Introduction
Cardiac myxomas are the most common benign primary neoplasm of the heart. They have the potential to embolize and grow at the site of implantation, which can cause infarction in other organs such as the brain1. It is crucial to perform a timely diagnosis and treatment to prevent complications that endanger life and affect functionality2.
The aim of this article is to conduct a comprehensive review of cardiac myxomas and their relationship with neurological complications and to present a series of reported cases in a third-level center in Mexico City.
Epidemiology
Cardiac tumors are extremely rare, their prevalence is higher in Europe and North America, with 0.117 and 0.114 cases, respectively, and lower in Turkey and South America, with 0.0413 and 0.058 cases, respectively2. The incidence of these tumors is 1.38-30 cases per 100,000 inhabitants/year3. Of the total primary cardiac tumors, 80-85% are benign. Among them, 70% are myxomas, 15-20% are lipomas, and 10-15% are papillary fibroelastomas, which are the most common. The remaining 10-20% of primary tumors are malignant1,4,5.
The prevalence of cardiac myxoma is 0.03% in the general population and the annual incidence is 0.5-1 case/million. Epidemiologically, they are divided into sporadic or familial cases (corresponding to the Carney complex), with the former being the most common, accounting for 95% of myxoma cases6. These conditions can occur at any age, with preference for females, with a ratio of 3:16,7.
Anatomy
Myxomas have a preference for the atrium as the most common location for growth, although they can affect any cavity of the heart. The affected sites include 60-90% in the left atrium, 12.7 to 28% in the right atrium, 1.7 to 8% in the right ventricle, 0.6-4% in the left ventricle, and 0.8-1.6% are multifocal4. Commonly, they originate in the limbus of the oval fossa of the atrial septum. However, they can also arise from the posterior atrial wall, the anterior atrial wall, and the atrial appendage6.
Myxoma can appear as polyps in up to two-thirds of cases while having a solid or papillary structure in one-third of cases. Compared to papillary myxomas, they are softer. The polyps are pedunculated, more compact, and less prone to fragmentation and subsequent embolization. On the other hand, papillary or villous myxomas are gelatinous, more fragile, and less compact. They have a higher potential for fragmentation and embolization toward the central nervous system, kidney, spleen, limbs, and coronary vessels6,8.
Histology
Cardiac myxomas consist of spindle-shaped and polygonal star-shaped cells embedded in an amorphous myxoid stroma. Multinucleated cells may also be present in some cases. These cells are arranged in chains or clusters around the capillaries9. The tumor surface is often covered by flattened endothelium, while the tumor mass is abundantly supplied with vessels that have thin walls and lack pericytes10.
The histogenesis of the myxoma is not well understood, but the current understanding is that it originates from primitive pluripotent mesenchymal cells. The genes encoding cardiac precursor markers can reactivate and express themselves in cells of the cardiac myxoma, causing differentiation along endothelial or endocardial lines11. A previous hypothesis was that myxomas originated from Prichard structures. These structures are microscopic, lined by thick endothelial cells, and are found in the oval fossa. Another hypothesis suggested that myxomas originated from neuroendocrine tissue12.
Clinical presentation
The clinical manifestations of myxoma can be divided into three groups. The first is the systemic group, which includes constitutional symptoms such as fever, arthralgia, weight loss, and fatigue. The second group is the cardiac group, caused by the mass effect that interferes with cardiac function and blood flow. This can lead to arrhythmias, regurgitation, or pericardial effusion, with or without tamponade, also affecting the cardiac valves and causing dyspnea, chest discomfort, and syncope. Finally, the third group is the group of embolic complications, which can include pulmonary or systemic thromboembolism caused by the tumor1.
Neurological complications occur as a result of embolic events. These complications can manifest in various ways, such as neurological syncope, headache, dizziness, seizures, transient ischemic attacks, ischemic or hemorrhagic strokes caused by aneurysm rupture, and brain metastases13,14. These complications can initially occur in up to 80% of myxoma cases13.
Diagnosis
The diagnostic protocol for cardiac myxomas includes imaging studies such as echocardiography, computed tomography (CT), and cardiovascular magnetic resonance (CMR). Less commonly used tests, such as positron emission tomography (PET) and angiography, may also be employed. Each of these studies offers specific information that is valuable for the diagnostic approach, preoperative planning, assessing the diseases extent, and establishing a definitive histopathological diagnosis.
