Introduction
Parasite diseases have enormous health, social, and economic impact worldwide [1] because of their high prevalence rates and morbidity. Indeed, it has been reported estimations regard 20 % of the world’s population is infected by at least one parasite [2]. In this sense, trichomoniasis, a sexually transmitted protozoal infection caused by Trichomonas vaginalis, affects both women and men with a high incidence/prevalence worldwide. The WHO estimated that in 2016, the global prevalence and incidence estimates of trichomoniasis were 5.9% and 156 million cases, respectively [3].
Trichomoniasis has been catalogued as a mild and curable disease that, in most cases, courses without symptoms. However, it can cause pelvic inflammatory disease, infertility, and adverse pregnancy outcomes such as premature rupture of membranes, preterm birth, and low birth weight [4]. Importantly, an aspect of concern of this disease is its association with a predisposition to cervical intraepithelial neoplasia or prostate cancer, as well as with an increased risk of acquisition of human papillomavirus (HPV) and human immunodeficiency virus (HIV) [4,5].
Metronidazole and tinidazole, 5-nitroimidazoles developed decades ago [6], are the current drugs approved by the Food and Drug Administration for the systemic treatment of trichomoniasis. Unfortunately, since 1990, the appearance of resistance to these drugs has been described [7,8]. Thus, these facts show the obvious necessity to search for new trichomonacidal compounds.
It is well known that thiazole derivatives exhibit a wide range of biological activities, including anti-protozoal properties; consequently, this heterocycle system is considered a privileged structural motif [9]. As part of our search for anti-protozoal heterocyclic compounds and accordingly to those data, few years ago, in our laboratory were synthesized a series of 2-amino-4-phenylthiazole derivatives which showed to have interesting activity against trophozoites of Giardia lamblia [10]. More recently, we found that two acetamide derivatives also have potent activity against Trichomonas vaginalis (IC50 = 0.60 µM) [11].
On the other hand, one strategy used in the search for new bioactive compounds is fusing a privileged structure with relevant biological active scaffolds. In this context, sulfonamide is considered relevant scaffold because its derivatives have, besides their well-known antibacterial properties, important biological activities. Also, it is remarkable that hybrids obtained by adding a sulfonamide group to biologically active scaffolds showed new activities or an enhancement of the previous showed activities [12]. An excellent example of those hybrids is sulphimidazole, which presents sulfonamide and 5-nitroimidazole moieties in its structure and exhibits a remarkable activity against T. vaginalis strains [13,14].
Thereby, based on our previous findings and the antitrichomonal activity exhibited by sulphimidazoles, we decided to synthesize several benzenesulfonamide derivatives of 2-amino-4-phenyl thiazole, to evaluate their antitrichomoniasic capability their activity against trophozoites of T. vaginalis, and to perform a docking study on Trichomonas vaginalis ferredoxin (TvFn).
Materials and methods
All commercial reagents were obtained from Sigma-Aldrich (Saint Louis, MO, USA) and used as received. Microwave reactions were conducted in 25 mL open glass vessels using a CEM Discover microwave reactor. Analytical thin-layer chromatography (TLC) was performed on 25 μm particle size silica gel GF-254 aluminum plates. Column chromatography was undertaken with 0.063-0.037 mm particle size silica gel. FT-IR spectra were recorded on a Nicolet iS5 FTIR spectrometer, using an accessory for attenuated total reflectance (ATR). 1H-NMR and 13C-NMR spectra were recorded in DMSO-d6 on Bruker Avance 400 spectrometer at 400 and 100 MHz, respectively, and the residual solvent peak was used as an internal reference. The δ values are given in ppm. Mass spectra (MS) were obtained on a Jeol GC-Mate II under electron impact (EI) at 70 eV.
General procedure for the synthesis of thiazole derivatives
Thiazole derivatives were synthesized following the methodology previously reported by us [13]. Briefly, a mixture of thiourea (17.2 mmol, 1.3 g), p-substituted acetophenone (8.6 mmol), and iodine (8.6 mmol, 2.2 g) were placed in an open vessel and subjected to MW irradiation (50 W) at indicated temperature for 10 min. After, the crude mixture was cooled to 70 °C and then it was triturated, filtered, and washed with Et2O. The crude product was dissolved in hot water at pH 11-12. The precipitated was filtered and crystallized from EtOH-H2O (1:4) to obtain the 2-amino-4-aryl-1,3-thiazole derivative.
General procedure for the synthesis of compounds 5 ,6a, 6c-f, 7a, 7c-f
A thiazole derivative (1.7 mmol), DMAP (0.17 mmol), and triethylamine (3.4 mmol) were added to a solution of a 2.5 mmol of the suitable sulphonyl chloride in 9 mL of anhydrous dichloromethane. The mixture was stirred at room temperature. The reaction was monitored by TLC until completion, then the formed precipitated was washed with acetone, dried at room temperature, and purified by column chromatography on silica gel using a gradient elution with n-hexane-ethyl acetate (10-100 % of ethyl acetate) to yield the desired compound.
