1. Introduction
Mangroves are forest ecosystems that grow on the ocean-continent ecotone. They are important not only because of the goods and services they provide to human communities, but also because of their role as nurseries for fauna. In addition, like many other wetlands, they are significant sinks of carbon and act as coastal protective barriers against storms and hurricanes. Mangrove forest dynamics are determined by oceanic, atmospheric, and terrestrial drivers. Whereas the latitudinal distribution of mangroves is bounded by minimum air temperatures, precipitation seasonality, intertidal range, and geomorphological settings play critical roles at determining the distribution of mangroves at more regional geographic scales (Krauss et al., 2008).
The sea-level increase that has characterized last century has been identified as the main threat to the stability and persistence of modern mangroves (Alongi, 2015). However, decreasing precipitation and increasing temperatures are also important threats as they cause salinization of soils and pore water, causing substantial decreases in productivity and biodiversity of mangrove forests (Alongi, 2008; Gilman et al., 2008). Changes in the frequency and intensity of extreme events such as storm surges and hurricanes have also been identified as important factors affecting the integrity and health of mangrove ecosystems.
The sedimentary dynamics of areas occupied by mangroves are critical to offsetting some of the deleterious effects of sea level rise on mangrove forests. Whereas coastal erosion processes related to sea-level rise occur in zones impacted directly by wave energy, sediment input may cause progradation of the surface (Parkinson et al., 1994; Krauss et al., 2014). Biotic processes such as increases in the root mat may also contribute to elevate the surface, representing another important mechanism for mangroves to adjust to sea level rise (Mckee et al., 2007). Thus, accretion of sediments by mangroves may counteract sea-level rise if their rates are similar. Conversely, if rates of sea-level rise are higher than accretion rates, and there is available space, mangrove ecosystems may migrate landward. When coastal erosion causes significant loss of land or when landward accommodation space is limited by infrastructure or other non-suitable conditions, a net loss of mangrove area occurs.
Based on future climatic scenarios of the IPCC (2013), several authors have postulated important changes in distribution and structure of mangrove ecosystems by the end of this century (e.g.Krauss et al., 2014; Alongi, 2015; Ellison, 2015). Changes in forest structure, composition, and function will be mostly associated with increases in sea surface temperature, atmospheric CO2 concentrations, sea level, and frequency of extreme meteorological events. Also, changes in precipitation and substrate salinity, and unavailability of landward sites for mangrove colonization, are expected to substantially impact these ecosystems. The Caribbean and Central America are expected to face the biggest losses of mangrove areas in the world. Negative impacts of both sea level and temperature rise combined with decreasing precipitation and related river discharges are expected. The combination of these factors will cause net losses in the stands of the three main mangrove species present in the Caribbean (Rhizophora mangle, Avicennia germinans, Laguncularia racemosa) (Record et al., 2013).
Because of the specific environmental preferences of mangrove species, their pollen record along sediment cores can be used to reconstruct past changes of sea level, precipitation, and salinity (Engelhart et al., 2007). Rhizophora mangle dominates fringe mangroves closer to shores, and has been recognized as an indicator of coastal line displacements. Avicenna germinans tolerates high salinities, and is therefore considered a good indicator of coastal drought and evaporative environments. On the other hand, the presence of pollen grains of Laguncularia racemosa and/or spores of the fern Acrostichum aureum may represent open canopies created by either natural or anthropogenic disturbances (Medina et al., 1990). Because the establishment of mangrove populations requires coasts protected from direct wave impact (Hogarth, 1999), rapid and strong displacements of coast lines may not be accurately reconstructed based solely on mangrove pollen spectra. In such cases, the pollen of marsh vegetation is a good indicator of environmental variability (e.g.González and Dupont 2009). In fact, pollen of Amaranthaceae, Cyperaceae and Poaceae has been used to reconstruct past sea-level fluctuation (Hodell et al., 1991; Higuera-Gundy et al., 1999; González and Dupont, 2009). Other taxa such as Batis maritima, Sesuvium portulacastrum, and herbaceous Asteraceae and Fabaceae taxa are abundant along beaches, and their pollen in sedimentary sequences usually indicates environmental stages prior to mangrove establishment (e.g. Peros et al., 2007; Urrego et al., 2013).
