Introduction
Convolvulus arvensis L. (Convolvulaceae) and Portulaca oleracea L. (Portulacaceae) are among the most harmful weeds to agriculture in Mexico (Espinoza and Villaseñor, 2017; Carrascosa et al., 2023). The specie C. arvensis, also known as bindweed, is a creeping habit plant with a high colonizing and regenerative potential, turning it into a serious problem in northwestern Mexico (Baja California Norte, Baja California Sur, Chihuahua, Durango, Sinaloa y Sonora), where reduces by 40 - 50 % the production of Jalapeño pepper (Capsicum annuum L.) (Solanaceae), chickpea (Cicer arietinum L.) (Fabaceae) and wheat (Triticum aestivum L.) (Poaceae), widely growth crops in the region (Rodríguez et al., 2015; Tamayo et al., 2021; Ávila, 2022). Similarly, P. oleracea, commonly named as purslane, maintains a strong competition with horticultural crops, such as pepper (C. annuum L.) and eggplant (Solanum melongena L.) (Solanaceae), where depending on its population density and the association it maintains with other species, reduces fruit production down by 96 % (Blanco et al., 2018).
Among the methods for managing these and other weeds is the use of chemical products; however, its use has generated environmental and public health problems, encouraging the search for technology based on sustainability. One of the strategies with the greatest projection and history of success in agriculture, is the use of plants with allelopathic properties (Galán, 2023). In this regard, the genus Eucalyptus (Myrtaceae) highlight as one of the main exponents of this characteristic, mainly due to the secretion of different essential oils (Barbosa et al., 2016), substances whose interference in the seed germination process has been observated in species such as Amaranthus retroflexus L. (Amaranthaceae), Echinochloa crus-galli (L.) P. Beauv. (Poaceae) and Lactuca sativa L. (Asteraceae) (González, 2017; Puig et al., 2018). With similar characteristics is Schinus terebenthifolius Raddi (Anacardiaceae), a species rich in monoterpenes and sesquiterpenes, with a record of interference in the germination of Eucalyptus camaldulensis Denham (Myrtaceae), Eragrostis plana Nees (Poaceae) and Urochloa brizantha (A.Rich.) R.D. Webster (Poaceae) (Maldaner et al., 2020; Fernandes et al., 2023).
Given the need to contribute with information on species with allelopathic potential, as well as weeds susceptible to them, this study aimed to evaluate the in vitro effect of aqueous extracts of E. globulus and S. terebinthifolius on the germination and initial growth of the weeds C. arvensis and P. oleracea.
Materials and methods
This research was carried out at the “Carlos Darwin” Herbarium of the Faculty of Agriculture of Valle del Fuerte, attached to the Autonomous Universidad Autónoma de Sinaloa, from August 2021 to October 2022. The samples of E. globulus and S. terebenthifolius were obtained from natural vegetation located on the border of the municipalities of Ahome and El Fuerte, Sinaloa, Mexico (25°51’39’’N and 108°57’27’’W), an area characterized by a warm semi-dry climate, with an average temperature between 24 - 26 °C and maximum rainfall of 700 mm per year (Cortés et al., 2013). Weed seeds were obtained from plants developed in agricultural crops in the area. To corroborate the taxonomic identity of the plants, representative samples were collected, based on what was described by Sánchez and González (2007). Taxonomic identification was determined by the staff assigned to the Herbarium.