General procedure for the synthesis of compounds 6b, 7b
A thiazole derivative (1.7 mmol), DMAP (0.34 mmol), and triethylamine (5.1 mmol) were added to a solution of a 4.25 mmol of the suitable sulphonyl chloride in 9 mL of anhydrous dichloromethane. The mixture was stirred at room temperature for 48 h, then were added, for 10 minutes, 10 mL of a 2.5 M NaOH solution, and the mixture were extracted with dichloromethane. The organic layer was dried under vacuum and subject column chromatography on silica gel using a gradient elution with n-hexane-ethyl acetate (10-100% of ethyl acetate) to yield the desired compound.
N-(4-phenyl-1,3-thiazol-2-yl) benzenesulfonamide (5): Yield 20 %. ATR-FTIR: ((max, 1598 (Csp2-Csp2), 1499 (Csp2-Csp2), 1291 (SO2), 1139 (SO2) cm-1.1H NMR (400 MHz, DMSO-d6) δ: 13.27 (s, 1H, H-1), 7.87 (d, J = 8 Hz, 2H, H-2´´´, H-6´´´), 7.70 (d, J = 7.6 Hz, 2H, H-2´, H-6´), 7.62-7.54 (m, 3H, H-3´, H-4´, H-5´), 7.44-7.39 (m, 3H, H-3´´´, H-4´´´, H-5´´´), 7.21 (s, 1H, H-5´´) ppm. 13C NMR (100 MHz, DMSO-d6) δ 168.8 (C-2´´), 142.2 (C-4´´), 136.7 (C-1´), 132.2 (C-4´), 129.2 (C-4´´´), 129.1 (C-3´, C-5´), 129.0 (C-3´´´, C-5´´´), 128.7 (C-1´´´), 125.9 (C-2´, C-6´), 125.6 (C-2´´´, C-6´´´), 103.5 (C-5´´). MS (EI, 70 eV): 316 [M+] (100), 285 (2), 251 (4), 175 (30), 134 (22), 77 (16), 18 (5) m/z.
N-(4-phenyl-1,3-thiazol-2-yl)-4-methyl benzenesulfonamide (6a) [25]: Yield 30 %. ATR-FTIR: ((max 3024 (Csp2-H), 2919 (Csp3-H), 1594 (Csp2-Csp2), 1486 (Csp2-Csp2), 1384 (SO2), 1167 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 7.72 (d, J = 8 Hz, 2H, H-2´´´, H-16´´´), 7.50-7.40 (m ,5H, H-2´, H-3´, H-5´, H-6´, H-4´´´), 7.21 (s, 1H, H´´-5), 7.12 (d, J = 8 Hz, 2H, H-3´´´, H-5´´´), 2.29 (s, 3H, C-4´-CH3); 13C NMR (100 MHz, DMSO-d6) δ 170.5 (C-2´´), 144.7 (C-4´´), 139.3 (C-1´), 138.4 (C-4´), 129.5 (C-4´´´), 129.1 (C-3´, C-5´), 128.8 (C-3´´´, C-5´´´), 128.4 (C-1´´´), 125.8 (C-2´´´, C-6´´´), 125.5 (C-2´, C-6´), 102.9 (C-5´´), 20.9 (C-4´-CH3). MS (EI, 70 eV): 331 [M++1] (2), 177 (100), 134 (8), 93 (2), 68 (20), 51 (44), 32 (32) m/z.
N-[4-(p-tolyl)-1,3-thiazol-2-yl]-4-methyl benzenesulfonamide (6b) [25]: Yield 40 %. ATR-FTIR: ((max 1601 (Csp2-Csp2), 1448 (Csp2-Csp2), 1318 (SO2), 1131 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 13.17 (1H, H-1), 7.73 (d, J = 7.68 Hz, 2H, H-2´´´, H-6´´´), 7.58 (d, J = 7.44 Hz, 2H, H-2´, H-6´), 7.34 (d, J = 7.68 Hz, 2H, H-3´, H-5´), 7.23 (d, J = 7.72 Hz, 2H, H-3´´´, H-5´´´), 7.12 (s, 1H, H-5´´), 2.34 (s, 3H, C-4´-CH3), 2.30 (s, 3H, C-4´´´-CH3). 13C NMR (100 MHz, DMSO-d6) δ 169.1 (C-2´´), 142.4 (C-4´´), 139.4 (C-1´), 138.8 (C-4´), 136.7 (C-4´´´), 129.5 (C-3´´´, C-5´´´), 129.4 (2CH, C-3´, C-5´), 129.9 (C-1´´´), 126.0 (C-2´, C-6´), 125.5 (C-2´´´, C-6´´´), 102.3 (CH-5´´), 21.0 (C-4´-CH3), 20.8 (C-4´´´-CH3). MS (EI, 70 eV): 344 [M+] (75), 279 (30), 189 (100), 148 (85), 91 (60), 39 (8) m/z.
