performed on silica gel F254 plates (Merck). All compounds were detected using UV light. Melting points were obtained on an Electrothermal 88629 apparatus and are not corrected. Infrared spectra (IR) were recorded on a Perkin Elmer FT-IR 1600 spectrometer. 1H and 13C NMR spectra at 400 MHz and 100 MHz, respectively, were recorded on a Bruker Avance III spectrometer in CDCl3, DMSO-d6 with TMS as the internal standard. Mass spectra were obtained on an Agilent Technologies 5975C MS Spectrometer at 70 eV by direct insertion and an Agilent HPLC (Mod 1100) coupled to MSD version SL. UV/Vis absorption spectra were obtained using Cary 50 spectrophotometer. Fluorescence spectra were recorded on a Photon Technology International Fluorescence System (USA) with a 1 cm standard quartz cell. Single crystal X-Ray diffraction Smart Apex. Gel permeation chromatography (GPC) was performed on a Variant 9002 chromatograph equipped with a series of three columns (Phenogel: OH-646-K0, OH-645-K0, and OH-643-K0) and two detectors: a refractive index detector (Varian RI-4 and a triangle light scattering detector (LS detector MINI-DAWN, Wyatt). The measurements were performed in THF at 35 ℃. Polystyrene standards were used for the calibration of the LS detector. THF was used for the mobile phase at a 0.7 mL/min flow rate. Sample solutions were prepared using 20 mg/mL concentration and filtered through a 0.45 µm PTFE membrane filter before analysis.
Synthesis of 4,4-dimethyl-2-vinyloxazol-5(4H)-one (4). VDMA (4) monomer was synthesized using procedures reported by Rivero et al. [26]
-
26ACS Omega, 2020
2-acrylamide-2-methylpropanoic acid (3). 2-Methylalanine (2) (2.0 g, 0.0194 mol) in NaOH (1.8 g, 0.0442 mol), 2,3-di-tert-butyl-4-methoxyphenol (2.0 mg, 0.009 mmol) was weighed into a 100-mL round-bottomed flask equipped with a magnetic stir bar and dissolved in distilled water (4.8 mL). The solution was stirred in an ice bath until the temperature was ~ 4 ℃. Acryloyl chloride (1) (2.0 g, 0.0221 mol) was added slowly using an addition funnel for approximately 5 min. The reaction was stirred for 3 hours. After that, concentrated HCl (~ 2.5 mL) was added slowly to the reaction solution until the solution reached pH 2, and a white precipitate formed. The solution was stirred for an additional 30 min on ice. The product was filtered in a Buchner funnel and rinsed with cold acetone. White solid. Yield: 2.25 g, 74%. Rf = 0.66 (ethyl acetate 100 %). mp 187-189 ºC. FT-IR (ATR) v / cm-1 3340(N-H), 3073(C-H), 2991(C-H, CH3), 1705(C=O), 1649(C=O), 1599(C=C). 1H NMR (400 MHz, DMSO d6) δ 8.35 (s, 1H, NH), 6.26 (dd, Htrans, J=17.0 Hz, JHtrans-Hcis=10.0 Hz, 1H, Vin), 6.02 (dd, Htrans, J=17.2 Hz, JHtrans-Hgem=2.4 Hz, 1H, Vin), 5.55 (dd, Hcis, J=10.0 Hz, JHcis-Hgem=2.4 Hz, 1H, Vin), 1.34 (s, 6H, C(CH3)2). 13C NMR (100 MHz, CDCl3) δ 176.0, 132.0, 126.0, 125.9, 55.67, 25.60. MS m/z: 157.2+ (4 %), 112.2+(100 %) amu.
