Abstract
Irrigation with water contaminated with arsenic (As) is a risk to human health since it causes the accumulation of this element in the soil and affects crop yield. The objective of this study was to evaluate the effect of phosphorus (P) concentration on irrigation water contaminated with As and its accumulation in the cultivation of barley (Hordeum vulgare L.). The hypothesis was that the accumulation of As by the barley plant is inverse to the concentration of P in the irrigation water. The effect of P was evaluated with a multifactorial experimental design, with a variable level of type 32. Forty-five seeds were planted in soil piles of 0.126 m2, with 20.16 L of irrigation with aqueous solutions of As (50, 200 and 400 μg L-1) and P (120, 210 and 300 μg L-1). We determined the content of As in the soil at the beginning and end of the study, and in the plant at 45 and 90 d after sowing. The analysis of the elements was carried out by ICP-AES after the digestion of the materials with microwaves. The average accumulation of As in the plant (14 101 ± 1 813 μg kg-1) with the treatment of 400 μg L-1 of As was 2.4 times higher than in the treatment with 50 μg L-1. In the treatment with higher concentration of P (300 μg L-1) the plant accumulated an average of 6 871 ± 1 051 μg kg-1, that is, 41.4 % less As than the treatment with 120 μg L-1. The concentrations of P and As in the irrigation water had a significant effect (p(0.05) in the accumulation of as in the barley plant; in addition, P inhibits the absorption of As in barley.
Key words::
barley, irrigation water, soil
Introduction
The presence of arsenic (As) in concentrations of 500 to 500,000 μg L-1 in surface water and wells compromises its use for human and agricultural consumption (Núñez et al., 2016; Rosas-Castor et al., 2016; Yazdani et al., 2016). In this regard, the World Health Organization recommends a maximum allowable limit of 10 μg L-1 and 20,000 μg kg-1 of As in water for human consumption and in soil, respectively (Ng et al., 2003). In response to these recommendations, Mexico gradually reduced the concentration of As allowed in drinking water from 50 to 25 μg L-1 (NOM, 2000).
-
Núñez et al., 2016Determination of arsenic in the presence of copper by adsorptive stripping voltammetry using pyrrolidine dithiocarbamate or diethyl dithiophosphate as chelating-adsorbent agents. Effect of CPB on the sensitivity of the methodMicrochem. J., 2016
-
Rosas-Castor et al., 2016An evaluation of the bioaccessibility of arsenic in corn and rice samples based on cloud point extraction and hydride generation coupled to atomic fluorescence spectrometryFood Chem., 2016
-
Yazdani et al., 2016Adsorptive removal of arsenic (V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorptionJ. Mol. Liq., 2016
-
Ng et al., 2003A global health problem caused by arsenic from natural sourcesChemosphere, 2003
-
NOM, 2000Norma Oficial Mexicana, NOM-127-SSA-1994, 2000
Mexico occupies one of the first places in the world in contamination of water bodies due to high concentrations of As, mainly in groundwater. Armienta and Segovia (2008) indicated that the main sources of As in water reservoirs are natural, such as rock weathering, mineralization, geothermalism and volcanic eruptions (Bundschuh et al., 2008).
-
Armienta and Segovia (2008)Arsenic and fluoride in the groundwater of MexicoEnviron. Geochem. Hlth., 2008
-
Bundschuh et al., 2008Fuentes geogénicas de arsénico y su liberación al medio ambienteDistribución de Arsénico en las Regiones Ibérica e Iberoamericana, 2008
One of the areas with this problem is the Bajío region of Guanajuato, in the physiographic province of the Trans-Mexican Volcanic Belt (FVTM). In this area there is active volcanism, its relief is formed by basaltic volcanic cones and geological faults of extension prevail (Ferrari, 2000). This combination of geological and geomorphological factors allows high contents of As and its easy transport through fractures. There are few regional studies that address the origin and mobilization mechanisms of As in aquifers used for crop irrigation.
-
Ferrari, 2000Avances en el conocimiento de la Faja Volcánica Transmexicana durante la última décadaBol. Soc. Geol. Mex., 2000
In aquifers of the Cuenca La Independencia, in the north of the Guanajuato state, the concentrations of As are 25 to 120 μg L-1, which could be due to the dissolution of minerals rich in this element within the aquifers (Ortega-Guerrero, 2009). Morales-Arredondo et al. (2015) indicated a geogenic origin of As in the groundwater, associated especially with rhyolitic rocks and the influence of geothermal activity in the municipality of Juventino Rosas, in the center of Guanajuato. Rodríguez et al. (2016) quantified 330 μg As L-1 in well water for irrigation in the town of El Copal, community of Irapuato, Guanajuato.