N-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl) 4-methyl benzenesulfonamide (6c) [25]: Yield 60 %. ATR-FTIR: ((max 3099 (Csp2-H), 2980 (Csp3-H), 1621 (Csp2-Csp2), 1448 (Csp2-Csp2), 1300 (SO2), 1172 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.91 (brs, 1H, H-1), 7.65 (d, J = 8.8 Hz, 2H, H-2´, H-6´), 7.51 (d, J = 8 Hz, 2H, H-2´´´, H-6´´´), 7.14 (d, J = 7.9 Hz, 2H, H-3´, H-5´), 7.08 (s, 1H, H-5´´), 7.04 (d, J = 8.8 Hz, 2H, H-3´´´, H-5´´´), 3.80 (s, 3H, C-4´´´-OCH3), 2.29 (s, 3H, C-4´-CH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.6 (C-2´´), 160.3 (C-4´´´), 144.6 (C-4´´), 139.0 (C-1´), 138.7 (C-4´), 128.5 (C-3´, C-5´), 127.5 (C-2´´´, C-6´´´), 125.7 (C-2´, C-6´), 121.3 (C-1´´´), 114.6 (C-3´´´, C-5´´´), 100.8 (C-5´´), 55.5 (C-4´´´-OCH3), 21.0 (C-4´-CH3). MS (EI, 70 eV): 246 [M+-114] (66), 205 (100), 175 (8), 149 (35), 121 (20), 77 (11), 39 (5) m/z.
N-[4-(4-fluorophenyl)thiazol-2-yl]-4-methyl benzenesulfonamide (6d): Yield 50 %. ATR-FTIR: ((max 3094 (Csp2-H), 1615 (Csp2-Csp2), 1443 (Csp2-Csp2), 1311 (SO2), 1135 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.79 (s, 1H, H-1), 7.77 (t, J = 8 Hz, 2H, H-2´´´, H-6´´´), 7.54 (d, J = 8 Hz, 2H, H-2´, H-6´), 7.33-7.29 (t, J = 8.8, 2H, H-3´´´, H-5´´´), 7.20 (s, 1H, H-5´´), 7.15 (d, J = 7.88, 2H, H-3´, H-5´), 2.28 (s, 3H, C-4´-CH3); 13C NMR (100 MHz, DMSO-d6) δ 170.2 (C-2´´), 162.4 (d, J = 245.42 Hz, C-4´´´), 144.8 (C-4´´), 140.0 (C-1´), 138.4 (C-4´), 128.4 (C-3´, C-5´), 128.1 (d, J = 8 Hz, C-2´´´, C-6´´´), 126.4 (C-1´´´), 125.6 (C-2´, C-6´), 116.0 (d, J = 22 Hz, C-3´´´, C-5´´´), 102.6 (CH-5´´), 20.9 (C-4´-CH3). MS (EI, 70 eV): 348 [M+] (75), 283 (40), 193 (100), 152 (100), 122 (6), 91 (36), 65 (10), 39 (4) m/z.
N-[4-(4-chlorophenyl)thiazol-2-yl]-4-methyl benzenesulfonamide (6e) [25]: Yield 70 %. ATR-FTIR: ((max 3089 (Csp2-H), 1616 (Csp2-Csp2), 1448 (Csp2-Csp2), 1308 (SO2), 1167 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.92 (s, 1H, H-1), 7.73 (d, J = 8.4 Hz, 2H, H-2´´´, H-6´´´), 7.55 (d, J = 8.6 Hz, 2H, H-2´, H-6´), 7.52 (d, J = 7.9 Hz, 2H, H-3´´´, H-5´´´), 7.31 (s, 1H, H-5´´), 7.14 (d, J = 8 Hz, 2H, H-3´, H-5´), 2.29 (s, 3H, C-4´-CH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.5 (C-2´´), 144.8 (C-4´´), 138.3 (C-1´), 138.3 (C-4´), 134.0 (C-4´´), 129.1 (C-3´´´, C-5´´´), 128.3 (C-3´, C-5´), 127.8 (C-1´), 127.6 (C-2´´´, C-6´´´), 125.5 (C-2´, C-6´), 103.8 (C-5´´), 20.9 (C-4´-CH3). MS (EI, 70 eV): 250 [M+-114] (42), 209 (100), 168 (50), 133 (30), 89 (36), 41(15) m/z.
N-[4-(4-nitrophenyl)-1,3-thiazol-2-yl]-4-methyl benzenesulfonamide (6f) [25]: Yield 65 %. ATR-FTIR: ((max 3077 (Csp2-H), 1598 (Csp2-Csp2), 1537 (-NO2), 1348 (-NO2), 1499 (Csp2-Csp2), 1349 (SO2), 1139 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.28 (d, J = 8.9 Hz, 2H, H-3´´´, H-5´´´), 8.02 (d, J = 8.9 Hz, 2H, H-2´´´, H-6´´´), 7.49 (d, J = 7.9 Hz, 2H, H-2´, H-6´), 7.49 (s, 1H, H-5´´), 7.12 (d, J = 7.9 Hz, 2H, H-3´, H-5´), 2.29 (s, 3H, C-4´-CH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.3 (C-2´´), 147.1 (C-4´´´), 144.6 (C-4´´), 139.3 (C-1´), 138.5 (C-4´), 135.8 (C-1´´´), 128.4 (C-3´,C-5´), 126.7 (C-3´´´, C-5´´´), 125.6 (C-2´, C-6´), 124.3 (C-2´´´, C-6´´´), 107.4 (C-5´´), 20.90 (C-4-CH3). MS (EI, 70 eV): 278 [M+-97] (8), 253 (2), 221 (6), 172 (4), 157 (100), 139 (28), 121 (73), 89 (74), 77 (20), 51 (8). 28 (8), 18 (22) m/z.