4,4-dimethyl-2-vinyloxazol-5(4H)-one (4). N-Acryloyl-2-methylalanine (3) (1.9 g, 0.0122 mol), triethylamine (2.5 mL, 0.0183 mol), and acetone (44.0 mL) were combined in a two-neck flask round-bottomed flask. The reaction mixture was purged with argon while cooling in an ice bath until the temperature was about 5 °C. Ethyl chloroformate (1.7 mL, 0.0183 mol) was added very slowly using a pressure-equalizing addition funnel. Once the addition was complete the reaction was stirred on ice under an inert atmosphere for 3 hours. The solution was filtered using a Buchner funnel, and the precipitate was washed with cold acetone. The filtrate was concentrated via rotary evaporation and purified by vacuum distillation (bp ~ 70 ℃, 60 mbar). Clear liquid. Yield: 0.86 g, 45%. Rf = 0.83 (ethyl acetate 100%). FT-IR (ATR) v / cm-1 2984(C-H), 2938(C-H, CH3), 2868(C-H, CH3), 1818(C=O, ester), 1666(C=N imine), 1596(C=C). 1H NMR (400MHz DMSO d6) δ 6.30 (dd, Htrans, J=17.6 Hz; JHtrans-Hcis= 9.60 Hz, 1H, Vin), 6.23 (dd, Htrans, J=17.6 Hz; JHtrans-Hgem=2.0 Hz, 1H, Vin), 5.92 (dd, Hcis, J=9.60 Hz, JHcis-Hgem=2.0 Hz, 1H, Vin), 1.47(s, CH3, 6H). 13C NMR (100 MHz, CDCl3) δ 180.6, 159.1, 129.0, 124.1, 65.5, 24.4. MS m/z: 139.2+(5%), 111.2+ (45%), 95.2+ (43%) amu.
Synthesis of Poly(2-vinyl-4,4’-dimethylazlactone) (PVDMA) (5). AIBN, 2,2’-azobisisobutyronitrile (2.6 mg, 0.02 mmol) was added into a 10-mL Schlenk flask equipped with a stir bar, anhydrous 1,4-dioxane (0.7 mL) was added. The mixture was stirred until the AIBN was dissolved completely. Later, VDMA (4) (0.2 g, 1.6 mmol) was added to the flask, and the flask was capped with a septum and purged with argon for 25 min. The reaction mixture was stirred at 70 ℃ for 16 hours. At the end of the reaction, the flask was cooled in an ice bath, acetone (~1 mL) was added, and hexane was added to precipitate the product. The polymer was precipitated twice in hexanes. White solid. Yield 0.2 g, 95%. GPC: Mn=67,300 g/mol; PDI=1.03. FT-IR (ATR) ν / cm1 2981, 2933 (C-H), 1818 (C=O), 1666 (C=N). 1H NMR (400 MHz, CD3COCD3) δ 2.84(m, 2H, CH, -CH2CH-), 2.01(m, 1H, -CH2CH-), 1.37(s, 6 H, -CH3).
Layer-by-Layer fabrication of films
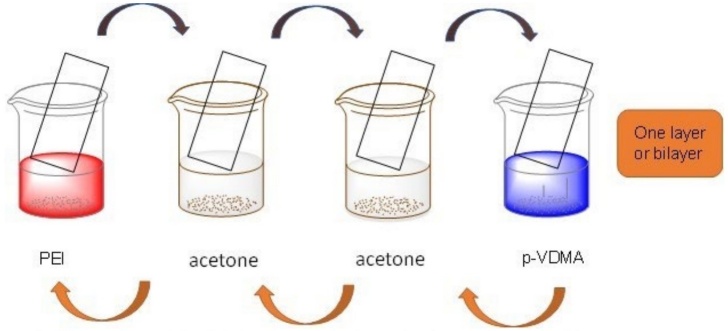
Solutions of PEI and PVDMA were prepared in acetone (20 mM regarding the molecular weight of the polymer repeat segment). All the ultrathin films were fabricated layer-by-layer on glass substrates automatically based on the following: (1) the glass was introduced into the solution of PEI for 20 s, (2) the glass was removed and submerged in an acetone bath for 20 s followed by a second acetone bath for another 20 s, (3) glass was introduced in a solution of PVDMA for 20 s, and (4) glass was rinsed in the form described above. This sequence was repeated in 35 cycles. Films were dry under a stream of compressed air and stored in a vacuum desiccator. All films were fabricated at room temperature.
Post-fabrication functionalization of thin films
PEI / PVDMA films 35 bilayers were functionalized post-fabrication by immersing film-coated substrates in solutions of either aniline, 4-nitroaniline, benzylamine, and 2-methoxyphenethylamine 20 mM in DMSO at room temperature for ~24 hours. Films were soaked in DMSO for ~1 hour after functionalization and the DMSO was changed at least once during the soak, washed with ETOH, and dried with compressed air before being analyzed or being used to grow β-cell cultures.