At millennial time scales, mangrove ecosystems have expanded and contracted in response to both eustatic and climatic changes. For instance, through the last deglaciation, the Caribbean was characterized by arid and cool conditions, and lower-than-present sea levels (Hodell et al., 1991; Curtis et al., 1998; Higuera-Gundy et al., 1999; Haug et al., 2001). Given this environmental scenario, only spared patches of mangroves were recorded worldwide (Woodroffe and Grindrod, 1991). At the beginning of the Holocene, around 11000 yr BP, fast sea level rise and increases in air temperature and precipitation (Haug et al., 2001) facilitated the earliest mangrove colonization in several places, as recorded in the Panamá Basin (González et al., 2006). Around 6000 BP the relative rapid decline of sea level led to the formation of several Caribbean coastal lakes, lagoons, estuaries, and back swamps that were colonized by mangroves (e.g., Raasveldt and Tomic, 1958; Hendry and Digerfeldt, 1989; Hodell et al., 1995; Curtis et al., 1998; Higuera-Gundy et al., 1999; Ramcharan and McAndrews, 2006; Peros et al., 2007; Wooller et al., 2007; Monacci et al., 2009, 2011; Carrillo-Bastos et al., 2010; Urrego et al., 2013; Joo-Chang et al., 2015).
The major expansion of mangrove forests during the Holocene in the Caribbean region took place around 4000 - 3000 yr BP, when sea levels stabilized, reaching modern conditions (e.g.Ellison, 1996; Rull et al., 1999; Urrego et al., 2013; Cohen et al., 2016). Precipitation and river discharges, although variable, probably contributed to stabilize sediment input and salinity in the newly mangrove-colonized areas. However, the decreasing precipitation trend in the northern Neotropics and increasing climatic variability, probably associated with El Niño-Southern Oscillation (ENSO) (Haug et al., 2001), caused several severe droughts along the Caribbean coasts, from Mexico to Venezuela. These ENSO-related droughts caused the decline of flooding events (Ramcharan and McAndrews, 2006), and in turn reductions in mangrove extensions. Alternatively, mangroves forests experienced compositional changes with dominance of saline tolerant species (e.g. Avicennia germinans) (Ramcharan and McAndrews, 2006; Wooler et al., 2007; Carrillo-Bastos et al., 2010; Monacci et al., 2011).
There is growing evidence of human populations larger than previously thought in South America since the early Holocene to Pre-Columbian times (Goldberg et al., 2015). Thus, human activities have played an important role as a disturbance factor to natural ecosystems through the last millennia. However, the strongest changes in natural resources associated with anthropogenic factors have been recorded during the last few centuries, especially after the industrial revolution by the end of the eighteenth century. Besides land use changes that have caused deforestation worldwide, other human activities have caused an increase of greenhouse gases in the atmosphere (mainly CO2, CH4, and N2O), dramatically affecting climate. Also, the biogeochemical cycles of crucial elements, such as carbon, nitrogen, phosphorous, and sulfur, have been altered by anthropogenic activities (Steffen et al., 2011), driving unprecedented losses of biodiversity. Given this environmental scenario, the Anthropocene has been proposed as a new geological epoch characterized by the deep imprint of humans on the Earth system, although it has not been formally defined. During the last ~200 years environmental changes associated with human activities have taken place in an accelerated manner, with greenhouse gases in the atmosphere reaching record concentrations (Crutzen and Stoemer, 2000; Steffen et al., 2011). Thus, this time interval offers a good scenario for studying the interplay of human and natural stressors on mangrove ecosystems in the Colombian Caribbean. Through the use of palynological evidence we illustrate the disturbance patterns that have characterized the evolution of regional mangrove ecosystems, highlighting the high potential of anthropogenic activities to disrupt ecological and environmental dynamics.
