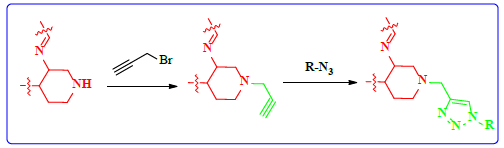
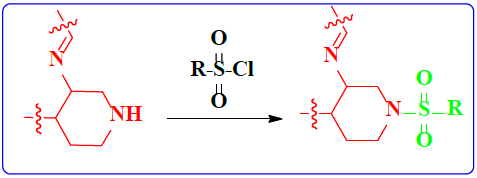
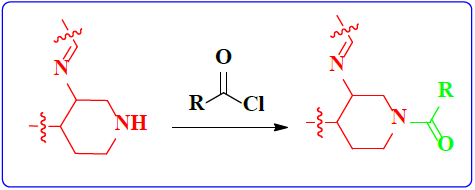
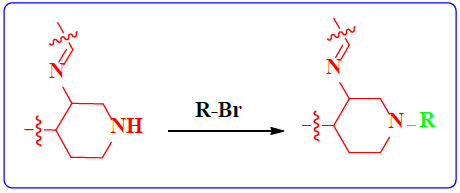
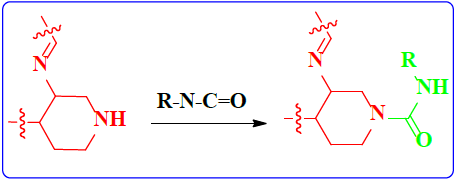
1. Introduction
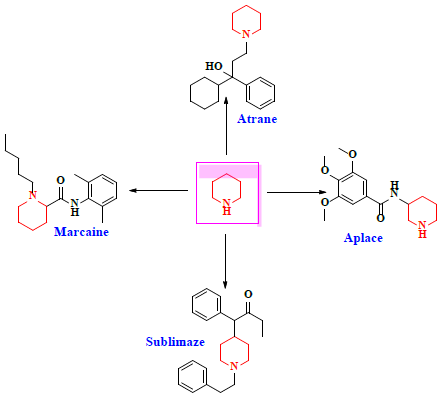
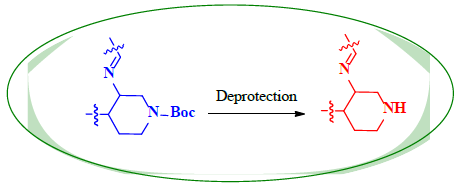
Schiff bases functionalized organic compounds are found to be one of the potent biomedical and industrially important organic derivatives (Sangle, 2022) exhibiting various applications such as antimicrobial (Belay et al., 2024), anticancer (Janowska et al., 2023), antidiabetic (Saeed et al., 2020), antioxidant (Al Zoubi et al., 2016), antituberculosis (Cordeiro & Kachroo, 2020), along with anticorrosive (Betti et al., 2023), fingerprint probe (Murugan et al., 2022), chemosensor (Khan et al., 2022), super capacitor (Saravanan et al., 2022) and other wide spread functionalities, and synthetic compounds with piperidine as a core nuclei which is abundant in nature exhibit various biological activities (see Fig.1), and naturally occurring alkaloids are represented in Table 1 and 2. Clubbing of these two functionalities may result in enhancement of the potency of various applications. A Boc protected Schiff base derivative which can be further utilized as building block for other organic transformation was synthesized using simple condensation method which upon deprotection (Scheme 1) can be converted into 1, 2, 3-triazoles (Scheme.2), sulphonamides (Scheme 3), acid amides (Scheme 4), alkylated derivatives (Scheme 5), carboxamide derivatives (Scheme 6) and other plausible moieties by causing reaction at N-site are depicted as shown in Figure 1.
Table 1 Piperidine containing FDA approved drugs.