-
Ortega-Guerrero, 2009Presencia, distribución, hidrogeoquímica y origen de arsénico, fluoruro y otros elementos traza disueltos en agua subterránea, a escala de cuenca hidrológica tributaria de Lerma-Chapala, MéxicoRev. Mex. .Cienc. Geol., 2009
-
Morales-Arredondo et al. (2015)Geological, hydrogeological, and geothermal factors associated to the origin of arsenic, fluoride, and groundwater temperature in a volcanic environment ‘‘El Bajío Guanajuatense’’, MexicoEnviron. Earth Sci., 2015
-
Rodríguez et al. (2016)Geological differentiation of groundwater threshold concentrations of arsenic, vanadium and fluorine in El Bajío Guanajuatense, MéxicoGeofís. Int., 2016
We humans are continuously exposed to natural sources of As by anthropogenic activities, such as food consumption with this metalloid from the water-soil-cultivation system (Ongley et al., 2007).
-
Ongley et al., 2007Arsenic in the soils of Zimapán, MexicoEnviron. Pollut., 2007
Agriculture is the anthropogenic activity that uses the highest proportion of water, so the absorption of As by the crops through its root system, from the soil and irrigation water should be investigated (Dahal et al., 2008; Marques et al., 2009; Rothwell et al., 2009). The increase of As concentration in the water-soil-cultivation system by agricultural activity, such as irrigation with deep waters, use of pesticides containing As and residual water from mining is evident (Zhao et al., 2008; Vamerali et al., 2009; Lu et al., 2010). The As increase is exacerbated in crops of higher consumption, such as barley (Hordeum vulgare L.), and Guanajuato produces one third of the national volume of barley, which is why it occupies the first place in Mexico.
-
Dahal et al., 2008Arsenic contamination of soils and agricultural plants through irrigation water in NepalEnviron. Pollut., 2008
-
Marques et al., 2009Arsenic, lead and nickel accumulation in Rubus ulmifolius growing in contaminated soil in PortugalJ. Hazard. Mater., 2009
-
Rothwell et al., 2009Arsenic retention and release in ombrotrophic peatlandsSci. Total Environ., 2009
-
Zhao et al., 2008Arsenic uptake and metabolism in plantsJournal compilation New Phytol., 2008
-
Vamerali et al., 2009Phytoremediation trials on metal- and arsenic- contaminated pyrite wastes (Torviscosa, Italy)Environ. Pollut., 2009
-
Lu et al., 2010Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South ChinaEnviron. Pollut., 2010
Tolerance to As is variable among agricultural crops; limits change in the same plant and depend on water saturation, aeration and other soil characteristics (Srivastava et al., 2012). The accumulation of As in plants also depends on the presence in the medium of phosphates, sulfates, carbonates and other chemical compounds (Lu et al., 2010, Brackhage et al., 2014). Phosphate and arsenate are chemically similar and compete for exchange of ions in the interstices of the soil; thus, in soils with high phosphate content there may be fewer spaces for the adsorption of arsenate (Violante and Pigna, 2002). Arsenate in the plant interrupts the phosphate metabolism and reacts with the hydrogen sulphide groups of enzymes, which inhibits cell function and causes plant death (Wang et al., 2002). Arsenate and phosphate are transported by the same route in higher plants, with transporters that have higher affinity for phosphate (Zhao et al., 2002; Zhao et al., 2008).
-
Srivastava et al., 2012Mechanisms of arsenic tolerance and detoxification in plants and their application in transgenic technology: a critical appraisaInt. J. Phytoremediation, 2012
-
Lu et al., 2010Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South ChinaEnviron. Pollut., 2010
-
Brackhage et al., 2014Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.)Sci. Rep., 2014
-
Violante and Pigna, 2002Competitive sorption of arsenate and phosphate on different clay minerals and soilsSoil Sci. Soc. Amer. J., 2002
-
Wang et al., 2002Mechanism of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciationPlant Physiol., 2002
-
Zhao et al., 2002Arsenic hyper accumulation by different fern speciesNew Phytol., 2002
-
Zhao et al., 2008Arsenic uptake and metabolism in plantsJournal compilation New Phytol., 2008
Mineral fertilizers with phosphate salts are common in the cultivation of cereals, but there are few studies about the effect of P content on irrigation water regarding the accumulation of As in plants. Smith et al. (2002) showed that P applied to crops can displace As from the soil and leaves it available for the plant, in which As concentration is increased. In malted barley plants grown in pots, with soils having 10 450 to 13 580 μg As kg-1, from Zimapán, Hidalgo, Mexico, and irrigated with well water contaminated with 400 μg As kg-1, the As concentration reached up to 33 130 μg kg-1 (Prieto et al., 2010).