Preparation of extracts
The fresh material (4.7 kg) was separated by leaf, fruit and bark structures, and was left to dry for 15 d in the shade at a room temperature of 28 ± 2 °C. Each plant structure was ground with the help of a grain mill (Estrella®). The powder obtained was weighed in portions of 2.5, 5, 7.5, 10, 15 and 20 g, incorported separately into amber-colored flasks and mixed with 100 mL of distilled water (w/v) (Ávalos et al., 2019). The colloid was stored in the dark at 25 ± 2 °C. After 24 h, it was filtered through Whatman # 40 paper and deposited in 100 mL polyethylene bottles, each solution making up a treatment: T2 (2.5 %), T3 (5.0 %), T4 (7.5 %), T5 (10.0 %), T6 (15.0 %) and T7 (20.0 %). A control treatment to which only distilled water was applied (T1= 0 %) was included. The breaking of dormancy in C. arvensis seeds was achieved by previous treatment based on sulfuric acid, according to Amani et al. (2015). Because dormancy was not observed in P. oleracea, no pre-germination procedure was necessary. The seeds of both species were sterilized for 5 min in a commercial chlorine solution (Cloralex®) and distilled water (1:10), with subsequent washing to eliminate residues of the chemical solution.
Bioassay
Germination and growth inhibition was evaluated according to Xuan et al. (2004). It consisted of placing 25 weed seeds on interfolded (Kimberly-Clark®) paper arranged in 10 cm diameter Petri dishes. Afterwards, the seeds were sprinkled with 7 mL of the corresponding treatment, sealed with Parafilm paper, and placed inside a Cooling Incubator Model IRH-150F germination chamber with a photoperiod of 16:8 h and a temperature of 30 ± 0.5 °C. The effect of the treatments on the germination of the seeds was measured every 24 h for a period of 14 d through the response variables, initial time of germination (ITG), mean germination time (T50), initial percentage of germination (IPG), and final percentage of germination (FPG), while the initial growth of the seedlings was evaluated with the total length (TL) of the shoot, in both species, and the hypocotyl length (HL) and radicle lenght (RL) alone in P. oleracea.
Experimental design and data analysis
The experiment was established through three independent bioassays, under a completely randomized design. In each bioassay, seven treatments with four repetitions (25 seeds/replication) were used. The response variables did not meet the assumptions of normality and homogeneity of variances, so the data was subject to a non-parametric analysis using the Kruskal-Wallis test with Pearson’s χ2 statistic (p < 0.05), and then to a test of multiple comparison of means using the Wilcoxon Rank Sum test at 5 % with the statistical program SAS online (SAS® OnDemand For Academics).
Results and discussion
In general, the weeds Convolvulus arvensis and Portulaca oleracea were susceptible to the extracts of the evaluated arboreal species, although this varied depending on the species, structure and concentration. The extracts of S. terebinthifolius were found to be more effective than those of E. globulus (p < 0.05), and P. oleraceae showed greater susceptibility than C. arvensis.
Germination
The 94.7 % of E. globulus formulations, did not show a significant effect (p < 0.05) on the ITG of the evaluated weeds (Figure 1). Twenty percent of treatment placed the ITG of C. arvensis in 2.7 ± 1.2 d (d), while 15 % of treatment delayed the germination of P. oleracea until 12.0 ± 6.0 d. Regarding T50, leaf and fruit structures were a highlight since delayed the germination of 50 % of the C. arvensis seed population at concentrations between 5 to 20 %, in a lapse of 7.92 ± 0.0 y 9.68 ± 0.2 d; meanwhile, on P. oleracea (T50 = 13.38 ± 3.2 d) the leaf extract did so at a concentration of 15 % (Figure 1). In this sense, Kandhro et al. (2016) found a suppressive effect on the germination of C. arvensis with the application of aqueous extracts of Eucalyptus camaldulensis Dehnh., behavior emulated by P. oleracea, where although germination is affected depending on the concentration used, the interaction of the components occurs at a lower level than expected, translating the inhibitory effect into a temporary deficit at the beginning of the process. In both cases, this situation may be due to the release of phenolic, benzoic, cinnamic acids, flavonoids, tannins and other substances, whose transfer to the target species induces the modification of the normal germination and growth mechanism of the plant (Pinto et al., 2021; Shahzad et al., 2023).