2. The sedimentary record of the Colombian Caribbean mangroves
2.1. THE COLOMBIAN CARIBBEAN AND THE STUDY SITES
The Colombian Caribbean is a 1760-km-long coastline that extends from the Gulf of Urabá, northwestern Colombia, to the Guajira Peninsula, close to the Gulf of Venezuela (Figure 1). Warm climates characterize the region, whereas the precipitation is modulated by a gradient that goes from wet (mean annual precipitation of 2500 mm, Gulf of Urabá) to arid conditions (mean annual precipitation of 550 mm, Guajira Peninsula). The Intertropical Convergence Zone (ITCZ) modulates the bimodal distribution of precipitation and consequently the fluvial sediment supply to the coastal environments. Our review of the evolution of Colombian Caribbean mangrove forests is based on six sedimentary records from four locations in the Colombian Caribbean: core Calancala from the Guajira Peninsula (Urrego et al., 2013), cores La Flotante and Navío from Cispatá Bay (Castaño et al., 2010), cores Candelaria and El Uno from the Gulf of Urabá (this study), and core Honda Bay from San Andrés Island (González et al., 2010).
Guajira Peninsula (northernmost Colombia): The Calancala core (11°34’30’’N, 72°52’36’’W) was retrieved from a branch of the Ranchería River delta that is the main source of freshwater of the Guajira Peninsula. The river reaches the coastal plain near the city of Riohacha (Figure 1), and the Caribbean Low Level Jet that blows parallel to the Guajira coast induces coastal upwelling (Andrade and Barton, 2005). The sediments of the area are mostly alluvial, underlain by aeolian sediments. The coastal sedimentary plain is slightly undulated and is mainly fed by alluvial sediments from the Cesar and Ranchería rivers. An estuary that opens only during the short rainy season is formed by the river mouth (Instituto Geográfico Agustín Codazzi, IGAC, 2009).
Gulf of Urabá: The Gulf of Urabá is the largest semi-enclosed sea inlet along the Colombian Caribbean coastline. The coastline of the gulf is characterized by low intertidal ranges, whereas rivers such as the Atrato at the west and the Turbo at the east exert a strong influence on the geomorphic dynamics because of their high discharge. Thus, the coastline is heavily affected by sediment accumulation and erosion caused by rising sea levels, alluvial sediment transport, and biogenic factors (Bernal et al., 2005). Because of the high fluvial input, soil salinity and interstitial salinity are very low. While the Candelaria Bay core is located on the west side of the gulf close to the Atrato River delta, El Uno Bay core is placed on the east coast of the gulf close to the mouth of the Turbo River (Figure 1).
Cispatá Bay: This bay is located on the northwestern coast of Colombia and encloses a big lagoon complex surrounded by the largest mangrove area of the region. The Sinú River is the main freshwater source, and thus the dynamics of the delta exert high influence on the regional geomorphology. Indeed, the delta has changed its position six times during the last ~350 years. Lagoons La Flotante (9°23’37’’N, 75°48’49’’W) and Navío (9°24’19’’N, 75°51’41’’W), where the cores were retrieved, are part of this system (Figure 1). Regional mean annual precipitation is 1338 mm (Serrano, 2004; Castaño et al., 2010).
San Andrés Island: The island is part of the archipelago comprised of San Andrés, Providencia, and Santa Catalina Islands, lying about 800 km from the Colombian continental coast and about 150 km off Nicaragua (IGAC, 1986). The Honda Bay (12°33’N, 81°42’W) is located on the eastern coast of the island, and is surrounded by a broad mangrove area, with direct communication with the sea on an emerged marine terrace. The mean annual precipitation is 1900 mm (Díaz et al., 1995).