-
Smith et al. (2002)Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorptionJ. Environ. Qual., 2002
-
Prieto et al., 2010Bioacumulación de arsénico en las etapas de desarrollo de la cebada malteraRev. Mex. Cienc. Agríc., 2010
Due to the chemical affinity of As and P, the presence of both elements in the irrigation water could generate competition between them, both in the soil and plant (Moreno-Jiménez et al., 2012; Bolan et al., 2013; Lee et al. al., 2016). Therefore, the objective of our research was to evaluate the effect of P concentration on the irrigation water contaminated with As on P accumulation in barley plants grown in soil contaminated with As, in Irapuato, Guanajuato. The transporters in the plant are more related to P (Zhao et al., 2008), so the hypothesis was that the barley plant accumulates less As from the soil and water if the concentration of P is higher in the irrigation water.
-
Moreno-Jiménez et al., 2012The fate of arsenic in soil-plant systemsReviews of Environmental Contamination and Toxicology, 2012
-
Bolan et al., 2013Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic bioavailability and mobilitySci. Total Environ., 2013
-
Lee et al. al., 2016Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soilsGeoderma, 2016
-
Zhao et al., 2008Arsenic uptake and metabolism in plantsJournal compilation New Phytol., 2008
Materials and Methods
Characteristics of the experiment site
This study was carried out at the locality El Copal, located 10 km from Irapuato, in the south-central part of the state of Guanajuato (20° 44’ 42.10” N, 101° 19´39.63” W and average altitude of 1800 m). In this region, there are extensive intermontane valleys, filled with modern continental sediments.
The climate is subhumid warm, with annual precipitation between 550 and 700 mm and average annual temperature of 18° C. The average annual evaporation is 1300 mm, with a water deficit of 600 mm, so the depth of extraction of well water dedicated to irrigation increases every year. Due to rocky bodies and their origins, irrigation water can be a source of heavy metals.
The soils cultivated at El Copal area are vertisols (USDA, 2014), of dark color, formed in temperate climates under a rustic humidity regime and characterized by a high content of clay minerals (Eash, et al., 2008).
-
USDA, 2014Keys to Soil Taxonomy: Natural Resources Conservation Services, 2014
-
Eash, et al., 2008Soil Science Simplified, 2008
Soil used
We used 1.82 m3 of soil in the layer of 0.3 m deep, of an agricultural plot with bulk density of 1.11 g cm-3, 55.96 % clay, pH of 8.17, and 2.76 % of organic matter. Irrigation at El Copal is with deep well water and its annual average concentration is 330 μg L-1.
Experimental design
The effect of P was evaluated with a multifactorial experimental design, with a variable level of type 3x3x2. In this stage, we evaluated the concentration of As and P in the irrigation water and growth of barley over time. The levels of P in the irrigation water (PAR) were 120, 210 and 300 μg L-1 and those of As (AsAR) were 50, 200 and 400 μg L-1; and the development time (t) was 45 and 90 d after sowing. A control or target (B) was distilled water (As and P free) for irrigation (Table 1). The response variable was the concentration of As in the plant (AsPP). All treatments were performed in triplicate.
Table 1
Watering treatments for barley with As and P in the irrigation water.
Watering treatments for barley with As and P in the irrigation water.
| Arsénico | Fósforo | ||
|---|---|---|---|
| 120 μg L-1 | 210 μg L-1 | 300 μg L-1 | |
| 50 μg L-1 | As1P1 | As1P2 | As1P3 |
| 200 μg L-1 | As2P1 | As2P2 | As2P3 |
| 400 μg L-1 | As3P1 | As3P2 | As3P3 |
Plant cultivation
We conducted sowing with certified seed (var. Esmeralda) on December 20, 2009, in 57 piles of soil of 0.126 m2 land, with 38 kg each. In each pile, 45 seeds were manually sown, by the staggered method (plants in parallel rows in which each plant of a row faces a hole, between two plants of the next row). Ammonium sulfate at 19 % per ha (75 kg N) was applied at the beginning and 45 d after sowing. The plants were kept in the field near the laboratory.
Irrigation
Irrigation was performed with distilled water mixed with As (Na2HAsO4·7H2O 99.98% pure; Sigma-Aldrich, Mexico) and P (NaH2PO4 99.00 % pure; Sigma Aldrich, Mexico) in defined concentrations for each treatment (Table 1). Na2HAsO47H2O in water dissociates into arsenate (AsO4 -3), the form commonly present in irrigation water and in the soil under aerobic conditions (Zhao et al., 2008). NaH2PO4 in water dissociates into phosphate (PO4 -3), as it occurs with phosphate fertilizers in soil under aerobic conditions. Irrigation was applied at the beginning of sowing (day 0) and 30 and 51 d after sowing (dds). In the first irrigation, we applied a 40 mm thick sheet of aqueous solution (5.04 L), in the second and third irrigation we used 60 mm (7.56 L). According to the area of the piles (0.126 m2), a total volume of 20.16 L of solution with As and P concentrations was applied according to the treatments used (Table 1).