Characterization of adhesion and growth of β cells on PEI/PVDMA films
β-cell line RIN-m5F cells (American Type Culture Collection, ATCC, USA) were grown in RPMI-1640 medium supplemented with 10 % fetal bovine serum, 10 U/mL penicillin, 10 µg/mL streptomycin, and 25 µg/mL amphotericin B. The culture medium was changed every 3 to 4 days and also passaged once per week, according to previous work.[33] Cultures were maintained at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2. Cells were seeded onto 100 mm plates at 230,000 cells/mL density and allowed to grow at 90 % of confluence. Next, cell cultures were recovered and grown on coverslips composed of PEI/PVDMA films 35 bilayers at a density of 2.3 X106 cells in a 10 mL RMPI culture medium. Furthermore, at 72 of incubation, cells were rinsed twice with cold PBS 1X, fixed with paraformaldehyde (2 %), and crystal violet stained. In previous work, this cell line was a cellular model to characterize physiopathological conditions such as metabolic overload.[34]
-
33Metabolites, 2022
-
34Biomolecules, 2020
Results and discussion
The LBL technique enables the assembly of reactive polymer thin films in a wide variety of substrates. This film was created in acetone and is driven by an interfacial reaction between poly (2-alkenyl azlactone) and branched poly (ethylene imine). We created ultrathin films that are crosslinked and chemically reactive, making them easy to modify with aromatic amines. The films can promote β-cell attachment by presenting a broad range of chemical or biological functionality. The approach uses azlactone-functionalized polymers, which are highly versatile and reactive, to create surface coatings. This innovative technique could lead to significant advancements in the field of cell adhesion and communication, then represent an adequate model to characterize the physiology of Langerhans β-cells.
Surface reactivity is essential for creating materials that can be chemically modified with molecules like aromatic amines to be used for seeding cells.[35]
-
35Adv. Funct. Mater, 2016
As mentioned, the ultrathin film was made in an automated way, which has 4 stations as shown in Fig. 3; this cycle is repeated until the desired number of layers is obtained, for this study, it was 70 layers or 35 bilayers.
Thumbnail
Fig. 3
Schematic illustration of the fabrication of the layer-by-layer ultra-thin films.
Schematic illustration of the fabrication of the layer-by-layer ultra-thin films.
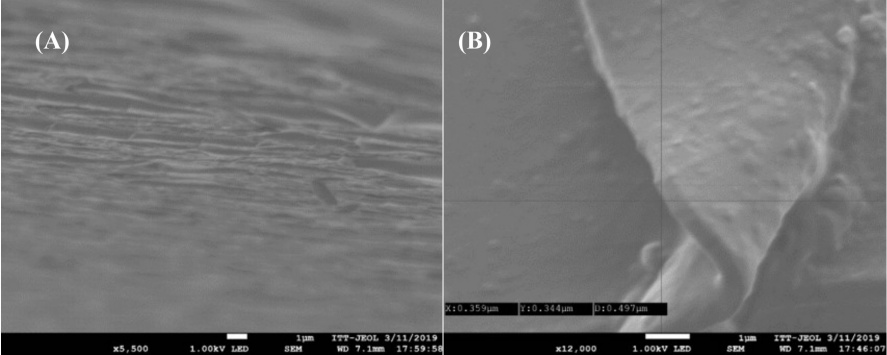
All films used in these initial studies were deposited on a glass slide substrate to allow the characterization of film thicknesses and growth profiles using FESEM. Fig. 4 shows the appearance, thickness, and topography of the ultrathin films.
Thumbnail
Fig. 4
Representative FESEM images of the film: (A) image of a 35-bilayer thin film, (B) and its measure.
Representative FESEM images of the film: (A) image of a 35-bilayer thin film, (B) and its measure.