N-(4-phenylthiazol-2-yl)-4-nitro benzenesulfonamide (7a): Yield 40 %. ATR-FTIR: ((max 2985 (Csp2-H), 1640 (Csp2-Csp2), 1561 (-NO2), 1501 (Csp2-Csp2), 1167 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 13.52 (brs,1H, H-1), 8.38 (d, J = 8.8 Hz, 2H, H-3´, H-5´), 8.10 (d, J = 8.8 Hz, 2H, H-2´, H-6´), 7.71 (d, J = 6.8 Hz, 2H, H-2´´´, H-6´´´), 7.44-7.41 (m, 3H, H-3´´´, H-4´´´, H-5´´´), 7.28 (s, 1H, H-5´´). 13C NMR (100 MHz, DMSO-d6) δ: 169.7 (C-2´´), 149.4 (C-4´), 147.6 (C-4´´), 137.1 (C-1´), 129.3 (C-1´´´), 129.1 (C-2´, C-6´), 128.5 (C-4´´´), 127.4 (C-3´´´, C-5´´´), 125.7 (C-2´´´, C- 6´´´), 124.6 (C-3´, C-5´), 104.1 (C-5´). MS (EI, 70 eV): 296 [M+-65] (100), 250 (30), 179 (37), 134 (13), 105(7), 76(40), 45(7) m/z.
N-[4-(p-tolyl)-1,3-thiazol-2-yl]-4-nitro benzenesulfonamide (7b): Yield 45 %. ATR-FTIR: ((max 2918 (Csp3-H), 1598 (Csp2-Csp2), 1538.01 (-NO2), 1322 (SO2), 1142 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 13.48 (s, 1H, H-1), 8.36 (d, J = 8.5 Hz, 2H, H-3´, H-5´), 8.08 (d, J = 8.6 Hz, 2H, H-2´, H-6´), 7.58 (d, J = 7.8 Hz, 2H, H-2´´´, H-6´´´), 7.22 (d, J = 7.8 Hz, 2H, H-3´´´, H-5´´´), 7.18 (s, 1H, H-5´´), 2.29 (s, 3H, C-4´´´, -CH3). 13C NMR (100 MHz, DMSO-d6) δ: 169.7 (C-2´´), 149.4 (C-4´), 147.7 (C-4´´), 139.0 (C-1´), 132.3 (C-4´´´), 129.6 (C-2´, C-6´), 127.4 (C-3´´´, C-5´´´), 125.9 (C-1´´´), 125.6 (C-2´´´, C-6´´´), 124.6 (C-3´, C-5´), 103.1 (C-5´´), 20.9 (C-4´´´-CH3). MS (EI, 70 eV): 310 [M+-65] (100), 264(30), 223 (3), 193 (29), 147 (13), 115(9), 76(23), 45(3) m/z.
N-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl)-4-nitro benzenesulfonamide (7c) [25]: Yield 47 %. ATR-FTIR: ((max, 2962 (Csp2-H), 2840 (Csp3-H), 1617 (Csp2-Csp2), 1526 (-NO2), 1460 (Csp2-Csp2), 1351 (-NO2), 1296 (SO2), 1182 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.96 (s, 1H, H-1), 8.21 (d, J = 8.84 Hz, 2H, H-3´, H-5´), 7.87 (d, J = 8.88 Hz, 2H, H-2´, H-6´), 7.63 (d, J = 8.8 Hz, 2H, H-2´´´, H-6´´´), 7.09 (s, 1H, H-5´´), 7.04 (d, J = 8.8 Hz, 2H, H-3´´´, H-5´´´), 3.79 (s, 3H, C-4´´´-OCH3). 13C NMR (100 MHz, DMSO-d6) δ: 170.4 (C-2´´), 160.2 (C-4´´´), 153.8 (C-4´), 147.4 (C-4´´), 138.8 (C-1´), 127.4 (C-2´, C-6´), 127.0 (C-2´´´, C-6´´´), 123.5 (C-3´, C-5´), 121.1 (C-1´´´), 114.5 (C-3´´´, C-5´´´), 100.8 (C-5´´), 55.4 (C-4´´´-OCH3). MS (EI, 70 eV): 346 [M+-46] (100), 283 (3), 205 (94), 164 (90), 121 (11), 77 (35), 45 (5) m/z.