Echocardiography
Echocardiography is the primary diagnostic study and is performed using two modalities: transesophageal echocardiogram (TEE) and transthoracic echocardiogram (TTE). TEE demonstrates superior sensitivity at 100%, compared to 95% for TTE. It also has a higher capability to identify insertion points, with 95.2% compared to 64.5% for TTE. Furthermore, TEE offers advantages in observing small lesions15.
Echocardiography enables the characterization of tumors based on their size, morphology, location, extent, and hemodynamic effects, as well as the shape of the mass (Fig. 1). Myxomas appear as spherical masses attached to the endocardial surface, sometimes with hypoechoic internal areas, speckled echogenic spots, and typically echogenic calcifications6,15.
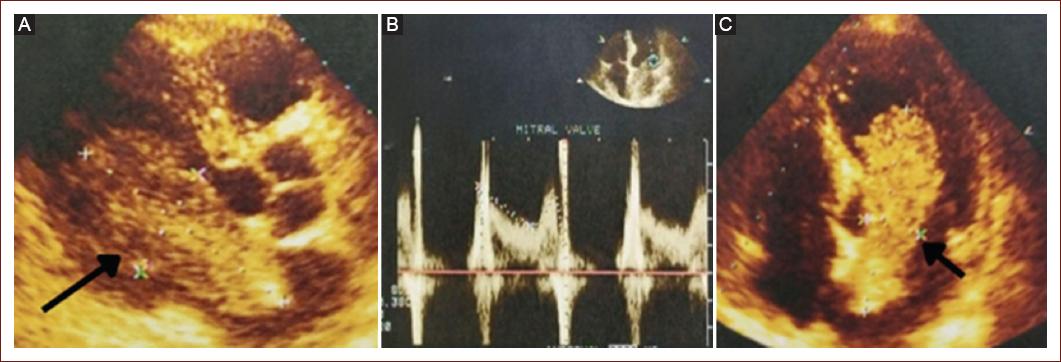
Figure 1 Transthoracic echocardiogram. Two-dimensional echocardiography, parasternal long axis view. A: an intracardiac mass is observed, compatible with intracardiac myxoma measuring 74 × 24 mm, with an implantation base at the level of the oval fossa, which protrudes into the ventricular cavity. Apical four-chamber view. B: transmitral flow is shown in pulsed Doppler mode, with a mean transmitral gradient of 3.5 mmHg, without valvular obstruction. C: an intracardiac mass is observed in the left atrium that protrudes into the ventricular cavity.
In addition, it enables the assessment of myocardial perfusion and the comparative perfusion of a cardiac mass, enhances the clarity of intracavitary structures, and evaluates vascularization distinguishing between vascular and non-vascular tumors or thrombi relies on how cardiac masses differ in perfusion. Myxomas often have poor blood irrigation, with quantitatively lower perfusion than the surrounding myocardium. Thrombi are avascular and do not perfuse in the echocardiogram, while malignant tumors are highly vascularized due to abnormal neovascularization, resulting in greater enhancement compared to adjacent myocardium1,7.
In a study involving 27 patients with cardiac myxoma, TTE revealed several findings. Liquefaction was observed in 18.5% (5/27) of the cases, characterized by an irregular anechoic cystic mass with a small fraction of hypoechoic basement. Calcifications were found in 70.4% (19/27) of the cases, presenting a hyperechoic appearance with shadow, multiple nodules in different positions of the heart in 11.1% (3/27), and high proliferative activity was observed in 7.4% (2/27) with irregular masses of large size and wide base, accompanied by abundant blood supply. These echocardiographic characteristics resemble those of malignant carcinoma, and a predilection for the right ventricle is noted16.
CT
CT is a diagnostic method that can be used as an alternative to echocardiography and CMR. It offers high spatial and temporal resolution, the ability to reconstruct multiplanar images, and rapid acquisition times. These features enable precise delineation of lesion margins and their relationship with tissue planes, which are valuable for surgical planning1,6.