-
Zhao et al., 2008Arsenic uptake and metabolism in plantsJournal compilation New Phytol., 2008
Determination of total arsenic
The AsPP was determined in each pile at 45 and 90 dds. From each pile we extracted 15 plants (with root), washed them with distilled water and dehydrated them at 70 ° C for 72 h, crushed in a mortar and passed through a 1 mm sieve. The soil As content was determined at the beginning and end of the study and in the nitrogen fertilizer.
For the digestion of the plant, soil and fertilizer samples, we used the EPA3051A/2007 method. The total As was quantified with the method EPA 6010C 2007/ICP: 0.5 g of homogenized sample were weighed, 10 mL of concentrated HNO3 (metal trace degree, 69 %, Fisher Scientific) were added and placed in a microwave oven (CEM, Model MARSX 230/60). The digestion took place in two stages: 1) with pressure of 0.827 MPa (120 psi) at 90 °C and constant temperature for 15 min; 2) the mixture was heated at 130 °C (reached in about 5 min) and kept for 25 min. The digested samples were cooled to room temperature, filtered (Watman paper 41) and 50 mL of tridstilled water were added. The P and As content was determined in an induction-coupled plasma atomic emission spectroscope (ICP-AES, model Iris Intrepid Thermo Elemental, Thermo Scientific Company, Waltham, MA, USA), which was calibrated with one white target and five standards.
Results and Discussion
Arsenic in soil
The average concentration of As in the soil at the beginning of the study was 1 372 ± 20 μg kg-1. This concentration was low compared to that reported by Dahal et al. (2008) (6 100 and 16 700 μg kg-1) in surface soil of the Nawalparasi district, Nepal, and by Roychowdhury et al. (2005) (up to 19 400 μg kg-1) in India. In the area of mining activity in Zimapán, Hidalgo, the soil As concentration was 52 000 μg kg-1 (Ongley et al., 2007) and the As accumulation in the plant tissue was 8 440 to 12 390 μg kg-1, in some of the 62 crops, including epazote (Chenopodium ambrosoides), chayote (Sechium edule), chilacayote (Cucurbita ficifolia) and parsley (Petroselinum crispum), (Prieto et al., 2005).
-
Dahal et al. (2008)Arsenic contamination of soils and agricultural plants through irrigation water in NepalEnviron. Pollut., 2008
-
Roychowdhury et al. (2005)Effect of arsenic-contaminated irrigation water on agricultural land soil and plants in West Bengal, IndiaChemosphere, 2005
-
Ongley et al., 2007Arsenic in the soils of Zimapán, MexicoEnviron. Pollut., 2007
-
Prieto et al., 2005Acumulación en tejidos vegetales de arsénico provenientes de aguas y suelos de Zimapán, estado de Hidalgo, MéxicoBioagro, 2005
In our experiment the concentration of As in the soil (1 372 ± 20 μg kg-1) was lower than in the studies mentioned, mainly because the cultivation of rice, vegetables and bulbs requires more irrigation than grains, such as barley, wheat, corn and sorghum. In this regard, Mukherjee et al. (2017) noted that the volume of irrigation water for rice production in India is 12,000 to 14,000 m3 ha-1 and, according to Perez et al. (2011), barley in Guanajuato utilizes a maximum irrigation volume of 7,540 m3 ha-1. Although in Guanajuato there is a mining zone, our study area is more than 45 km away, so it is not an As source for agricultural soils; in addition, the fertilizer used did not provide As.
-
Mukherjee et al. (2017)Arsenic load in rice ecosystem and its mitigation through deficit irrigationJ. Environ. Manage., 2017
-
Perez et al. (2011)Contaminación agrícola y costos en el Distrito de Riego 011, GuanajuatoRev. Mex. Cienc. Agríc. Pub. Esp., 2011
Arsenic in plants
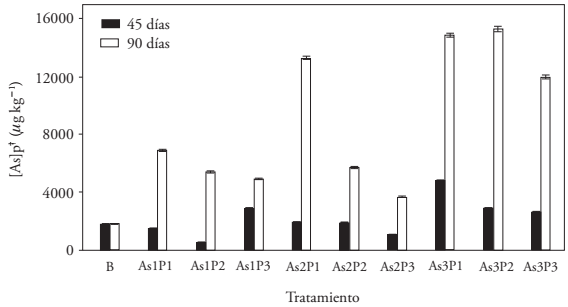
The average concentration of AsPP at 90 dds was higher than at 45 dds, but in the control (B) increased 1.1 % (Figure 1). This indicates that the rate of As absorption by the plant increased and accumulated 4.7 times more As in the last 45 d (between tillering and stem elongation). Malhi et al. (2012) indicated that barley absorbs P in sigmoid form, from its emergence until the stage of grain filling. The competition of P and As explains the increase in the speed of absorption of As by the plant. The concentration of AsPP in B did not increase between 45 and 90 dds, and the plant grew keeping the As absorption rate of the soil per kg of tissue.