N-[4-(4-fluorophenyl)-1,3-thiazol-2-yl]-4-nitro benzenesulfonamide (7d): Yield 15 %. ATR-FTIR: ((max, 2985 (Csp2-H), 1643 (Csp2-Csp2), 1565 (-NO2), 1166 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.23 (d, J = 8.6 Hz, 2H, H-3´, H-5´), 7.83 (d, J = 8.64 Hz, 2H, H-2´, H-6´), 7.66 (dd, J = 8.36 & 5.36 Hz, 2H, H-2´´´, H-6´´´), 7.18 (t, J = 8.64 Hz, 2H, H-3´´´, H-5´´´), 6.92 (s, H-5´´). 13C NMR (100 MHz, DMSO-d6) δ 170.4 (C-2´´), 162.5 (d, J = 245.93 Hz, C-4´´´), 154.0 (C-4´´), 147.4 (C-4´´), 138.5 (C-1´), 128.4 (d, J = 8.59 Hz, C-2´´´, C-6´´´), 127.0 (C-2´, C-6´), 125.2 (C-1´´´), 123.5 (C-3´, C-5´), 116.1 (d, J = 21.84, C-3´´´, C-5´´´), 102.9 (C-5´´). MS (EI, 70 eV): 356 [M+-65] (5), 314 (100), 281 (9), 243 (37), 197 (46), 152 (17), 122 (16), 76 (23), 30 (10) m/z.
N-[4-(4-chlorophenyl)-1,3-thiazol-2-yl]-4-nitro benzenesulfonamide (7e) [26]: Yield 35 %. ATR-FTIR: ((max, 1605 (Csp2-Csp2), 1539 (-NO2), 1387 (-NO2), 1296 (SO2), 1138 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.20 (d, J = 8.6 Hz, 2H, H-3´, H-5´), 7.86 (d, J = 8.6 Hz, 2H, H-2´, H-6´), 7.73 (d, J = 8.6 Hz, 2H, H-2´´´, H-6´´´), 7.55 (d, J = 8.6 Hz, 2H, H-3´´´, H-5´´´), 7.29 (s, 1H, H-5´´). 13C NMR (100 MHz, DMSO-d6) δ 170.4 (C-2´´), 154.0 (C-4´), 147.4 (C-4´´), 138.7 (C-1´), 133.9 (C-4´´´), 129.1 (C-3´´´, C-5´´´), 128.0 (C-1´´´), 127.7 (C-2´´, C-6´´), 127.0 (C-2´´´, C-6´´´), 123.5 (C-3´, C-5´), 103.6 (C-5´´). MS (EI, 70 eV): 361 [M+-33] (100), 322 (4), 298 (40), 251 (4), 223 (4), 175 (44), 134 (48), 76 (17), 51 (14) m/z.
N-[4-(4-nitrophenyl)-1,3-thiazol-2-yl]-4-nitro benzenesulfonamide (7f): Yield 70 %. ATR-FTIR: ((max, 1592 (Csp2-Csp2), 1522 (-NO2), 1344 (-NO2), 1158.53 (SO2) cm-1. 1H NMR (400 MHz, DMSO-d6) δ: 8.27 (d, J = 9 Hz, 2H, H-3´´´, H-5´´´), 8.20 (d, J = 8.8 Hz, 2H, H-3´, H-5´), 8.03 (d, J = 8.98 Hz, 2H, H-2´´´, H-6´´´), 7.83 (d, J =8.8 Hz, 2H, H-2´, H-6´), 7.46 (s, 1H, H-5´´). 13C NMR (100 MHz, DMSO-d6) δ: 170.5 (C-2´´), 154.4 (C-4´), 147.8 (C-4´´´), 147.4 (C-4´´), 139.5 (C-1´´´), 136.8 (C-1´), 127.4 (C-2´´´, C-6´´´), 127.2 (C-2´, C-6´), 124.6 (C-3´´´, C-5´´´), 123.9 (C-3´, C-5´), 107.6 (C-5´´). MS (EI, 70 eV): 405 [M+-1] (4), 221 (100), 202 (88), 191 (50), 175 (99), 156 (48), 133 (50), 109 (50), 89 (99), 75 (48), 50 (8), 18 (23) m/z.
Trichomonicidal assay
In vitro susceptibility assays used Trichomonas vaginalis strain GT3 and the sub-culture method of Cedillo-Rivera et al [15]. The compounds were dissolved in 1 mL of dimethylsulfoxide (DMSO) and added to microtubes containing 1.5 mL of medium in order to reach concentrations of 1, 2, 10, and 20 μg/mL. The solutions were inoculated with T. vaginalis trophozoites to achieve an inoculum of 4 × 104 trophozoites/mL and then were incubated for 48 h at 37 °C. Each test included metronidazole as positive control, a control (culture medium plus trophozoites and DMSO), and a blank (culture medium). After incubation, the trophozoites were detached by chilling and samples of each tube were sub-cultured in fresh medium for another 48 h, without anti-protozoal drugs or compounds. The final number of parasites was determined with a haemocytometer and the 50 % inhibitory concentration (IC50) was calculated by probit analysis (GraphPad Prim 4 software). The experiments were performed in duplicate and replicated.