Similarly to E. globulus, 36.8 and 26.3 % extracts of S. terebenthifolius expressed a significant delay (p < 0.05) in the germination onset times of C. arvensis and P. oleracea, respectively (Figure 2). Both species were susceptible to fruit extract concentrations of 7.5 %, and reached their greatest time lag with the leaf and/or fruit structure formulations at 20 %. C. arvensis registered a significant minimum ITG of 2.0 ± 0.0 d and a maximum of 13.5 ± 3.0 d, while P. oleracea observed a delay between 2.0 ± 0.0 d and 15.0 ± 0.0 d. The T50 of both species showed significant differences (p < 0.05) when using the leaf, fruit or bark structure extracts. With an amplitude between 8.13 ± 0.0 and 14.1 ± 1.7 d, C. arvensis responded negatively to the fruit and leaf extracts at 5 %, while P. oleracea responded negatively to fruit (2.5 - 20 %), leaf (10 - 20 %) and bark (15 - 20 %) extracts, all with a ITG>8.13 ± 0.0 d.
Figura 1 Medias del tiempo inicial (TIG) y tiempo medio de germinación (T50) de semillas de a) Convolvulus arvensis L. y b) Portulaca oleracea L., tratadas con extractos acuosos a distintas concentraciones de fruto (-), hoja (---) y corteza (···) de Eucalyptus globulus Labill. Los colores de las barras y líneas se corresponden al orden presentado para las estructuras utilizadas de cada planta.
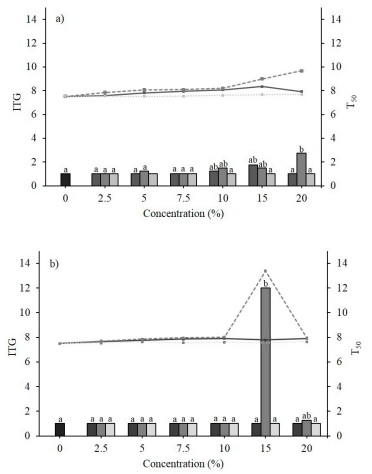
Figure 1. Means of the initial time (ITG) and mean germination time (T50) of the seeds of a) Convolvulus arvensis L. and b) Portulaca oleracea L., treated with aqueous extracts at different concentrations of the fruit (-), leaf (---) and bark (···) structures of Eucalyptus globulus Labill. The colors of the bars and lines correspond to the order presented for the structures used on each plant.
Figura 2 Medias del tiempo inicial (TIG) y tiempo medio de germinación (T50) de semillas de a) Convolvulus arvensis L. y b) Portulaca oleracea L., tratadas con extractos acuosos a distintas concentraciones de fruto (-), hoja (---) y corteza (···) de Schinus terebinthifolius Raddi. Los colores de las barras y líneas se corresponden al orden presentado para las estructuras utilizadas de cada planta.
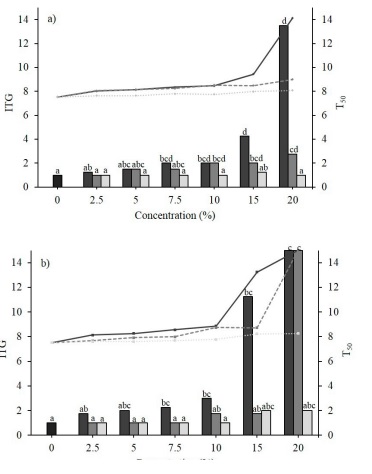
Figure 2. Means of the initial time (ITG) and mean germination time (T50) of the seeds of a) Convolvulus arvensis L. and b) Portulaca oleracea L., treated with aqueous extracts at different concentrations of the fruit (-), leaf (---) and bark (···) structures of Schinus terebinthifolius Raddi. The colors of the bars and lines correspond to the order presented for the structures used on each plant.