Thumbnail
Figure 1
As concentration in the barley plant (B: control; n = 3).
As concentration in the barley plant (B: control; n = 3).
In addition, the average concentration of AsPP in the treatments with 400 μg L-1 of AsAR, at 90 dds, was 2.4 times higher than that of the treatments with 50 μg L-1 of AsAR. The average of As accumulated in the plants of the treatments with 300 μg L-1 of PAR was 6 871 ± 1051 μg kg-1, and the average of AsPP for the treatments with 120 μg L-1 of PAR was 11 733 ± 4 229 μg kg-1. This represented 41.4 % less As accumulated in barley due to a higher PAR concentration. Besides, the AsPP of barley with the AS3P1 treatment was 2.2 times higher than with As1P1. Also, the As1P3 treatment showed less AsPP accumulation than As1P1 and As1P2; similarly, in As2P3 with respect to As2P1 and As2P2, and in As3P3 compared to As3P1 and As3P2. The highest accumulation of As in the plant was found at 90 dds in the treatments As3P2 (15 354 ± 91 μg kg-1) and As3P1 (14 926 ± 67 μg kg-1) that used irrigation water with the highest amount of As and the lowest of P. The two minor AsPP concentrations (at 90 dds) were observed in the treatments As1P3 (4 920 ± 39 μg kg-1) and As2P3 (3 670 ± 35 μg kg-1), which had the highest concentration of PAR. This behavior is similar to that described by Saldaña-Robles et al. (2012).
-
Saldaña-Robles et al. (2012)Transfer concentrations of arsenic in the structures of barley (Hordeum vulgare)Understanding the Geological and Medical Interface of Arsenic-As 2012: Proceedings of the 4th International Congress on Arsenic in the Environment, 22-27 July 2012, 2012
At the beginning of the study, the soil contained 1 372 ± 20 μg As kg-1 (As0, Figure 2) and by the end (CASFIN) 1 386 ± 2, 1 469 ± 12 and 1 568 ± 5 μg kg-1 in the treatments with irrigation water having 50, 200 and 400 μg As kg-1, respectively, which were increments of 1.1, 7.1 and 14.2 % in CASFIN, with a significant linear correlation (R2 = 0.999) between percentage and concentration of AsAR. The piles of each treatment (same AsAR) had a low variation, with a maximum of 20 μg kg-1. Therefore, the accumulated amount of As in the soil depended only on the AsAR concentration and not on PAR concentration. These results coincided with those by Dahal et al. (2008), who monitored the influence of As contamination by irrigation water on alkaline soils and the intake of As by the plants in the field, and concluded that the concentration of As in the soil and plant is influenced by the content of As in the irrigation water.
-
Dahal et al. (2008)Arsenic contamination of soils and agricultural plants through irrigation water in NepalEnviron. Pollut., 2008
Thumbnail
Figure 2
Concentration of As in the soil (± SD) at the end of the barley crop (B: control).
Concentration of As in the soil (± SD) at the end of the barley crop (B: control).
Plants with the same AsAR concentration showed differences in the As absorbed and different PAR concentration, suggesting that PAR affected the concentration of AsPP, but not in CASFIN. The effect of PAR is explained by its wide interval in the mass difference of As accumulated between the soil and plant (mainly at early ages), and the increased occurrence of ion exchange processes, in which As in the irrigation water greatly accumulates in terms of total mass in the soil, compared to the amount of As absorbed by the plant. However, the concentration of As in the barley plant can be up to 12 times greater than that of the soil.
The concentration of As and P in the irrigation water and as a result of the effect of t on the development of plants was significant (p ≤ 0.05) in the concentration of As in the plant. The interactions AsAR-AsAR, AsAR-t and PAR-t were also significant (Table 2).
Table 2
Analysis of variance for As plant.