The thickness of the film was determined by FESEM by measuring in at least five different points, giving an average value of 524 nm. It should be mentioned that the transparent films were obtained in an automated system so that each layer of the film was built up uniformly. The fabrication of ultrathin films on glass substrates allowed the characterization of the chemical structure of these materials using reflective infrared (IR) spectroscopy. Inspection of these data reveals an absorbance peak at 1820 cm-1 corresponding to the carbonyl in the azlactone group of PVDMA. Closer inspection reveals the presence of a second absorbance peak at 1647 cm-1, which corresponds to C-N functionality on the azlactone ring. Functionalized PVDMA−PEI ultrathin films have been used in other studies to enhance cell performance and manage cell growth, and they are reliable and sturdy even in biological settings. [36,37]
-
36Fabrication of Amine-Reactive Polymer Multilayers on Microwell Cell Culture Arrays: Combining Methods for the Topographic Patterning of Cell Substrates with Approaches to Facile Surface Functionalization, 2011
-
37J. Biomed. Mater. Res. A, 2017
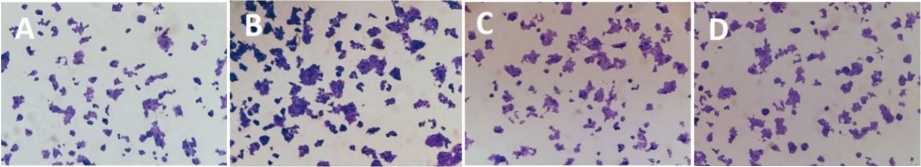
To move our research ahead, we looked into how well β-cells adhere and grow on PVDMA−PEI films with aromatic amines (2a-d).
Fig. 5 shows optical images of the ultrathin films functionalized with aromatic amines. In this sense, we performed the growth of the β-cell cultures on the PVDMA−PEI films, representative images are shown, which correspond to 2a (Fig. 5(A)), 2b (Fig. 5(B)), 2d (Fig. 5(C)), and 2c (Fig. 5(D)). The results suggest an adhesion process and, therefore, a differential growth between the different samples, particularly a higher capacity for 2b (Fig. 5(B)). The images show normal growth in 2D and less growth in 2A and 2C. It is the nitro group (electron withdrawing group) that influences the greater growth of beta cells. The mechanisms are complex, but they could be associated with the adsorption and sequestration of signaling molecules such as growth factors and membrane receptors.
Thumbnail
Fig. 5
Schematic representation of the ultrathin films functionalized with: (A) aniline, (B) 4-nitroaniline. (C) benzylamine and (D). 2-methoxyphenetylamine.
Schematic representation of the ultrathin films functionalized with: (A) aniline, (B) 4-nitroaniline. (C) benzylamine and (D). 2-methoxyphenetylamine.
Conclusions
The layer-by-layer technique has recently been validated as a practical and versatile approach to constructing functional films. The process of Lbl assembly is straightforward, and its interest has grown due to its flexibility and ability to control the thickness of the film growth.[26,38] Based on that, ultrathin polymeric films of 35 bilayers of PVDMA/PEI were constructed. The ultrathin films were robust, transparent, and resistant again mechanical manipulation and pressure, with smooth and uniform surfaces. The films were functionalized with aromatics amines like aniline, 4-nitroaniline, benzylamine, and 2-methoxyphenethylamine and β cells were seeded. The results of this work suggest that the PVDMA/PEI ultrathin films can be used to modify surfaces with chemical or biological functionality, like proteins, peptides, or small molecules like aromatics amines, to design ultrathin films to investigate β-cells or other broad areas of medicine or biotechnology. It is worth mentioning that the nitro group interacts with the membrane proteins of the β-cells, resulting in cytocompatibility for the cells.
-
26ACS Omega, 2020
-
38Chem. Soc. Rev, 2012
Acknowledgements
We gratefully acknowledge the support for this project of the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT, GRANT No. CF-2023-I-327) and postdoctorate scholarship (No. 559644), we also acknowledge Tecnológico Nacional de México for the support of this project (No. 7814-20P).