The allelopathic activity of the genus Schinus has been documented in the germination of cultivated and unwanted species (Bañuelas, 2019; Nunes et al., 2019). In termination experiments of L. sativa,Bündchen et al. (2015) observed a similar behavior, where the concentration of the aqueous leaves extracts delays in 5 % the germination process, even when the final percentage (70 %) does not present significant differences (p < 0.05) with respect to the control (75 %). This delay is part of a dose-dependent relationship observed in species such as Gledtschia amorphoides Taub, where an increase in T50 and a decrease in germination speed were recorded as a function of the concentration of Schinus leaves aqueous extract (Buturi et al., 2015). The aqueous extracts of E. globulus significantly (p < 0.05) reduced the germination percentages of C. arvensis and P. oleracea. As germination inhibitor extracts, the leaf formulations at 15 - 20 % stood out (p < 0.05), observing greater susceptibility of P. oleracea (FPG ≤ 17.0 ± 3.8 %) with respect to C. arvensis (FPG ≤ 38.0 ± 9.5 %) (Table 1). This situation responds to the abundance of phenolic compounds in the extracts of Eucalyptus, whose interaction with the recipient organisms results in interference with the cell division and growth processes (El-Ghit and Hanan, 2016; Morsi and Abdelmigid, 2016; González, 2017). However, a close relationship is observed between the concentration and the capacity of the extract to completely inhibit the FPG of weeds, with 89.4 % of the treatments losing their effectiveness at the end of the experiment; probably due to the degradation of the compounds at a lower concentration in the aqueous formulation, and whose residence period does not exceed five days (Sáez, 2019; Pinto et al., 2021).
Tabla 1 Promedios del porcentaje inicial (PIG) y final (PFG) de germinación de semillas de Convolvulus arvensis L. y Portulaca oleracea L. tratadas con extractos acuosos a distintas concentraciones de fruto, hoja y corteza de Eucalyptus globulus Labill. y Schinus terebenthifolius Raddi.
Table 1. Means of the initial (IPG) and final (FPG) percentage of germination of Convolvulus arvensis L. and Portulaca oleracea L. seeds, treated with aqueous extracts at different concentrations of the fruit, leaf and bark structures of Eucalyptus globulus Labill. and Schinus terebenthifolius Raddi.
| Arvense species | Concentration (%) | Structure | Arboreal species | |||
| Eucalyptus globulus | Schinus terebenthifolius | |||||
| IPG | FPG | IPG | FPG | |||
| Convolvulus arvensis | 0 | 64.0±8.6a | 95.0±3.8abc | 64.0±12.6bc | 96.0±0.0a | |
| 2.5 | 73.0±13.6abcd | 89.0±6.0abc | 22.0±28.5defg | 69.5±40.7abcdef | ||
| 5 | 32.0±13.4cdefg | 88.0±6.5abcde | 33.0±33.5cdef | 79.0±3.8cdefgh | ||
| 7.5 | Fruit | 17.0±5.0defg | 89.0±12.3abc | 42.0±18.0abcde | 88.0±8.6abcde | |
| 10 | 21.0±28.9fg | 81.0±8.2cdefg | 26.0±2.3cdef | 83.0±7.5abcdefg | ||
| 15 | 39.0±33.5bcdefg | 91.0±5.0abc | 5.0±2.0fg | 8.0±3.2fg | ||
| 20 | 15.0±3.8efg | 84.0±3.2bcdef | 1.0±2.0g | 1.0±2.0g | ||
| 2.5 | 26.0±12.4defg | 78.0±2.3efg | 15.0±10.0defg | 87.0±10.0abcde | ||
| 5 | 21.0±28.9fg | 87.0±8.8abcde | 36.0±36.9bcdef | 92.0±3.2abc | ||
| 7.5 | Leaf | 7.0±3.8g | 86.0±4.0abcdef | 33.0±33.8cdef | 84.0±8.0abcdef | |
| 10 | 20.0±19.6efg | 75.0±10.5defg | 34.0±7.6abcdef | 81.0±9.4bcdefg | ||
| 15 | 9.0±6.0g | 72.0±6.5fg | 19.0±11.0cdefg | 75.0±2.0efgh | ||