Analysis of variance for As plant.
| Fuente | Suma de cuadrados (108) | F | Grados de libertad | p ≤ |
|---|---|---|---|---|
| AsAR | 2.31527 | 111.02 | 1 | 0.0000† |
| PAR | 0.67871 | 32.54 | 1 | 0.0000† |
| t | 6.68840 | 320.71 | 1 | 0.0000†/ |
| AsAR-AsAR | 0.19412 | 9.31 | 1 | 0.0039† |
| AsAR-PAR | 0.05268 | 2.53 | 1 | 0.1193 |
| AsAR-t | 0.99640 | 47.78 | 1 | 0.0000† |
| PAR-PAR | 0.04137 | 1.98 | 1 | 0.1662 |
| PAR-t | 0.41813 | 20.05 | 1 | 0.0001† |
| Bloques | 0.00114 | 0.03 | 2 | 0.973 |
| Error total | 0.89675 | 43 | ||
| Total (correl.) | 12.1168 | 53 |
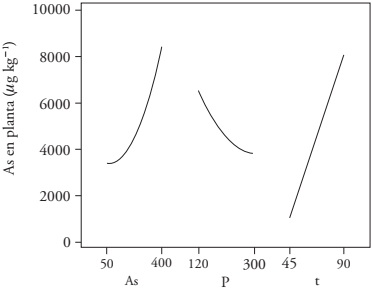
The simple statistical effects showed that the combination of the highest concentration of AsAR (400 μg L-1), the lowest of PAR (120 μg L-1) and the highest t (90 d) tended to accumulate more AsPP (> 7 400 μg) kg-1). On the contrary, the combination of the lowest concentration of AsAR (50 μg L-1), the highest concentration of PAR (300 μg L-1) and the lowest t (45 d) showed that AsPP decreased (<3 400 μg kg-1). (Figure 3). The effect of PAR on accumulated AsPP could be due to the similarity of the chemical form of phosphates and arsenates, which would generate competition in their absorption by the plant, and in adsorption sites in the soil. Lee et al. (2016) confirmed this mechanism by describing arsenate as analog of phosphate and the access route to the rice plant, with the same transporters. Smith et al. (2002) indicated that the addition of phosphates to the soil can release arsenates and favor their absorption by the plant. In our study, we observed an opposite effect, which strengthens previous findings that show that arsenate competes with phosphate as a substrate in the nutrient intake of the plant, with greater affinity for phosphates (Zhao et al., 2008; -Jiménez et al., 2012). Then, the addition of phosphate competed with arsenate for the soil adsorption sites, which in turn affected As availability for the plant that preferably incorporated P, and the soil was the medium that accumulated most of the As. Bolan et al. (2013) observed that P can inhibit the absorption of As by the plant; therefore, the presence of P in the irrigation water decreased the accumulation of As in the rice plant, according to Lu et al. (2010).
-
Lee et al. (2016)Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soilsGeoderma, 2016
-
Smith et al. (2002)Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorptionJ. Environ. Qual., 2002
-
Zhao et al., 2008Arsenic uptake and metabolism in plantsJournal compilation New Phytol., 2008
-
Bolan et al. (2013)Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic bioavailability and mobilitySci. Total Environ., 2013
-
Lu et al. (2010)Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South ChinaEnviron. Pollut., 2010
Thumbnail
Figure 3
Main effects (AsAR, PAR and t) of As concentration on the barley plant.
Main effects (AsAR, PAR and t) of As concentration on the barley plant.
The ASAR-PAR interaction was not significant (p>0.05) for the accumulation of As in the plant, whereas the AsAR-AsAR interaction actually was (Table 2 and Figure 4). This indicates an exponential increase of AsPP concentration dependent on the increase of AsAR concentration. The significance of the AsAR and t (p ≤ 0.05) interaction was observed in the increase of As in the plant, which on average rose 4.7 times between sowing, at 45 d and 90 d of crop development; PAR-t had a significant effect on the accumulation of As in the plant because at 45 d of development, AsPP accumulated in the plant almost in the same amount as any PAR concentration, and at 90 d, the higher the concentration of PAR the lower that of AsPP (Figure 4).
Thumbnail
Figure 4
Interaction effects of AsAR-PAR, AsAR-t and PAR-t.
Interaction effects of AsAR-PAR, AsAR-t and PAR-t.
Conclusions
The barley plant increases its As content with the concentration of this element in the irrigation water; in contrast, the higher the P content in the irrigation water, the lower the accumulation of As in the barley plant. This effect is accentuated with the development of the crop. Arsenic and P compete in the soil and the plant, but the affinity is greater for P in the plant. The soil accumulates the As coming from the irrigation water in a linear way, without significant influence of P concentration on that water.