2.2. SEA LEVEL RISE AND GEOMORPHOLOGICAL DYNAMICS
Following the worldwide trend, sea levels have risen steadily along the Caribbean Colombian coast through the last 200 years. The rates of mean regional sea level rise have been estimated at 0.20 cm/yr from 1907 to 1997, and 0.36 cm/ yr for the last five decades (data from two recording stations: Cristóbal, Panama and Cartagena, Colombia, Torres et al., 2008). This sea level rise record coincides with the trends of climate indices (SOI and NOI) reported by Mendelson et al. (2005).
Since the end of the Little Ice Age (~1850 AD) to present, mangroves have shown an increasing representation at several locations of the Colombian Caribbean (Figure 2): El Uno and Candelaria bays in the Gulf of Urabá, La Flotante and Navío in the Cispatá Bay, and Calancala in the Guajira Peninsula. Such expansions have been mainly characterized by a steady increase of Rhizophora mangle pollen and to a lesser extent Avicennia germinans and Laguncularia racemosa. The mean sedimentation rates of these cores have been high since 1850 AD (Figure 3). In El Uno and Candelaria bays the mean annual sedimentation rates have been 0.79 and 0.89 cm/yr, respectively, and in the Cispatá bay these rates were 0.33 and 0.26 cm/ yr in La Flotante and Navío cores, respectively (Castaño et al., 2010). In contrast, a sedimentation rate of 1.44 cm/yr was recorded for the Calancala core (Urrego et al., 2013). High sedimentation rates are related to the high sediment loads of the rivers, a common feature of deltas (Turbo River, 403.29 t km2 yr-1; Atrato River, 