| 20 | 6.0±4.0g | 38.0±9.5g | 14.0±12.4defg | 52.0±8.6fgh | ||
| 2.5 | 84.0±8.6abc | 88.0±5.6abcde | 74.0±8.3a | 95.0±5.0ab | ||
| 5 | 92.0±5.1ab | 93.0±5.0ab | 75.0±12.3ab | 94.0±5.1ab | ||
| 7.5 | Bark | 84.0±5.6ab | 88.0±3.2abcd | 51.0±15.4abcd | 93.0±6.8abc | |
| 10 | 67.0±5.0abcde | 84.0±5.6bcdef | 50.0±7.6abcd | 91.0±6.8abcd | ||
| 15 | 64.0±8.6abcdef | 84.0±8.6abcdef | 27.0±30.5cdef | 79.0±5.0cdefgh | ||
| 20 | 54.0±12.4abcdef | 83.0±3.8bcdefg | 6.0±2.3efg | 74.0±9.5defgh | ||
| p<0.05 | <0.0001 | 0.0006 | 0.0002 | <0.0001 | ||
| Portulaca oleracea | 0 | 98.0±2.3a | 98.0±2.3ab | 72.0±15.3ab | 100.0±0.0a | |
| 2.5 | 67.0±8.8abcde | 98.0±4.0ab | 58.0±37.0abcd | 99.0±2.0ab | ||
| 5 | 48.0±7.3bcdefg | 99.0±2.0ab | 49.0±19.7abcde | 99.0±2.0ab | ||
| 7.5 | Fruit | 28.0±9.8defgh | 98.0±2.3ab | 26.0±41.3bcdef | 100.0±0.0a | |
| 10 | 19.0±3.8efgh | 96.0±3.2sbcd | 33.0±14.0bcdef | 98.0±2.3ab | ||
| 15 | 40.0±8.6cdefgh | 99.0±2.0ab | 2.0±2.3f | 4.0±5.6c | ||
| 20 | 49.0±13.2bcdefg | 99.0±2.0ab | 0.0±0.0f | 0.0±0.0c | ||
| 2.5 | 58.0±10.5abcdef | 100.0±0.0a | 67.0±11.9abc | 98.0±2.3ab | ||
| 5 | 27.0±6.8defgh | 100.0±0.0a | 21.0±2.0cdef | 99.0±2.0ab | ||
| 7.5 | Leaf | 16.0±6.5fgh | 97.0±3.8abc | 11.0±6.0ef | 99.0±2.0ab | |
| 10 | 12.0±5.6gh | 98.0±4.0ab | 14.0±6.9def | 98.0±4.0ab | ||
| 15 | 1.0±2.0h | 1.0±2.0d | 23.0±14.3cdef | 94.0±4.0c | ||
| 20 | 8.0±4.6gh | 17.0±3.8cd | 0.0±0.0f | 0.0±0.0c | ||
| 2.5 | 93.0±11.4a | 100.0±0.0a | 82.0±6.9a | 100.0±0.0a | ||
| 5 | 90.0±4.0ab | 99.0±2.0ab | 78.0±2.3a | 98.0±2.3ab | ||
| 7.5 | Bark | 87.0±8.2zbc | 98.0±2.3ab | 64.0±9.8abcd | 100.0±0.0a | |
| 10 | 83.0±11.0abc | 100.0±0.0a | 48.0±19.8abcde | 99.0±2.0ab | ||
| 15 | 89.0±12.3ab | 94.0±7.6abcd | 67.0±6.8abc | 99.0±2.0ab | ||
| 20 | 72.0±8.0abcd | 92.0±5.6bcd | 57.0±10.5abcde | 91.0±6.0bc | ||
| p<0.05 | <0.0001 | 0.0013 | <0.0001 | <0.0001 | ||
With a similar pattern, the fruit and leaf formulations of S. terebinthifolius significantly reduces (p < 0.05) the IPG of both weeds. As observed in Table 1, the initial germination of P. oleracea showed a greater susceptibility to the extracts, expressing a negative response with the 36.8 % of treatments, while C. arvensis did so with 31.0 %. In both displays, the IPG was less than or equal to 23.0 ± 14.3 %. This register increased substantially when calculating the FPG. The differentiation between treatments (p < 0.05), showed effective in those treatments with germination lower than 81.0 ± 9.4 and 91.0 ± 6.0 %, for C. arvensis and P. oleracea, respectively. The fruit extracts at 15 and 20 % stood out with a FPG ≤ 8.0 ± 3.2 %, highlighting the inability of P. oleracea to germinate with the concentrate at 20 %. This situation, according to Reinaldo et al. (2012), has been observed in similar species such as Schinus molle L., attributing the response to the reduction in the reproduction rates of meristematic cells. The sum, and in some cases the greater effectiveness of the fruit extracts, coincides with the suggestion about a greater proportion of allelochemicals in the reproductive structures in relation to its leaves (Carvalho et al., 2013). Said components, mainly of the phenolic type, generate instability in the permeability of the cell membrane, translating its effect into an alteration of the water level within the plant, potentiating its effect based on the increase of solutes in the solution (Buturi et al., 2015; Oviedo, 2020).