Literatura citada
- Armienta, M A., and N. Segovia. 2008. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Hlth. 30: 345-353. Links
- Bolan, N S., S. Mahimairaja, A. Kunhikrishnan, and G. Choppala. 2013. Phosphorus-arsenic interactions in variable-charge soils in relation to arsenic bioavailability and mobility. Sci. Total Environ. 463-464: 1154-1162. Links
- Brackhage, C., J H. Huang, J. Schaller, E J. Elzinga, and E G. Dudel. 2014. Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.). Sci. Rep. 4: 9444. Links
- Bundschuh, J., F E. Giménez, R. Guerequiz, A C. Pérez, M E. García, J. Mello, y E. Deschamps. 2008. Fuentes geogénicas de arsénico y su liberación al medio ambiente. In: Bundschuh, J., A. Pérez Carrera, y M I. Litter (eds). Distribución de Arsénico en las Regiones Ibérica e Iberoamericana. Ed.: CYTED, Buenos Aires, Argentina. pp: 33 - 48. Links
- Dahal, B M., M. Fuerhacker, A. Mentler, K B. Karki, R R. Shrestha, and W E H. Blum. 2008. Arsenic contamination of soils and agricultural plants through irrigation water in Nepal. Environ. Pollut. 155: 157-63. Links
- Eash, N S., C J. Green, A. Razvi, and W F. Bennett. 2008. Soil Science Simplified. Fifth edition. Blackwell Publishing, 9600 Garsington Rd., Oxford OX4 2DQ UK 246. 272 p. Links
- Ferrari, L. 2000. Avances en el conocimiento de la Faja Volcánica Transmexicana durante la última década. Bol. Soc. Geol. Mex. 53: 84-92. Links
- García, E. 1988. Modificaciones al sistema de clasificación climática de Köppen (para adaptarlo a las condiciones de la República Mexicana). Offset Larios. México D.F. pp: 46-52. Links
- Lee, C H., C H. Wu, C H. Syu, P Y. Jiang, C C. Huang, and D Y. Lee. 2016. Effects of phosphorous application on arsenic toxicity to and uptake by rice seedlings in As-contaminated paddy soils. Geoderma 270: 60-67. Links
- Lu, Y., F. Dong, C. Deacon, H. Chen, A. Raab, and A A. Meharg. 2010. Arsenic accumulation and phosphorus status in two rice (Oryza sativa L.) cultivars surveyed from fields in South China. Environ. Pollut. 158: 1536-1541. Links
- Malhi, S S., A M. Johnston, J J. Schoenau, Z H. Wang, and C L. Vera. 2006. Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozem soil in Saskatchewan. Can. J. Plant Sci. 86: 1005-1014. Links
- Marques, A P., H. Moreira, A O. Rangel, and P M L. Castro. 2009. Arsenic, lead and nickel accumulation in Rubus ulmifolius growing in contaminated soil in Portugal. J. Hazard. Mater. 165: 174-179. Links
- Morales-Arredondo, I., R E. Villanueva-Estrada, R. Rodríguez, and M A. Armienta. 2015. Geological, hydrogeological, and geothermal factors associated to the origin of arsenic, fluoride, and groundwater temperature in a volcanic environment ‘‘El Bajío Guanajuatense’’, Mexico. Environ. Earth Sci. 74: 5403-5415. Links
- Morales-Arredondo, I., R. Rodríguez, A. Armienta, and R E. Villanueva-Estrada. 2016. A low-temperature geothermal system in central Mexico: Hydrogeochemistry and potential heat source. Geochem. J. 50: 211-225. Links
- Moreno-Jiménez, E., E. Esteban, and J M. Peñalosa. 2012. The fate of arsenic in soil-plant systems. Whitacre, D M. (ed). Reviews of Environmental Contamination and Toxicology. pp: 1-37. Links
- Mukherjee, A., M. Kundu, B. Basu, B. Sinha, M. Chatterjee, M D. Bairagya, U K. Singh, and S. Sarkar. 2017. Arsenic load in rice ecosystem and its mitigation through deficit irrigation. J. Environ. Manage. 197: 89-95. Links
- Ng, J. C., J. Wang, and A. Shraim. 2003. A global health problem caused by arsenic from natural sources. Chemosphere 52: 1353-1359. Links
- Norma Oficial Mexicana, NOM-127-SSA-1994. 2000. Salud ambiental, agua para uso y consumo humano-límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. http://www.salud.gob.mx/unidades/cdi/nom/m127ssa14.html . (Consulta: mayo 2016). Links
- Núñez, C., V. Arancibia, and M. Gómez. 2016. Determination of arsenic in the presence of copper by adsorptive stripping voltammetry using pyrrolidine dithiocarbamate or diethyl dithiophosphate as chelating-adsorbent agents. Effect of CPB on the sensitivity of the method. Microchem. J. 126: 70-75. Links
- Ongley, L. K., L. Sherman, A. Armienta, A. Concilio, and C. Ferguson. 2007. Arsenic in the soils of Zimapán, Mexico. Environ. Pollut. 145: 793-799. Links