315 t km2 yr-1; and Sinú River, 167 t km km2 yr-1). It has been suggested that Caribbean mangroves could tolerate rates of sea level rise of about 0.08 - 0.09 cm/yr, and would be at risk of disappearing with increases between 0.09 and 0.12 cm/yr (Ellison and Stoddart, 1991; Ellison, 1993; Field, 1995). However, our data show that mangroves in the Colombian Caribbean have not only survived high mean rates of sea level rise (between 0.20 and 0.36 cm/yr) but have expanded in territory. Regional mangrove expansion has probably benefited from high fluvial sediment inputs that provide materials to offset sea-level rise (Parkinson et al., 1994; Krauss et al., 2014).
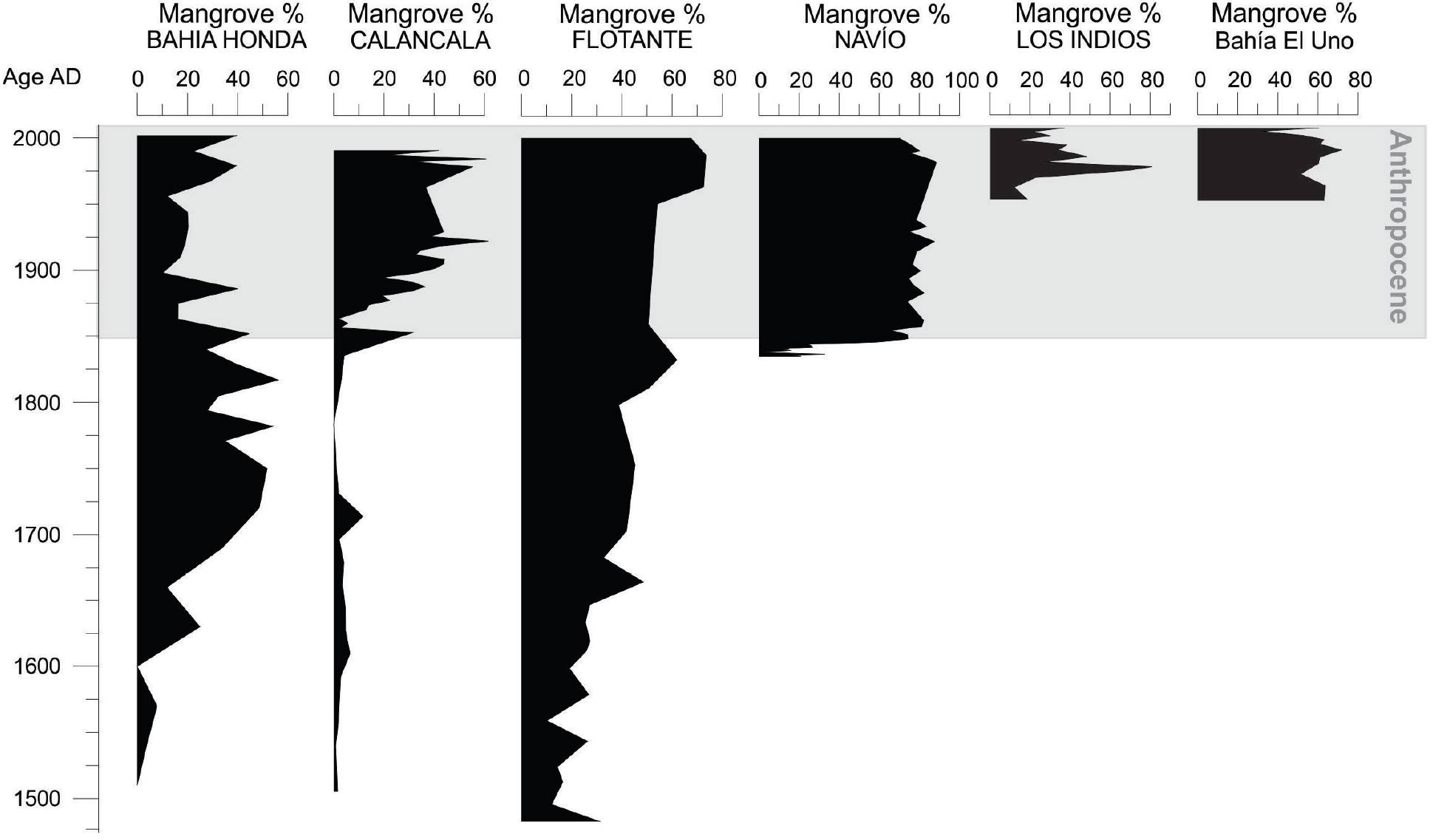
Figure 2. Diagrams of pollen percentages of mangroves from analyzed sediment cores from the Colombian Caribbean during the Anthropocene. A generalized increase of mangrove forests since 1850 AD and especially after 1950 is recorded in all of them.
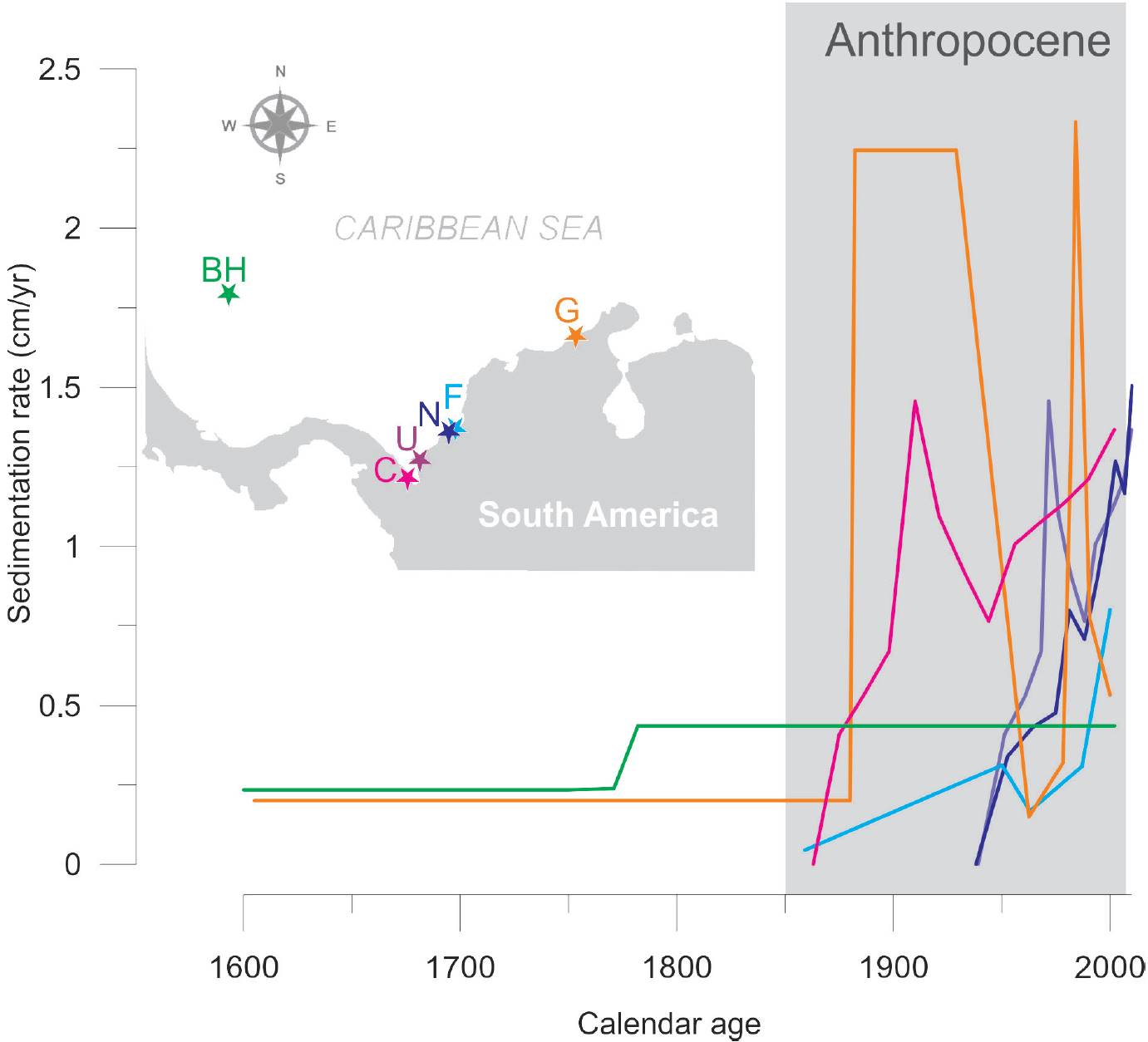
Figure 3. Comparison of sedimentation rates of mangroves from analyzed sediment cores from the Colombian Caribbean during the Anthropocene. Note the increase of sedimentation rates in the continental records located close to deltas, derived from changes in river discharges.
Despite the high sedimentation rates recorded at the Atrato River delta, there were differences in both sedimentation rates and mangrove ecological succession between the two localities sampled in the region. Differences between these locations (Candelaria and El Uno bays) are likely modulated by geomorphology, the