Cytotoxicity assay
Cytotoxic activities were determined by the sulforhodamine B technique [16] using African green monkey kidney (VERO, ATCC-CCL-81) cells, from the American Type Culture Collection (ATCC). Cells undergoing exponential growth were seeded in a 96-well cell culture plate; 100 μL of cell suspension at a concentration of 5 x 104 cells/mL were added to each well and it was incubated at 37 °C for 24 h in a CO2 incubator. Cytotoxicity (CC50) was measured until cells reached 90 % confluency without FBS. Each compound was tested at increasing concentrations in serial dilution (50-1.625 μg/mL) mL and incubated for 48 h. At the end of the exposure time, the medium was removed, and the cells were fixed by adding 50 μL of 10 % trichloroacetic acid solution to each well and incubated at 4 °C for 30 min. After incubation, the trichloroacetic acid was eliminated and 50 μL of sulforhodamine B (0.1 % sulforhodamine B in 1 % acetic acid) were added to each well and left in contact with the cells for 30 min, after which they were washed with 150 μL of 1 % acetic acid, and rinsed three times until only dye adhering to the cells was left. The plates were dried and 100 μL of 10 mM Tris base were added to each well to solubilize the dye. The plates were shaken gently for 10 min and the cellular proliferation was determined by measuring the optical density (OD) at 540 nm using a bioassay reader (BioRad, USA). Docetaxel (Taxotere®; Sigma- Aldrich Co.) was used as positive control, whereas untreated cells were used as a negative control. Each concentration was evaluated by triplicate in an assay by three independent experiments. The concentration of each compound that killed 50 % of the cells (CC50), was calculated by GraphPad Prism 4 software. The selectivity index (SI) of the compounds is defined as the ratio of cytotoxic activity on normal cells to antitrichomonal activity (SI = CC50 Vero cells/IC50 T. vaginalis).
Molecular docking
The molecular geometries of all compounds were fully optimized using density functional theory with a 6-31G (d, p) basis set. The exchange-correlation potential was evaluated using the hybrid functional B3LYP [17]. After optimization, a frequencies calculation was performed to characterize all the stationary points at the same computational level and no imaginary frequency was observed. All the calculations, including polar surface areas, were carried out using the SPARTAN 20 program [18].
The crystal structure of the Trichomonas vaginalis Ferredoxin (PDB-ID: 1L5P) at 2.2 Å resolution was obtained from the Protein Data Bank (http://www.rcsb.org/pdb) [19]. The molecular docking studies were performed using the software Autodock v 4.2 and the Autodock tools v 4.2 (ADT) [20] graphical user interface was used to calculate the Gasteiger-Marsili charges for the protein and to add polar hydrogens. The polar hydrogen charges of Gasteiger-type were assigned, and the nonpolar hydrogens were merged with the carbon atoms. The partial charges of the ligands were computed using PM6 semiempirical calculation employing the SPARTAN20 code. All the protein was considered as a rigid body and the ligands being flexible. All the torsion and rotatable bonds in the ligand were defined. The grid box for chalcones derivatives were centered at the residue Thr37, with a dimension of 52 × 52 × 52 with a spacing grid of 0.375 Å. The search was performed with the Lamarckian Genetic Algorithm as is implemented in Autodock v 4.2 code. The population of 150 individuals was mutated with a mutation rate of 0.02 and envolved for 10 generations. The number of the docking runs was 50. A cluster analysis was performed based on a rms deviation values lower than 2.0 Å referenced to the starting geometry. The best binding mode was selected based on the lowest energy binding and the more populated cluster. The visualization of the complex was done using Maestro [21].
Results and discussion
Chemistry
Firstly, the 2-amino-4-phenyl thiazoles were obtained by condensation of p-substituted acetophenones and thiourea according to our previously reported method [22]. As a second step, equimolar reactions between 2-amino-4-phenyl thiazol derivatives and arylsulfonyl chlorides (4) were performed in the presence of Et3N (2 eq.) and 4-dimethylaminopyridine (DMAP) (0.1 eq) [23]. Thus, eleven sulfonamides were obtained (5, 6a, 6c-f, 7a, 7c-f) (Fig. 1).
Another two sulfonamides (6b and 7b) were synthesized, increasing the concentration of promoters (Et3N at 3 eq. and DMAP at 0.2 eq) and, according to Greenfield et al. [24], the equivalents of arylsulfonyl chloride were increased up to 2.5, and basic hydrolysis was included (NaOH at 2.5 M).
Synthesized sulfonamides yields range from 15 to 70 %. Thirty percent of the sulfonamides were synthesized with yields over 50 %, being the yields of compounds 6e and 7f the highest; moreover, compound 6e exhibited a yielding 2.5-fold than when pyridine was used as catalyst but, contrastingly, compounds 5, 6b, and 7e exhibited the lowest yields. Interestingly, only the compound 7e yield was significant lesser than that reported in the literature. Therefore, the use of DMAP instead pyridine moderately improved the yielding.