Myxomas can be observed with CT scans as rounded, movable, lobulated, and well-defined masses, typically with a narrow pedicle. They often have a heterogeneous appearance and may contain areas of calcification. CT is a valuable tool for assessing the size, shape, location, and calcification of myxomas. The appearance of myxomas can vary depending on their composition, such as the presence of hemorrhage, calcification, necrosis, fibrosis, or cystic changes17. CT is also useful in tumor staging, as it can detect metastases in cases of suspected malignancy. However, it has some disadvantages, such as radiation exposure and limited temporal and soft tissue resolution when compared to MRI15.
In non-contrast CT scans, the tumor typically has lower attenuation compared to non-opacified blood. Myxomas often exhibit heterogeneity due to various factors such as hemorrhage, calcification, ossification, necrosis, cysts, or fibrosis. Tumors may also demonstrate visible enhancement after the administration of contrast, although this may be less pronounced than in magnetic resonance imaging (MRI) and could be challenging to observe due to the presence of highly contrasted surrounding blood. Dual-energy CT with a medium iodine concentration is effective in accurately determining if a mass exhibits visible growth15,17.
CMR
It provides a comprehensive and non-invasive evaluation of the mass, its potential involvement of the cardiac chambers, the pericardium, extracardiac structures, and its surrounding anatomy. This is useful in surgical planning6. CMR enables the evaluation of various characteristics of the heart, including morphology, dimensions, location, extent, homogeneity, and the presence of infiltration in the surrounding tissues. In addition, CMR can provide valuable information about signal characteristics that assist in histopathological characterization, such as fatty infiltration, necrosis, hemorrhage, calcification, and vascularity1,3.
To differentiate between tumors and thrombi, especially if they originate in the left atrial appendage, late gadolinium enhancement sequences performed 10-15 min after contrast administration is useful. Enhancement is usually heterogeneous, and it has been observed that enhanced areas correspond to regions rich in myxomatous tissue and focal inflammation. Internal cysts or necrosis could cause non-enhanced areas as well. First-pass perfusion studies may show mild, heterogeneous enhancement. Another sequence that can help differentiate between myxoma and thrombus is the inversion time. Steady-state free precession cine images are useful for evaluating myxoma function, as mobile lesions can prolapse through the AV valve during diastole. If there is associated mitral valve obstruction, features such as left atrial enlargement, pulmonary venous hypertension with pulmonary vascular redistribution, and pulmonary edema can be observed on X-rays, CT, and MRI6,15.
Some limitations of CMR are its lower temporal resolution, prolonged acquisition times (30 min-1 h), limited availability, and contraindications in hemodynamically unstable patients or those with older generation cardiac devices, as well as in patients with claustrophobia15.
PET
PET provides an accurate assessment of the metabolic activity of tumors using fluorodeoxyglucose (18F-FDG). It is useful in the staging of malignant tumors, the detection of possible involvement of the myocardium and pericardium, the evaluation of early responses to cancer therapies, the planning of radiation therapy, and the optimization of sites for biopsy sampling6,15.
The level of FDG uptake in tumors is a valuable tool for distinguishing between benign and malignant tumors. A study on the detection of benign versus malignant cardiac masses found that this method has a sensitivity of 100% and a specificity of 92%18.
Angiocardiography
Angiocardiography is rarely used as a diagnostic method due to the availability of non-invasive studies and greater accessibility to other tests such as echocardiography, in addition to the risk of embolization of tumor fragments during the procedure. In angiocardiography, tumors usually appear as filling defects. In cases of left atrial myxoma, a radiotransparent mass within the left atrium can be visualized on the pulmonary angiogram6,19. The usefulness of this lies in providing valuable pre-operative information by identifying the blood vessels that supply blood to the cardiac myxoma19.
Histopathology
In general, immunohistochemistry is used to detect cardiac myxomas. This technique utilizes a variety of biomarkers such as CD31, CD34, CD56, FVII Ag, S-100 protein, calretinin, vimentin, desmin, smooth muscle myosin, α1-antitrypsin, and alpha 1-antichymotrypsin20.
Myxoma surgery
Functionally, myxomas are considered malignant due to the embolic phenomena they can cause due to the mass effect6. To prevent complications, it is important to perform surgical resection as soon as possible. During the procedure, systemic anticoagulation is required for the resection of the myxoma. However, this can increase the risk of intracranial hemorrhage. Despite this risk, various studies and reported series have shown that the procedure is generally safe with low or no mortality21.