- Ortega-Guerrero, MA. 2009. Presencia, distribución, hidrogeoquímica y origen de arsénico, fluoruro y otros elementos traza disueltos en agua subterránea, a escala de cuenca hidrológica tributaria de Lerma-Chapala, México. Rev. Mex. .Cienc. Geol. 26: 143-161. Links
- Pérez, EyR., KyA. Jara Durán, y A. Santos Baca. 2011. Contaminación agrícola y costos en el Distrito de Riego 011, Guanajuato. Rev. Mex. Cienc. Agríc. Pub. Esp. 1: 69-84. Links
- Prieto, F., J. Callejas, M. Lechuga, J. Gaytán, y E. Barrado. 2005. Acumulación en tejidos vegetales de arsénico provenientes de aguas y suelos de Zimapán, estado de Hidalgo, México. Bioagro 17: 129-135. Links
- Prieto, F., J. Prieto, J. Callejas, A D. Román, y M A. Méndez. 2010. Bioacumulación de arsénico en las etapas de desarrollo de la cebada maltera. Rev. Mex. Cienc. Agríc. 1:37-44. Links
- Rodríguez, R., I. Morales-Arredondo, and I. Rodríguez. 2016. Geological differentiation of groundwater threshold concentrations of arsenic, vanadium and fluorine in El Bajío Guanajuatense, México. Geofís. Int. 55: 5-15. Links
- Rosas-Castor, J., L. Portugal, L. Ferrer, L. Hinojosa-Reyes, J L. Guzmán-Mar, A. Hernández-Ramírez, and V. Cerdá. 2016. An evaluation of the bioaccessibility of arsenic in corn and rice samples based on cloud point extraction and hydride generation coupled to atomic fluorescence spectrometry. Food Chem. 204: 475-482. Links
- Rothwell, J J., K G. Taylor, E L. Ander, M G. Evans, S M. Das, and T E H. Allott. 2009. Arsenic retention and release in ombrotrophic peatlands. Sci. Total Environ. 407: 1405-1417. Links
- Roychowdhury, T., H. Tokunaga, T. Uchino, and M. Ando. 2005. Effect of arsenic-contaminated irrigation water on agricultural land soil and plants in West Bengal, India. Chemosphere 58: 799-810 Links
- Saldaña-Robles, A., and R J. Guerra-Sánchez. 2012. Transfer concentrations of arsenic in the structures of barley (Hordeum vulgare). In Understanding the Geological and Medical Interface of Arsenic-As 2012: Proceedings of the 4th International Congress on Arsenic in the Environment, 22-27 July 2012, Cairns, Australia. CRC Press. 231 p. Links
- Smith, E., R. Naidu, and A M. Alston. 2002. Chemistry of inorganic arsenic in soils: II. Effect of phosphorus, sodium, and calcium on arsenic sorption. J. Environ. Qual. 31: 557-63. Links
- Srivastava, S., P. Suprasanna, and S F. D’Souza. 2012. Mechanisms of arsenic tolerance and detoxification in plants and their application in transgenic technology: a critical appraisal. Int. J. Phytoremediation 14: 506-17. Links
- United States Department of Agriculture, USDA, Soil Survey Staff. 2014. Keys to Soil Taxonomy: Natural Resources Conservation Services, 12th edition. 1400 Independence Avenue, S.W.; Washington, D.C. 314 p. Links
- Vamerali, T., M. Bandiera, L. Coletto, F. Zanetti, N M. Dickinson, and G. Mosca. 2009. Phytoremediation trials on metal- and arsenic- contaminated pyrite wastes (Torviscosa, Italy). Environ. Pollut. 157: 887-94. Links
- Violante, A., and M. Pigna. 2002. Competitive sorption of arsenate and phosphate on different clay minerals and soils. Soil Sci. Soc. Amer. J. 66: 1788-1796. Links
- Wang, J., F. Zhao, A. Meharg, A. Raab, J. Feldmann, and S. McGrath. 2002. Mechanism of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol. 130: 1552-1561. Links
- Yazdani, M R., T. Tuutijärvi, A. Bhatnagar, and R. Vahala. 2016. Adsorptive removal of arsenic (V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 214: 149-156. Links
- Zhao, FJ., J F. Ma, A A. Meharg, and S P. McGrath. 2008. Arsenic uptake and metabolism in plants. Journal compilation New Phytol. 181: 777-794. Links
- Zhao, F J., S J. Dunham, and S P. McGrath. 2002. Arsenic hyper accumulation by different fern species. New Phytol. 156: 27-31. Links

 † [As] p - Concentration of arsenic in the barley plant.
† [As] p - Concentration of arsenic in the barley plant. † [As] s: Concentration of As in the soil at the end of the experiment.¶ As0: Concentration of As in the soil at the beginning of the experiment.
† [As] s: Concentration of As in the soil at the end of the experiment.¶ As0: Concentration of As in the soil at the beginning of the experiment.