Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Chapingo. Serie horticultura
versión On-line ISSN 2007-4034versión impresa ISSN 1027-152X
Rev. Chapingo Ser.Hortic vol.21 no.2 Chapingo may./ago. 2015
https://doi.org/10.5154/r.rchsh.2015.01.004
Artículo de revisión
The organic acids that are accumulated in the flesh of fruits: occurrence, metabolism and factors affecting their contents - a review
Ácidos orgánicos acumulados en la pulpa de los frutos: ocurrencia, metabolismo y factores que afectan sus contenidos - una revisión
Franco Famiani1*; Alberto Battistelli2; Stefano Moscatello2; Juan G. Cruz-Castillo3; Robert P. Walker1
1 Dipartimento di Scienze Agrarie, Alimentari e Ambientali, Università degli Studi di Perugia, Perugia, ITALY. Correo-e: franco.famiani@unipg.it y rob.walker@talktalk.net (*Autor para correspondencia).
2 Istituto di Biologia Agroambientale e Forestale, CNR, Porano (TR), ITALY.
3 Centro Regional Universitario Oriente, Universidad Autónoma Chapingo, Huatusco, Veracruz, MÉXICO.
Received: January 17, 2014.
Accepted: May 27, 2015.
Abstract
Organic acids are abundant constituents of ripe fruits and are responsible for their sourness. In addition, they contribute to their flavour. In many fruits, the most abundant organic acids are malic and citric. The aims of this review are two-fold. The first is to provide a clear overview of malic and citric acids in the flesh of fruits. The abundance of different organic acids in commercially grown fruits is described. How this abundance changes during fruit development is outlined. The metabolic pathways used in the synthesis and dissimilation of malic and citric acids in fruits are described. The functions of malic and citric acids in the flesh of fruits are discussed. Secondly, how environmental and cultural practices can alter the organic acid content of fruits is considered.
Keywords: citrate, cultural practices, malate, malate dehydrogenase (MDH), malic enzyme, phosphoenolpyruvate carboxykinase (PEPCK), phosphoenolpyruvate carboxylase (PEPC), pyruvate orthophosphate dikinase (PPDK), temperature.
Resumen
Los ácidos orgánicos son componentes abundantes de los frutos maduros y responsables de la acidez, además contribuyen en su sabor. En muchos frutos, los ácidos más abundantes son el málico y el cítrico. Los objetivos de esta revisión son, en primer lugar, proporcionar una reseña clara respecto de los ácidos málico y cítrico en los frutos. Se describe la abundancia de diferentes ácidos en frutos cultivados comercialmente, la manera en que esta abundancia cambia durante el desarrollo de los frutos y las rutas metabólicas usadas en la síntesis y la desasimilación de los ácidos málico y cítrico en los frutos; adicionalmente, se discute sobre las funciones de estos ácidos en la pulpa de los frutos. En segundo lugar, se aborda la manera en que las prácticas ambientales y culturales pueden alterar el contenido de los ácidos orgánicos en frutos.
Palabras clave: citrato, prácticas culturales, malato, Malato deshidrogenasa (MDH), enzima málica, fosfoenolpiruvato carboxiquinasa (PEPCK), fosfoenolpiruvato carboxilasa (PEPC), piruvato ortofosfato diquinasa (PPDK), temperatura.
INTRODUCTION
Organic acids together with sugars are the main soluble components of ripe fruits and have a major effect on taste, being responsible for sourness and contributing to the flavour. Sourness is generally attributed to proton release from acids, whereas their different anions each impart a distinct taste (Johanningsmeiner et al., 2005). Acidity is also one of the main ripening indices that determines the harvest date of fruits used either for direct consumption or industrial processing (Neri, Pratella, & Brigati, 2003). In this article, organic acids are often referred to by the names of their anions, e.g. citrate and malate, and this is because at the pH of the cytoplasm the bulk of their content is in this form. The vast majority of the malate and citrate content of the flesh of fruits is located in the vacuole, and the pH of the vacuole determines the ratio of the undissociated:dissociated acid (Etienne, Génard, Lobit, Mbeguié-A-Mbéguié, & Bugaud, 2013).