Surgical resection of the myxoma is the preferred treatment to alleviate symptoms and prevent neurological complications. However, there is still uncertainty regarding the best timing for surgery following the occurrence of neurological complications, such as cerebral infarction6.
At present, there are new surgical approaches and minimally invasive techniques22, as well as technology used in surgeries such as robotic surgery23, which helps reduce post-operative complications, further decrease mortality, and restore quality of life early on, in addition to enabling early return to work and daily activities6. It has also improved the availability and types of treatments, with reports of up to 2.45% of patients undergoing resection of cardiac myxoma undergoing heart transplantation2.
The recurrence rate in the resection of benign atrial tumors is lower compared to malignant tumors from the same site (0.8% vs. 22.2%)24. Some factors that increase the risk of recurrence include incomplete tumor resection due to limited tumor exposure, multifocal, and genetic conditions13,22.
Up to 25.5% of patients may experience postoperative complications. These can include pulmonary infections in 5.1% of cases; arrhythmias in 2.3%; embolism in 1.5%; and less frequently, pulmonary hemorrhage, and cerebral hemorrhage secondary to embolism, as well as nerve injuries25. In another series, a frequency of 20.6% of arrhythmias during the immediate post-operative period and transient ischemic attacks in 6.7-10.5% was described26. The main causes of death in the post-surgical follow-up are heart failure, massive cerebral embolism, and pneumonia25.
Neurologic complications
Cerebrovascular disease
Embolism can lead to the development of ischemic cerebral infarctions (Fig. 2). In patients with myxoma, systemic embolism occurs in 30-50% of cases, with 50% of those embolizations affecting the central nervous system and the retinal artery. This is attributed to the biological ability of myxoma to detach and cause embolization or to the tumor's morphology, especially when it has an external velvety appearance, which independently increases the risk of embolism (OR = 8.7; 95% confidence interval [CI]: 2.4-42.1; p < 0.001)9.
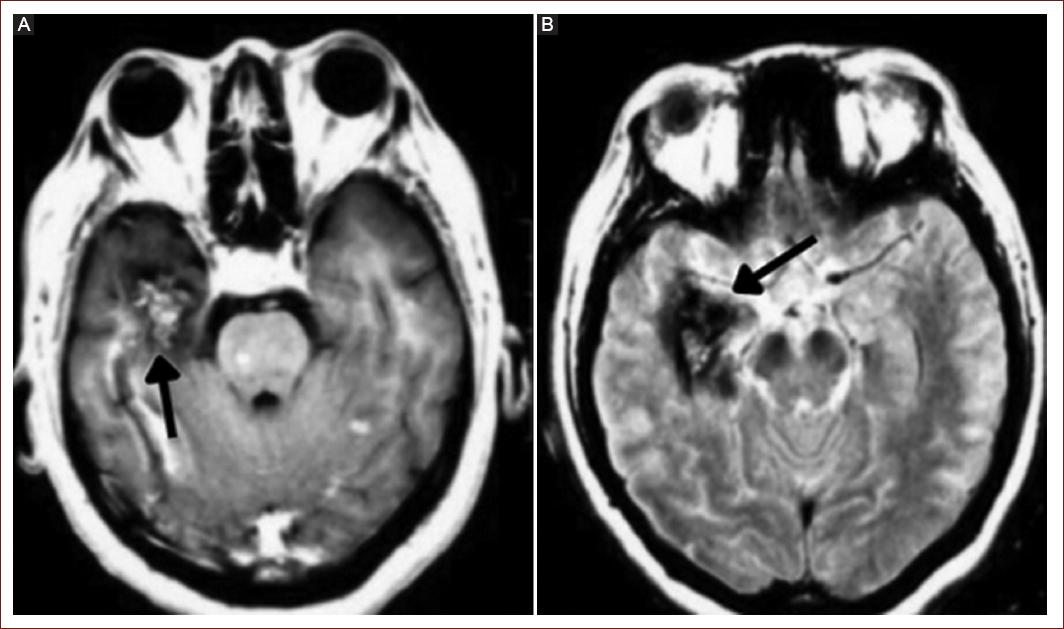
Figure 2 Cerebral magnetic resonance imaging. A and B: cerebral metastases are shown in the right temporal lobe.