specific location of the coring sites within the delta, and the sediment load that each river branch may carry according to the size of the drainage basin. At Candelaria Bay, north of the Atrato River Delta (Figure 2), mangrove pollen shows a decreasing trend towards the present, despite the high sediment load transported by the Atrato River. However, the bay is in direct contact with the sea, receiving high impact from waves (Suárez et al., 2015). This marine influence and the increasing sea level have caused coastal erosion and local mangrove losses. Contrastingly, El Uno Bay, located on the eastern side of the Gulf of Urabá (Figure 2), shows an expansion of mangrove forests during the last two centuries, probably related to a local reconfiguration of the coastal geomorphology. At this site, the Turbo River spit is advancing, causing a natural closure of the bay, which in turn is becoming a coastal lagoon more suitable for mangrove expansion. The eastern side of the Gulf of Urabá, where El Uno Bay lies, receives more sediment than the western side, mostly related to littoral drift and a lesser impact of strong winds (Suárez et al., 2015). This is also the case of Cispatá Bay where changes in mangrove and marsh vegetation reflect at least four migrations of the Sinú River delta. Hydrological and sedimentological dynamics changed the relative position of the lagoons with respect to the shorelines and the river delta. These examples show the critical role of coastal protection and/or geomorphological position for mangroves coping with sea level rise.