Initial growth
The E. globulus extract significantly reduces (p < 0.05) the growth of both weeds. The species C. arvensis was shown to be susceptible to the extracts from fruit and leaf structures, at 5 and 7.5 % concentrations, respectively (Figure 3). The total length of the seedling decreased between 28.3 - 44.3 % in relation to the control treatment (32.5 ± 1.9 mm). Meanwhile, P. oleracea responded in an homologous way to these treatments, however, the effective base concentration was established at 2.5 and 15 % for the leaf and fruit structure extracts, correspondingly. With a TL ≤ 7.6 ± 0.6 mm, seedling growth was reduced between 60 - 100 % in relation to the control treatment (19.0 ± 0.9 mm). The E. globulus leaf extract decreased the growth of the P. oleracea hypocotyl between 66.7 - 100.0 % from a concentration of 7.5 % (Figure 4). On the radicle, the leaf-based treatments stand out, starting from concentrations of 2.5 %, observing differences between 81.4 and 99.6 % with the HL spectrum of the control treatment (13.5 ± 1.3 mm).
Figura 3 Promedios de la longitud total (LT) de plántulas de a) Convolvulus arvensis L. y b) Portulaca oleracea L. germinadas tras ser tratadas con extractos acuosos a distintas concentraciones de fruto (-), hoja (---) y corteza (···) de Eucalyptus globulus Labill. (l) y Schinus terebenthifolius Raddi. (l).
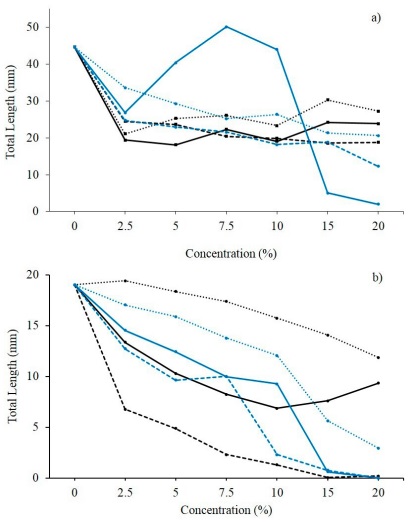
Figure 3. Means of the total length (TL) of seedlings germinated from a) Convolvulus arvensis L. and b) Portulaca oleracea L., after being treated with aqueous extracts at different concentrations of the fruit (-), leave (---) and bark (···) structures of Eucalyptus globulus Labill. (l) and Schinus terebenthifolius Raddi. (l).
Figura 4 Promedios de la longitud de a) hipocótilo (LH) y b) longitud de radícula (LR) de plántulas de P. oleracea L. germinadas tras ser tratadas con extractos acuosos a distintas concentraciones de fruto (-), hoja (---) y corteza (···) de E. globulus Labill. (l) y S. terebenthifolius Raddi. (l).