The risk factors associated with embolism in patients with myxoma include NYHA class I/II heart failure (OR = 2.98, 95% CI = 1.66-5.33, p < 0.01), hypertension (OR = 1.41, 95% CI = 1.04-1.92, p = 0.03), irregular tumor surface (OR = 3.99, 95% CI = 3.04-5.25, p < 0.01), atypical location (OR = 1.81, 95% CI = 1.13-2.88, p = 0.01), narrow-based tumors (MD = −0.36, 95% CI = −0.51-−0.22, p < 0.01), and increased levels of fibrinogen (MD = 0.62, 95% CI = 0.28-0.95, p < 0.01)27.
Myxomas are a rare cause of ischemic brain infarctions, accounting for < 1% of cases. Cardiac myxomas represent < 1% of cases of stroke. Risk factors associated with a higher risk of stroke include tumor size smaller than 30 mm (OR = 2.652, 95% CI: 1.061-6.627, p = 0.037), highly mobile tumors (OR = 2.700, 95% CI: 1.357-5.371, p = 0.005), thrombus on the tumor surface (OR = 1.856, 95% CI: 1.003-3.434, p = 0.049), and lower levels of BNP (OR = 0.995, 95% CI: 0.989-0.999, p = 0.047)25.
The American Heart Association, in conjunction with the American Stroke Association, considers thrombolysis as a viable treatment for patients with cardiac myxoma presenting with stroke28. This statement is based on reported cases that have demonstrated the effectiveness and safety of intravenous and intra-arterial thrombolysis treatment. Clinical improvement, defined by a reduction of 4 or more points on the NIHSS scale, has been observed in 52.2%-64.3% of multicenter series. It has also been found that standard-dose alteplase leads to a higher rate of neurological improvement compared to low doses (64.3% vs. 37.5%, respectively)29. In cases where proximal arterial occlusion is documented, mechanical thrombectomy is indicated30.
Resection of the myxoma prevents neurological complications and should be performed as soon as possible. In patients with cerebral infarction, it has been observed that a prolonged interval between cerebral infarction and myxoma resection is significantly associated with recurrent cerebral infarction (p = 0.021)13, as well as prolonged symptoms at the time of surgery (OR = 1.046, 95% CI: 1.005-1.088, p = 0.029)25.
Cerebral aneurysm
Neoplastic cerebral aneurysms are extremely rare but have high morbidity and mortality. The first aneurysm secondary to cardiac myxoma was reported in 196631. They occur in 13-56% of patients with myxoma, with a higher risk of aneurysm rupture (20-25%) compared to aneurysms of other etiologies32, which can result in intracerebral hemorrhage in 19.6% of cases. Typically, they are treated with open surgery, chemotherapy, radiation therapy, endovascular occlusion with coils, or conservatively33.
The prognosis of a neoplastic cerebral aneurysm depends on the histology of the primary tumor34. It is significantly better in the case of cardiac myxomas compared to choriocarcinoma or other carcinomas (p < 0.0001). The mortality rate for cardiac myxomas is 11.4%, while for choriocarcinoma and other carcinomas, it ranges from 60.9% to 92.3%, respectively. Furthermore, 77.3% of patients with cardiac myxomas remain stable, either with or without the disappearance of the aneurysm35.
At present, there are no established guidelines for the treatment of aneurysms caused by cardiac myxomas. However, a conservative approach and regular radiological monitoring are typically preferred. In most cases, the aneurysms remain stable, and in some instances, they may even regress spontaneously. It has been observed that the risk of delayed cerebral aneurysm formation is not reduced, even if a cardiac tumor resection is performed36.
Brain metastases
Brain parenchymal metastases are typically removed through surgical resection35. Studies have shown that the combination of temozolomide and radiosurgery can help eliminate and control the recurrence of metastatic myxoma14. In addition, it has been reported that low-dose radiation combined with chemotherapy can assist in the degradation of metastases. An alternative option is frameless stereotactic radiosurgery, which is less invasive compared to endovascular or open surgery and has fewer systemic effects from chemotherapy36.
Prognosis and surveillance
Benign primary neoplasms of the heart have a late mortality rate of 0.79%2. Among a series of 180 surgically treated patients, the mortality rate was 2.4% and the tumor recurrence rate was 0.8% during an average follow-up period of 48 months24.