References
-
1Hudish, L. I.; Reusch, J. E. B.; Sussel, L. J. Clin. Invest. 2019, 129, 4001-4008. DOI: https://doi.org/10.1172/JCI129188. Links
-
2Smith, G. I.; Mittendorfer, B.; Klein, S. J. Clin. Invest. 2019, 129 , 3978-3989. DOI: https://doi.org/10.1172/JCI129186. Links
-
3Reaven, G. M. Annu. Rev. 2003, 44, 121-131. DOI: https://doi.org/10.1146/ANNUREV.ME.44.020193.001005. Links
-
4Reaven, G. M. Diabetes 1988, 37, 1595-1607. DOI: https://doi.org/10.2337/DIAB.37.12.1595. Links
-
5O’Neill, S.; O’Driscoll, L. Obes. Rev. 2015, 16 , 1-12. DOI: https://doi.org/10.1111/OBR.12229. Links
-
6Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M. P.; Haffner, S. M. Diabetes Care- 2003, 26, 3153-3159. DOI: https://doi.org/10.2337/DIACARE.26.11.3153. Links
-
7Anuradha, R.; Saraswati, M.; Kumar, K. G.; Rani, S. H. DNA Cell Biol. 2014, 33, 743-748. DOI: https://doi.org/10.1089/DNA.2014.2352. Links
-
8Dotta, F.; Fondelli, C.; Di Mario, U. Acta Biomed. 2005, 76 Suppl 3, 14-18. Links
-
9Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Diabetes Care. 2004, 27, 1047-1053. DOI: https://doi.org/10.2337/DIACARE.27.5.1047. Links
-
10Pérez-Bravo, F.; Carrasco, E.; Gutierrez-López, M. D.; Martínez, M. T.; Lopez, G.; García De Los Rios, M. J. Mol. Med. (Berl). 1996, 74, 105-109. DOI: https://doi.org/10.1007/BF00196786. Links
-
11Krijnen, P. A. J.; Simsek, S.; Niessen, H. W. M. Apoptosis. 2009, 14, 1387. DOI: https://doi.org/10.1007/S10495-009-0419-6. Links
-
12Martinez, S. C.; Tanabe, K.; Cras-Méneur, C.; Abumrad, N. A.; Bernal-Mizrachi, E.; Permutt, M. A. Diabetes. 2008, 57, 846-859. DOI: https://doi.org/10.2337/DB07-0595. Links
-
13Randle, P. J.; Garland, P. B.; Hales, C. N.; Newsholme, E. A. Lancet. 1963, 1, 785-789. DOI: https://doi.org/10.1016/S0140-6736(63)91500-9. Links
-
14Newsholme, P.; Keane, D.; Welters, H. J.; Morgan, N. G. Clin. Sci. (Lond). 2007, 112, 27-42. DOI: https://doi.org/10.1042/CS20060115. Links
-
15Acosta-Montaño, P.; Rodríguez-Velázquez, E.; Ibarra-López, E.; Frayde-Gómez, H.; Mas-Oliva, J.; Delgado-Coello, B.; Rivero, I. A.; Alatorre-Meda, M.; Aguilera, J.; Guevara-Olaya, L.; García-González, V. Cells. 2019, 8. DOI: https://doi.org/10.3390/CELLS8080884. Links
-
16Lewis, G. F.; Carpentier, A.; Adeli, K.; Giacca, A. Endocr. Rev. 2002, 23, 201-229. DOI: https://doi.org/10.1210/EDRV.23.2.0461. Links
-
17Acosta-Montaño, P.; García-González, V. Nutrients. 2018, 10. DOI: https://doi.org/10.3390/NU10040393. Links
-
18Heimberg, H.; De Vos, A.; Vandercammen, A.; Van Schaftingen, E.; Pipeleers, D.; Schuit, F. EMBO J. 1993, 12, 2873-2879. DOI: https://doi.org/10.1002/J.1460-2075.1993.TB05949.X. Links
-
19Gupta, D.; Jetton, T. L.; LaRock, K.; Monga, N.; Satish, B.; Lausier, J.; Peshavaria, M.; Leahy, J. L. J. Biol. Chem. 2017, 292, 12449-12459. DOI: https://doi.org/10.1074/JBC.M117.781047. Links
-
20Poitout, V.; Amyot, J.; Semache, M.; Zarrouki, B.; Hagman, D.; Fontés, G. Biochim. Biophys. Acta. 2010, 1801, 289-298. DOI: https://doi.org/10.1016/J.BBALIP.2009.08.006. Links
-
21Donath, M. Y.; Shoelson, S. E. Nat. Rev. Immunol. 2011, 11, 98-107. DOI: https://doi.org/10.1038/NRI2925. Links
-