| Common Name | Chemical Name | Role |
| Artane | Trihexyphenidyl | An antispasmodic drug that is mostly used to treat Parkinson's disease symptoms. It aids in the relief of problems like tremors, spasms, stiffness, and lack of muscle control. It can also help with involuntary movements brought on by some psychiatric drugs' adverse effects. |
| Aplace | Troxipide | Drug used in the treatment of gastroesophageal reflux disease. Troxipide is a systemic non-antisecretory gastric cytoprotective agent with anti-ulcer, anti-inflammatory and mucus secreting properties irrespective of pH of stomach or duodenum. |
| Sublimaze | Fentanyl | A highly potent synthetic piperidine opioid primarily used as an analgesic. It is 20 to 40 times more potent than heroin and 100 times more potent than morphine its primary clinical utility is in pain management for cancer patients and those recovering from painful surgeries, Fentanyl-containing medicine Sublimaze is mostly utilized as a premedication for anaesthesia and for its analgesic effects during surgical procedures. In these circumstances, it is recommended for short-term pain treatment in adults and children older than two. |
| Marcaine | Bupivacaine | Marcaine, a drug also referred to by its generic name bupivacaine, is mostly used for post-operative pain management and local anaesthetic. It is frequently recommended for operations like lower extremities and perineal surgery that need for spinal anaesthesia. Markaine is beneficial in a variety of medical contexts due to its ability to provide anaesthetic effects that continue for a long time. |
Table 2 Naturally occurring piperidine bearing alkaloids.
Therefore, with the above based evidence, an attempt was made to synthesize the molecule bearing both Schiff base and piperidine functionality where the natural product was clubbed with synthetic functionality.
2. Materials and methods
All the chemicals and reagents were obtained from the company Spectrochem and Sigma Aldrich and were of analytical grade. The reaction was monitored by TLC (60F254) aluminum sheets and visualization was done by UV light. 1H NMR and 13C NMR spectra was recorded using an AV400 - Bruker 400 MHz high resolution multinuclear FT-NMR spectrometer with internal TMS and chemical shifts were expressed in parts per million (ppm) using DMSO-d6 as solvent.
2.1. Synthesis of tert-butyl 3-(((2-hydroxynaphthalen-1-yl)methylene)amino)-4-(4 (trifluoromethyl)phenyl)piperidine-1-carboxylate (RSB1)
tert-butyl 3-amino-4-(4-(trifluoromethyl)phenyl)piperidine-1-carboxylate (0.172g, 1mmol) was added to a methanolic solution of 2hydroxy naphthaldehyde (0.086g, 1mmol) maintained at room temperature, 4 drops of trifluroacetic acid (TFA) was added as catalyst, the reaction was monitored through visualization owing for the formation of light yellow-colored precipitate of tert-butyl 3-(((2-hydroxynaphthalen-1-yl)methylene)amino)-4-(4-(trifluoromethyl)phenyl)piperidine-1-carboxylate (RSB1) as shown in Scheme 7. After completion of the reaction, the contents were filtered, washed with distilled water, followed by diethyl ether and recrystallized using methanol.
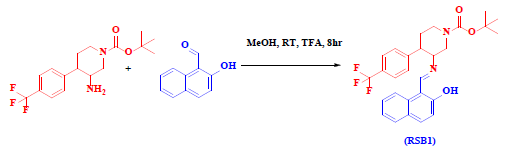
Scheme 7 Synthesis of tert-butyl 3-(((2-hydroxynaphthalen-1-yl)methylene)amino)-4-(4-(trifluoromethyl)phenyl)piperidine-1-carboxylate (RSB1)
1 H NMR (400 MHz, DMSO-d 6 δ, ppm) 10.66 (s, 1H), 8.77 (d, J = 8.6 Hz, 1H), 7.98 (s, 1H), 7.96 (s, 2H), 7.63 (dd, J = 69.4, 8.2 Hz, 3H), 7.58 - 7.35 (m, 4H), 7.26 - 6.82 (m, 2H), 4.28 (d, J = 9.1 Hz, 1H), 3.87 (d, J = 13.4 Hz, 4H), 3.31 - 2.87 (m, 2H), 1.27 (s, 9H). 13 C NMR (100 MHz, DMSO-d 6 δ, ppm): 27.866, 32.076, 39.081, 40.060, 45.902, 50.145, 79.444, 112.271, 118.575, 122.046, 124.144, 125.530, 127.448, 128.726, 128.849, 129.217, 131.564, 138.334, 144.625, 163.831, 186.027, 192.729.. HRMS (+ESI, m/z)found: 498.2352 (M+) (calculated for C28H29F3N2O3: 498.213)
3. Conclusions
A novel fluorine containing reactive building block tert-butyl 3-(((2-hydroxynaphthalen-1-yl)methylene)amino)-4-(4 (trifluoromethyl)phenyl)piperidine-1-carboxylate a versatile synthetic derivative was synthesized employing the widely used aldehyde amine condensation reaction and was characterized using different spectral techniques. The compound could be further carried to synthesize other functionalities.











 nueva página del texto (beta)
nueva página del texto (beta)