Cardiac myxomas have a disease recurrence rate of 2-3%37 and neurological complications in up to 12% of cases7. Therefore, some authors recommend performing annual echocardiographic follow-up after surgery for 4 years38 to detect recurrence of the disease or new neurological complications. However, since there are reports of recurrence occurring up to 3, 7, or more years later24,25, and of cerebral aneurysms appearing up to 7 and 25 years after surgery for myxoma31,39, it is important to actively monitor the patient for a longer period of time.
Case series presentation
A series of cases is presented with the presentation of cerebrovascular disease and cardiac myxoma diagnosed incidentally during the stroke study protocol and treated at a third-level center in Mexico City (Table 1). Eleven patients with a mean age of 37.5 years ± 13.1 at the time of cerebrovascular disease symptoms were included, with a range of 16-54 years. Fifty-five percent (n = 6) were women and 45% (n = 5) were men. All patients presented symptoms consistent with stroke, and up to 18% had a history of transient ischemic attacks before the first ischemic event.
Table 1 Summary of characteristics of patients with myxoma and cerebrovascular disease
| Patient | Age/Gender | Antecedent | Location | Clinical presentation | Affected arterial territory | Myxoma treatment | Treatment | mRS at egress | mRS at 6-month follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/M | Dyslipidemia and alcoholism | Left atrial myxoma | Dysarthrias, left central facial paresis, left hemiparesis NIHSS 4 | Right middle cerebral artery | Myxoma resection surgery | Thrombolysis | 1 | 1 |
| 2 | 37/M | Dyslipidemia | Left atrial myxoma | Loss of awake state, motor aphasia, right hemiparesis, initial NIHSS 4 | Left middle cerebral artery | Myxoma resection surgery and mitral valve plasty | Thrombolysis | 2 | 2 |
| 3 | 44/F | Acute arterial insufficiency and supracondylar amputation | Left atrial myxoma 36mm × 18 mm | Loss of awake state, seizures, initial NIHSS 10 | Left middle cerebral artery | Myxoma resection surgery | Thrombectomy | 3 | 3 |
| 4 | 54/F | Dyslipidemia | Left atrial myxoma | Right-hand paresis, dizziness, and decreased visual acuity | Multiple territories | Myxoma resection surgery and mitral valve plasty | ASA, statins | 4 | 4 |
| 5 | 39/M | Diabetes mellitus type 2, dyslipidemia, and active smoking | Atrial myxoma | Memory loss, headache, weakness | Middle cerebral artery | Myxoma resection surgery | Thrombectomy | 2 | 2 |
| 6 | 16/M | Migraine and ischemic heart disease | Atrial myxoma | Right hemiparesis, headache, campimetry deficit, initial NIHSS of 16 | Internal carotid | Myxoma resection surgery | Thrombolysis | 3 | 3 |
| 7 | 28/F | Unknown | Atrial myxoma | Left hemiparesis, initial N IHSS of 12 | Right middle cerebral artery | Myxoma resection surgery | Thrombolysis | 3 | 1 |
| 8 | 40/F | Unknown | Left atrial myxoma | Right hemiparesis and headache | Multiple territories | Myxoma resection surgery | Thrombolysis | 0 | 0 |
| 9 | 36/M | Family history of stroke and ischemic heart disease | Atrial myxoma | Seizures, headache, dizziness, hemiparesis | Middle cerebral artery and lenticulostriate arteries | Myxoma resection surgery | ASA, statins | 4 | 4 |
| 10 | 18/F | Dyslipidemia | Atrial myxoma | Hemiparesis, headache | Left middle cerebral artery | Myxoma resection surgery | Thrombolysis | 1 | 1 |
| 11 | 53/M | Type 2 diabetes, verte brobasilar stroke | Left atrial myxoma | Loss of awake state, deviation of the lip corner to the left, right central facial paralysis, dysarthria, right hemiparesis, motor aphasia, | Multiple territories Posterior communicating artery inferior posterior cerebellar artery | Myxoma resection surgery | Thrombectomy | 2 | 2 |
M: male; F: female; ASA: acetylsalicylic acid; mRS: modified Rankin Scale.
The studies carried out were part of the medical procedures. All patients underwent a brain tomography as part of the initial study protocol for stroke, and an echocardiogram in the early days of hospitalization was performed and interpreted by an echocardiologist. None of the patients were aware of their myxoma diagnosis before the first stroke. In some cases, cerebral MRI and angiography were performed for diagnostic and therapeutic purposes of the stroke. In some patients, CT or CMR was performed as part of the pre-surgical protocol for myxoma resection.