22Ito, Y. Biomaterials. 1999, 20, 2333-2342. DOI: https://doi.org/10.1016/S0142-9612(99)00162-3. Links
-
23Wancura, M. M.; Anex-Ries, Q.; Carroll, A. L.; Paola Garcia, A.; Hindocha, P.; Buck, M. E. J. Polym. Sci. A Polym. Chem. 2017, 55, 3185-3194. DOI: https://doi.org/10.1002/POLA.28664. Links
-
24Buck, M. E.; Breitbach, A. S.; Belgrade, S. K.; Blackwell, H. E.; Lynn, D. M. Biomacromolecules. 2009, 10, 1564. DOI: https://doi.org/10.1021/BM9001552. Links
-
25Singhvi, R.; Kumar, A.; Lopez, G. P.; Stephanopoulos, G. N.; Wang, D. I. C.; Whitesides, G. M.; Ingber, D. E. Science. 1994, 264, 696-698. DOI: https://doi.org/10.1126/SCIENCE.8171320. Links
-
26Ávila-Cossío, M. E.; Rivero, I. A.; García-González, V.; Alatorre-Meda, M.; Rodríguez-Velázquez, E.; Calva-Yáñez, J. C.; Espinoza, K. A.; Pulido-Capiz, Á. ACS Omega. 2020, 5, 5249-5257. DOI: https://doi.org/10.1021/ACSOMEGA.9B04313. Links
-
27Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Quesada, I.; Ropero, A. B. Mol. Cell Endocrinol. 2009, 304, 63-68. DOI: https://doi.org/10.1016/J.MCE.2009.02.016. Links
-
28Decher, G. Science (1979). 1997, 277, 1232-1237. DOI: https://doi.org/10.1126/SCIENCE.277.5330.1232. Links
-
29Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N. A. Adv.Mater. 2006, 18, 3203-3224. DOI: https://doi.org/10.1002/ADMA.200600113. Links
-
30Buck, M. E.; Zhang, J.; Lynn, D. M. Adv. Mater. 2007, 19, 3951-3955. DOI: https://doi.org/10.1002/ADMA.200700822. Links
-
31Kolb, H. C.; Sharpless, K. B. Drug Discov. Today. 2003, 8, 1128-1137. DOI: https://doi.org/10.1016/S1359-6446(03)02933-7. Links
-
32Mrksich, M. Chem. Soc. Rev. 2000, 29, 267-273. DOI: https://doi.org/10.1039/A705397E. Links
-
33Guevara-Olaya, L.; Chimal-Vega, B.; Castañeda-Sánchez, C. Y.; López-Cossio, L. Y.; Pulido-Capiz, A.; Galindo-Hernández, O.; Díaz-Molina, R.; Ruiz Esparza-Cisneros, J.; García-González, V. Metabolites. 2022, 12. DOI: https://doi.org/10.3390/METABO12080754. Links
-
34Martínez-Navarro, I.; Díaz-Molina, R.; Pulido-Capiz, A.; Mas-Oliva, J.; Luna-Reyes, I.; Rodríguez-Velázquez, E.; Rivero, I. A.; Ramos-Ibarra, M. A.; Alatorre-Meda, M.; García-González, V. Biomolecules. 2020, 10, 1-21. DOI: https://doi.org/10.3390/BIOM10091201. Links
-
35Manna, U.; Raman, N.; Welsh, M. A.; Zayas-Gonzalez, Y. M.; Blackwell, H. E.; Palecek, S. P.; Lynn, D. M. Adv. Funct. Mater. 2016, 26, 3599-3611. DOI: https://doi.org/10.1002/ADFM.201505522. Links
-
36Broderick, A. H.; Azarin, S. M.; Buck, M. E.; Palecek, S. P.; Lynn, D. M. in: Fabrication of Amine-Reactive Polymer Multilayers on Microwell Cell Culture Arrays: Combining Methods for the Topographic Patterning of Cell Substrates with Approaches to Facile Surface Functionalization. AIChE January 1, 2011, 72-73. https://experts.umn.edu/en/publications/fabrication-of-amine-reactive-polymer-multilayers-on-microwell-ce , accessed June 2023. Links
-
37Weeks, C. A.; Aden, B.; Zhang, J.; Singh, A.; Hickey, R. D.; Kilbey, S. M.; Nyberg, S. L.; Janorkar, A. V. J. Biomed. Mater. Res. A. 2017, 105, 377-388. DOI: https://doi.org/10.1002/JBM.A.35910. Links
-
38Li, Y.; Wang, X.; Sun, J. Chem. Soc. Rev. 2012, 41, 5998-6009. DOI: https://doi.org/10.1039/C2CS35107B. Links