The synthesized sulfonamides were split into two small series, one formed by the sulfonamides para-substituted at phenylthiazolyl moiety and the other one grouped those sulfonamides showing para-substitution at phenylsulfonyl moiety. All synthesized compounds were characterized by IR, 1H, 13C NMR, and mass spectra.
Antitrichomonal activity
The synthesized thiazole sulfonamides were tested in vitro against Trichomonas vaginalis trophozoites, as well as against Green monkey kidney cells (Vero cells). The results of the bioassay tests are summarized in Table 1.
Table 1 Antitrichomonal activity, selectivity index and lipophilic parameters of phenylthiazolyl benzenesulfonamides.
| C | R 1 | R 2 | T. vaginalis IC50 µM ± SD 1 | Vero cells CC50 µM ± SD | SI 2 |
| 6f | NO2 | Me | 2.05 ± 0.09 | 33.24 (±0.98) | 16.22 |
| 7a | H | NO2 | 0.58 ± 0.08 | 1136.97 (±7.22) | 1960.00 |
| 7b | Me | NO2 | 0.64 ± 0.01 | 37.59 (±0.76) | 58.73 |
| 7c | MeO | NO2 | 2.53 ± 0.02 | 1174.50(±3.45) | 464.23 |
| 7e | Cl | NO2 | 0.58 ± 0.04 | 238.96 (±2.35) | 412.00 |
| 7f | NO2 | NO2 | 0.27 ± 0.02 | >1230.31 | >4556.70 |
| MTZ 3 | 0.93 ± 0.02 | 367 (±2.87) | 394 |
1Standard deviation
2Selectivity index
3Metronidazole
Usually, small molecules are tested in in vitro cellular assays at concentrations of 1 - 50 μM [27], and any compound exhibiting activity at a concentration lower than 25 μM can be considered as having significant activity [28]. Therefore, in the present study, a compound with an IC50 < 25 μM was considered active. Thus, T. vaginalis were sensitive to six of 13 synthesized thiazole sulfonamides derivatives (6f, 7a-c, e, f). Noteworthy, the IC50’s showed by active thiazole sulfonamides ranged from 0.27 to 2.53 μM. Remarkably, four of those compounds (7a, 7b, 7e, and 7f) exhibited more potent anti-trichomonal activity than the used positive control, metronidazole (IC50 = 0.93 μM) (Table 1).
The synthesized trichomonicidal sulfonamides can be distinguished according to the position of their nitro group, then a set of four compounds bear a nitro group at phenylsulfonyl moiety (7a-c,e); while only one presents the nitro group located at phenylthiazolyl moiety (6f). The potency values of those two sets of sulfonamides showed marked differences, being three compounds belonging to the first set were 3-fold more potent than compound 6f. Curiously, sulfonamide 7c was almost equipotent to sulfonamide 6f.
Interestingly, compound 7f (N-[4-(4-nitrophenyl)-1,3-thiazol-2-yl)]-4-nitrobenzenesulfonamide) exhibited the highest trichomonicidal activity (0.27 μM), evidencing that the simultaneous presence of two nitro groups at the sulfonamide structure probably improves the anti-trichomonas activity. Furthermore, this compound was remarkably even 3.4-fold more potent than metronidazole.
Considering these findings and the fact that compound 5 was not active (IC50 > 25 μM); then, it is plausible to think that trichomonicidal activity is related to the presence of a nitro group in sulfomanide structure.
Cytotoxic activity
The non-specific cytotoxicity (CC50’s) of the synthesized trichomonicidal sulfonamides, evaluated against Green monkey kidney cells (Vero cells), ranged from 33.24 to >1230.31 μM. From those data, the harmfulness level for each compound was determined by calculating the selectivity index (SI = CC50/IC50). All sulfonamides exhibited notably low harmfulness levels (SI´s > 10), being four of them even less harmful than metronidazole (SI = 394) and, surprisingly, the most active compound (7f) also resulted in the most selective one with a prominent SI >4556.70.
It is important to note that all assayed sulfonamides exhibited remarkable trichomonicidal activities in vitro (IC50 ≤ 1 μg/mL), as well as prominent selectivity indexes (SI ≥ 10), therefore, they can be classified as antiparasitic hit compounds [29].
Molecular docking
Currently, 5-nitroimidazoles, mainly metronidazole, are the drugs of choice to treat trichomoniasis. Like all nitroaromatic drugs, these drugs owe their activity to high reactive oxygen species generated by the single-electron transfer reduction of their nitro group [30]. In T. vaginalis, TvFd (Trichomonas vaginalis ferredoxin) is a hydrogenosomal protein involved in the bioreduction of metronidazole. TvFd is a 9.8 kD protein constituted by 93 residues containing a [2Fe-2S] cluster responsible for electron transfer reactions [31, 32].