All patients presented with cardioembolic stroke (100%, n = 11), and all were incidentally diagnosed with myxoma during the search for the etiology of the stroke. Initially, thrombolysis was used (54%, n = 6) and mechanical thrombectomy was performed (27%, n = 3) without complications, adjusting the pharmacological treatment with antiplatelet and anticoagulant medications, and subsequently, the myxoma was resected.
The average length of hospital stay during the first stroke event was 10.67 ± 11.8. Up to 64% (n = 7) remained without cardiac symptoms after the resection and during follow-up. Patients were assessed for functional capacity using the modified Rankin Scale (mRS) at discharge and during follow-up at 3, 6, and 18 months. At discharge, 54% (n = 6) had a good functional outcome (mRS < 2 points) and 45% (n = 5) had a poor functional outcome (mRS > 3 points). At 18 months, 64% (n = 7) had a good functional outcome and 36% (n = 4) had a poor functional outcome.
The average follow-up period was 21.5 ± 17.2 months. Among the patients, 18% (n = 2) experienced recurrent strokes, and brain metastasis was found in one of these patients.
Discussion
Although cardiac myxomas have a low prevalence, several case series have been documented in Mexico. The Cardiology Hospital of Centro Médico Nacional Siglo XXI reported 51 primary cardiac tumor cases over 16 years, with 74% being myxomas40. The Centro Médico Nacional 20 de Noviembre reported 34 myxoma cases over 11 years41. In addition, the Centro Médico ABC also reported 12 cardiac tumor cases over 12 years, where 75.1% were myxomas, occurring more frequently in women at a ratio of 5:142. Our report includes 11 cases recorded over a 25-year period.
Diagnosis and treatment present significant challenges within our country's health systems. Regular long-term follow-up using imaging studies such as echocardiography and MRI is required to detect new tumors or brain lesions. While guidelines recommend a 4-year follow-up38, numerous cases have been reported beyond this timeframe39,43.
In our case series, two patients experienced recurrent stroke after myxoma resection. The first patient had three strokes at 3-, 8-, and 24-month post-surgery and the second had two at 5- and 51 months after the myxoma resection. In both cases, follow-up was lost multiple times, which prevented imaging studies from being performed to assess any tumor growth. As a result, we associate tumor growth with the recurrence of stroke.
The risk of recurrent stroke grows with increasing time intervals between the initial cerebral infarction and the surgical resection of the myxoma44. In our series, patients with recurrence had a time interval of 2-3 weeks between the first stroke and myxoma resection, and this may have increased the risk of recurrent strokes.
Myxoma resection is the only treatment that can prevent neurological complications24 since up to 46% of patients with recurrence of cerebral infarction were on antiplatelet or antiplatelet treatment45. In our case series, patients were given platelet and anticoagulant treatment in the acute stroke and long-term antiplatelet treatment was implemented.
Treatment of stroke with thrombolysis and thrombectomy seems to be effective treatment with a good prognosis, especially in the case of thrombolysis. Rao et al. (2022) found in a study involving patients with cerebral infarction and myxoma that the average mRS was 2 for patients treated with thrombolysis and 3 for those treated with mechanical thrombectomy at discharge and at the 3-month follow-up29,30.
In this case series, six patients underwent thrombolysis treatment. The average mRS at discharge was 2, dropping to 1.6 at the 6-month follow-up. On the other hand, three patients were treated with thrombectomy. The average mRS for these patients was 2.3 at both discharge and the 6-month follow-up. All patients remained free from complications.
Conclusion
Myxomas are a rare cause of cerebrovascular disease. Therefore, it is crucial to identify the cardiac tumor early on to allow for prompt resection as the initial treatment, to prevent neurological complications. Patients who experience a cardioembolic event should undergo a comprehensive diagnostic protocol to determine the cause, such as the presence of a blood clot or embolization due to an intracardiac tumor. Once the diagnosis is confirmed, the stroke is treated, and the myxoma is removed and patients should be closely monitored for any recurrence of the primary tumor and potential subsequent neurological complications.











 nueva página del texto (beta)
nueva página del texto (beta)