2.3. INCREASED SOIL AND PORE-WATER SALINITY AFFECTING MANGROVE SUBSTRATE
Precipitation increases have contributed to the resilience of Caribbean mangrove communities to sea level rise. During periods of high rainfall, mangroves face the indirect effects of increased erosion rates, high continental sediment loads and lower soil salinity. In contrast, when rainfall decreases for longer than the regular dry season (e.g., during strong El Niño events), mangrove forests might die off or change their species composition. Apart from the obvious consequences of rainfall reduction and temperature increases, droughts cause a substantial increase in both soil and interstitial salinity. When the substrate becomes more saline, mangrove community composition switches towards the dominance of Avicennia germinans, which is the most salt tolerant mangrove species (Sobrado, 2000). Despite its low representation in mangrove pollen spectra, during prolonged droughts this species increases at the expense of Rhizophora mangle, sometimes becoming the dominant species of the forest stand (Ellison, 1996; Monacci et al., 2009), a process that has been observed in some instances in the Colombian Caribbean (Figure 2, La Flotante, Calancala and Honda Bay; Castaño et al., 2010; González et al., 2010; Urrego et al., 2013). Similar high-salinity conditions can also be produced by increases in sand sedimentation that cause relief increases, but that are easily drained. This process combined with high evaporation rates and shallow water causes increases of soil salinity, and therefore the turnover from Rhizophora mangle to Avicennia germinans (Twilley et al., 1999; López-Medellín et al., 2011; Monacci et al., 2011). At El Uno Bay, high deposition of sand was recorded in the 1950s when the Turbo River bed was artificially deviated. This anthropogenic intervention changed the local sedimentary dynamics and the composition of the mangrove community, turning a typical fringe mangrove stand into a typical inland community (López-Medellín et al., 2011). Thus, the dominance of Avicennia germinans can either be a signal of mangroves adjusting to current climate change (e.g. López-Medellín et al., 2011), or alternatively a local change in the geomorphology and the sediment dynamics as shown for El Uno Bay.
Several palynological records have shown strong reduction of Caribbean mangroves since the Medieval Warm Period, possibly as a result of decreasing rainfall. Extreme events such as storm surges and hurricanes that have been recorded in several sites have also changed the mangrove forest composition reflected in the sedimentary sequences (López-Medellín et al., 2011; McCloskey and Liu, 2013). Increases in Avicennia germinans have been reported as indicators of early colonization after hurricanes and windstorms at Honda Bay (Figure 2) (González et al., 2010) and in La Guajira Peninsula (Bernal et al., 2016). The climates of both regions were characterized by a strong and prolonged dry season after the occurrence of the extreme event. Increases in Avicennia germinans were probably the result of the high resprout capacity of damaged stems and the increase of light availability caused by massive defoliation (Smith et al., 1994; Baldwin et al., 2001). Avicennia germinans has been regarded as the best-adapted mangrove species to conditions associated with current climate change (increasing temperatures, salinity, and drought frequency; Field, 1995; Snedaker, 1995). Thus, its expansion during the last century in San Andrés Island (Urrego et al., 2009; González et al., 2010) and other localities (Behling et al., 2001) is related not only to droughts and high salinity, but also the increased frequency of extreme storms and winds.
2.4. HUMAN DISTURBANCES
Human activities have affected the extension and composition of most Caribbean mangrove forests, obscuring the signals of current climate change on these ecosystems and therefore on their pollen spectra. Through the last few decades, substantial losses of mangrove forest extensions have been attributed to human disturbances (Rull et al., 1999; Martinuzzi et al., 2009). Nevertheless, clear evidence of the long-term changes, such as pollen records, are rather scarce. Human disturbances to mangrove ecosystems are represented by either the expansion of Laguncularia racemosa (Benfield et al., 2005) and Avicennia germinans at the expense of Rhizophora mangle, or mangrove replacement by herbaceous vegetation (grasslands or crops), especially the fern Acrostichum aureum (Medina et al., 1990). However, these responses have proven highly dependent on species’ differential tolerance to soil salinity and local conditions.