Since the anti-trichomonal activity of the synthesized sulfonamides depends on the presence of a nitro group in their structures, it is plausible to theorise that it is the result of the bioreduction of the nitro group [30].
Notably, the first electron transfer to form the ArNO2/ArNO2-· couple is a factor involved in the nitroreduction control, hence in the order of bioreduction of the most important groups of ArNO2, which is: nitropyridines > nitrofurans ≥ nitrothiophenes > nitrobenzenes > nitroimidazoles. Thus, this fact can explain the higher trichomonacidal activity exhibited by four of the synthesized sulfonamides.
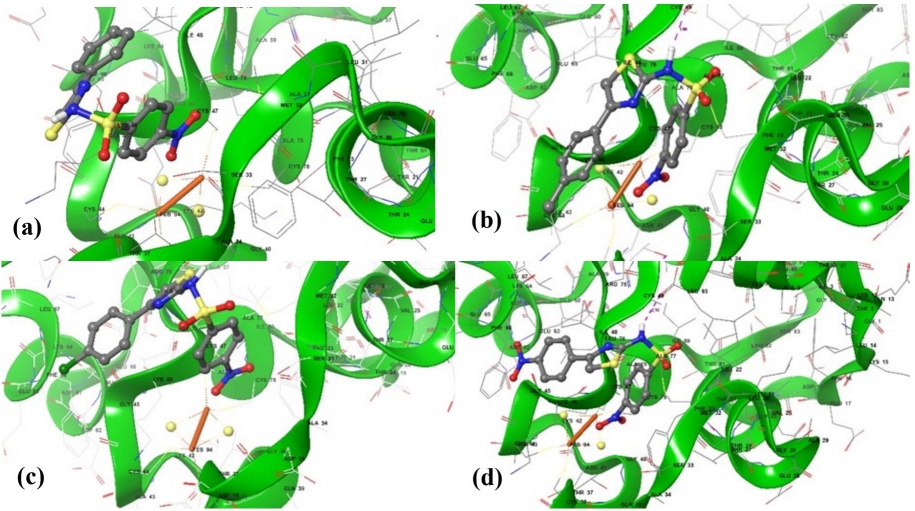
To explore the putative mechanism of action of the compounds with higher antitrichomonal activity than metronidazole, a molecular docking study was performed with Trichomonas vaginalis ferredoxin (PDB-ID: 1L5P) as target. Thus, the affinity of those compounds, including metronidazole, for the amphipathic binding site of the iron-sulfur cluster was studied.
Metronidazole showed a binding affinity of -2.50 Kcal/mol (Table 2); besides that, its nitro group is oriented toward Lys46 and Thr37, which together Asp36, Gln39, Asn41, and Lys42 are the hydrophilic residues forming the front, top, and bottom of the binding site [31].
Table 2 Docking scores of the most active phenylthiazolyl benzenesulfonamides and metronidazole in the binding site for Trichomonas vaginalis ferredoxin.
| Comp | Binding affinity (Kcal/mol) | Interactions with residues |
| 7a | -2.57 | Ser33, Met32, Cys47, Ile48 |
| 7b | -2.77 | Lys7, Ser33, Thr37, Met32, Cys47, Ile48, Leu93 |
| 7e | -2.72 | Ser33, Thr37. Phe23, Met32, Ile48 |
| 7f | -2.91 | Ser33, Thr37, Gln70, Leu31, Met32, Ile48, Leu93 |
| Metronidazole | -2.50 | Ser33, Met32, Met32, Cys47, Ile48, Lys46 |
The binding affinity of all docked sulfonamides (7a, 7b, 7e, 7f) ranged from -2.91 to -2.57 kcal/mol, being better than the one exhibited by metronidazole, and as was expected, their nitro group pointed toward the cluster binding loop of the [2Fe-2S] core. Interestingly, molecular docking showed these sulfonamides interact with the binding site in the same fashion as metronidazole (Table 2). Curiously, each sulfonamide interacts through interactions with residues Met32 and Ile48. These interactions are remarkable because are quite close to the residues 33-47, which form the loop covering the [2Fe-2S] cluster (Fig. 2) [31].
Conclusions
Two sets of 13 N-[(4-substituted phenyl)-1,3-thiazol-2-yl)]-4subtituted benzenesulfonamides were synthesized with modest yields, which not correlated to the chemical nature of the para-substituents in the starting sulfonyl chlorides. Four synthesized sulfonamides were more active against Trichomonas vaginalis than metronidazole, and remarkably, two of them were significantly more selective. Although antitrichomonal activity did not correlate with the chemical nature of the para-substituents at phenylthiazolyl moiety, it is clear that presence of a nitro group in the skeleton of the compound is essential for this activity. The docking study of the four more active sulfonamides revealed their interaction with the binding site of the Trichomonas vaginalis ferredoxin iron-sulfur cluster. Finally, it is necessary to expand the number of sulfonamides to improve the SAR analysis and perform a QSAR study.











 nueva página del texto (beta)
nueva página del texto (beta)