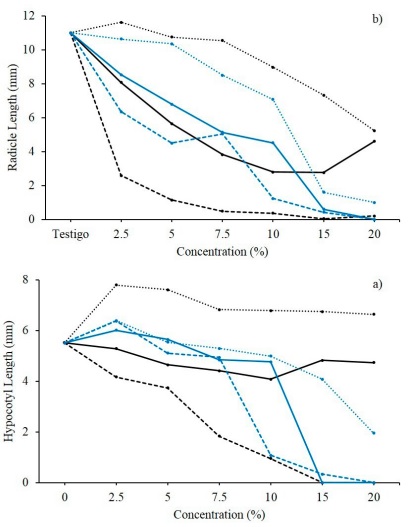
Figure 4. Means of a) hypocotyl length (HL) and b) radicle length (RL), of seedlings germinated from P. oleracea L. after being treated with aqueous extracts at different concentrations of fruit (-), leaf (---) and bark (···) structures of Eucalyptus globulus Labill. (l) and Schinus terebenthifolius Raddi. (l).
For both weeds, although there is initial growth in the seedling, the decrease in TL depending on the concentration and recording time, implies a lag in the translocation of the allelochemicals in the embryo, allowing initial growth but reducing the cell reproduction rate as these are accentuated. Specifically to C. arvensis, Kandhro et al. (2016) highlights a similar behavior of C. arvensis when treated with the aqueous extract of the leaf structure from the species E. camaldulensis, whose effect could be related to the interference of monoterpenes in mitotic activity, and its translations into abnormal growth of the radicle and hypocotyl (Singh et al., 2005; Khan et al., 2008). Meanwhile, Pinto et al. (2021) infers that the growth restriction in P. oleracea seedlings could be due to a homeostatic imbalance, resulting from the metabolic decompensations of the oxido-reducer system.
Regarding S. terebinthifolius, 47.3 % of the treatments registered a significant effect (p < 0.05) on the initial growth of C. arvensis. The TL was reduced between 50.8 and 97.5 % when using the extracts of fruit and bark structures at 15 and 20 %, as well as those of the leaf structure from 7.5 to 20 % (Figure 3). Similarly, the TL of P. oleracea decreased significantly (p < 0.05) when using the leaf and fruit structure treatments at 15 - 20 % (TL ≤ 0.7 ± 0.1 mm), expressing differences in length between 97.5 and 100 % with respect to the LT of the control treatment (16.5 ± 0.8 mm). These observations were complemented by the null hypocotyl growth (0.0 ± 0.0 mm) registered when using the fruit (15 - 20 %) and leaf (20 %) structure treatments, followed by a decrease between 68.8 and 94.4 % with the leaf structure extracts at 10 - 15 %, and bark structure extracts at 20 % (Figure 4). In addition to this, the RL was reduced to 0.0 ± 0.0 mm when it was treated with the 20 % fruit and leaf structure extracts. The formulated fruit (7.5 to 15 %), leaf (5 to 15 %) and bark (15 - 20 %) structure extracts are added, all with a RL between 0.4 ± 0.0 - 5.1 ± 0.4 mm, and whose value represents a decrease at 53.3 %.
The evaluation of total or partite growth is recognized as a process of greater sensitivity to allelochemical componentes (Castro et al., 2004; Bundchen et al., 2015). The susceptibility of smaller radicles to the accumulation of allelochemicals is translated into the absence of absorbent hairs and an abnormal growth of the structure, coinciding with what was observed in the present study (Fonseca et al., 2016; Bitencourt et al., 2021).
Conclusion
The extracts evaluated inhibited the germination and initial in vitro growth of C. arvensis and P. oleracea. According to the evaluated weed, P. oleracea is the species with the highest susceptibility. According to the source, E. globulus expresses better results with the leaf formulations, while S. terebenthifolius does so with its fruits. In all the formulations, a relationship is observed between the concentration and the level of effectiveness obtained.











 nueva página del texto (beta)
nueva página del texto (beta)