Around 1850 AD, at Honda Bay, San Andrés Island, Colombian Caribbean, mangroves and beach vegetation were replaced by coconut plantations (González et al., 2010), a practice that expanded widely through the Caribbean since that time (Parsons, 1985). The proliferation of Laguncularia racemosa after 1850 AD is also an indication of disturbances, especially during the last few decades, not only in San Andrés Island but also on the continental coastlines of the Colombian Caribbean (La Flotante and Navío localities from the Cispatá Bay, Castaño et al., 2010; and Calancala in the Guajira Peninsula, Urrego et al., 2013). While in San Andrés Island, Zea mays pollen indicating its cultivation was detected in the surroundings of the mangrove forest, in Cispatá, the pollen spectra revealed swampy areas covered by rice crops. Although after 1900 AD these crops were abandoned because of a marine incursion, Laguncularia racemosa has prevailed so far as an indication of pervasive anthropogenic disturbances. In the Gulf of Urabá, mangrove conversion into grasslands for cattle raising is indicated at El Uno Bay (Figure 2). Also, assessments of current vegetation in the Gulf of Urabá have reported expansions of Avicennia germinans, likely as a result of human disturbance resulting in higher sedimentation rates (Suárez et al., 2015).
3. Conclusions
The strongest changes in natural resources associated with anthropogenic factors have been recorded during the last few centuries, especially after the industrial revolution by the end of the eighteenth century. Thus, the need for defining a new geological epoch (Anthropocene) has been identified. In the Colombian Caribbean during this time period, pollen records from sedimentary sequences suggest vegetation changes mostly affected by three main drivers (sea level rise, salinity changes, and human disturbances). During the last two centuries, despite the accelerated rates of sea level rise, increases in sea surface and air temperatures, an invigorated ENSO, and precipitation decreases, mangroves have shown an increasing representation in pollen records at several continental locations of the Colombian Caribbean. Such is the case of El Uno and Candelaria bays in the Gulf of Urabá, La Flotante and Navío in the Cispatá Bay, and Calancala in the Guajira Peninsula. Such expansions are probably associated with the offsetting of growing sea levels by the increasing loads of fluvial sediments since 1850 AD. Mangrove communities of San Andrés Island that grow on carbonate substrates also expanded during this period, and in this case, sea level rises were counterbalanced by the increases in autochthonous peat accumulation. At local scales, differences in mangrove responses to sea level rise are modulated by geomorphology, specific location of the coring sites within deltas, sediment input, or coastal erosion.
When rainfall decreases for longer than the regular dry season (e.g. during strong El Niño intervals) mangroves forests might die off or suffer changes in species composition. During extremely dry climatic episodes, there are substantial increase in soil temperature and salinity, and interstitial salinity. This salinity increase causes mangrove community composition to switch towards the dominance of Avicennia germinans, which is the most salt-tolerant mangrove species. Anthropogenic changes in the river courses have also been associated with similar floristic and compositional changes, turning a typical fringe mangrove forest into a typical inland community. Increases in Avicennia germinans have also been associated with strong and prolonged droughts after the occurrence of extreme events such as strong winds, tropical storms, and hurricanes. High resprout capacity of mangrove species’ damaged stems, and the increase of light availability caused by massive defoliation, are a reasonable explanation for mangrove forest expansion after extreme events. Human disturbances are represented either by the expansion of Laguncularia racemosa and Avicennia germinans at the expense of Rhizophora mangle, or by the replacement of mangrove by herbaceous vegetation (grasslands or crops), especially the fern Acrostichum aureum. This footprint has also been recorded in the palynologycal record of the studied Colombian mangroves.











 nueva página del texto (beta)
nueva página del texto (beta)