Many fruits accumulate organic acids in their flesh at certain stages of their development. Usually either one or two acids account for the bulk of this content (Ulrich, 1971). Theorganic acid(s) that accumulate are dependent on the fruit in question (Ulrich, 1971). However, the most abundant organic acids in many fruits are citric and malic (Ulrich, 1971). A characteristic feature of this accumulation in many fruits is that the concentrations (mg∙g-1 fresh weight - FW) of these acids increase until the beginning of ripening and then decrease (Walker, Battistelli, Moscatello, Chen, Leegood, & Famiani, 2011a; Famiani, Baldicchi, Battistelli, Moscatello, & Walker, 2009; Famiani et al., 2012a; Famiani, Farinelli, Palliotti, Moscatello, Battistelli, & Walker, 2014a). However, the patterns of their changes during fruit development are often different when the total amount of these compounds per fruit is considered. In this case, in the flesh of several fruits, there is an increase in their content during most of fruit development and ripening (Walker et al., 2011a; Famiani et al., 2012a).
The bulk of the organic acids present in the flesh of fruits is not imported but rather synthesized in the flesh from imported sugars (Ruffner, 1982a, 1982b; Sweetman, Deluc, Cramer, Ford, & Soole, 2009; Etienne et al., 2013). If these acids are metabolized during ripening there are a number of fates. The main ones are: metabolism by the Krebs cycle (respiration), gluconeogenesis, fermentation to ethanol, amino acid synthesis/interconversion, and as a substrate for the synthesis of secondary metabolites such as pigments (Ruffner, 1982a, 1982b; Famiani, Walker, Tecsi, Chen, Proietti, & Leegood, 2000; Famiani et al., 2005; Famiani, Casulli, Proietti, Walker, & Battistelli, 2007; Famiani et al., 2014a; Famiani, Moscatello, Ferradini, Gardi, Battistelli, & Walker, 2014b; Sweetman et al., 2009; Etienne et al., 2013).
The organic acid content of the flesh of fruits is affected by environmental factors and cultivation practices (e.g. temperature, light intensity, cultivar, rootstock, mineral nutrition, water availability, fruit load/ pruning). However, how these factors alter metabolism to bring about changes in organic acid content is in most cases uncertain (Etienne et al., 2013).
This review focuses on malic and citric acids, which are the most abundant organic acids in many fruits. The aims of this review are two-fold. Firstly, to give a clear overview of the occurrence, metabolism and functions of these organic acids in fruits. Secondly, to outline how different environmental conditions and cultivation practices can alter the amount of these compounds in the fruits.
Changes in the organic acid content of fleshy fruits during their development and ripening
Organic acids accumulate in the flesh of many types of fruits at certain stages of their development (Hulme, 1971; Ruffner, 1982a, b; Famiani et al., 2005). The organic acids that are accumulated in fruits can be divided into several metabolic groups, and these are synthesized by different pathways. Firstly, the anions of citric, isocitric and malic acids, namely citrate, isocitrate and malate, are Krebs cycle intermediates (we refer to these in this review as Krebs cycle acids), and one or more of them (usually citric or malic) accounts for a large proportion of the organic acid content of the flesh of all fruits that have been studied (Ulrich, 1971). Nevertheless, other types of organic acids may also be abundant in certain fruits.
A second metabolic group is represented by ascorbic and tartaric acid. Tartaric acid is synthesized from ascorbic acid, and the latter is synthesized from imported sugars (Ruffner, 1982a; Ford, 2012). Grapes usually contain large amounts of tartaric acid in addition to malic acid (Ruffner, 1982a).
A third metabolic group includes quinic acid, which is synthesized by the shikimate pathway (Herrmann, 1995). Several fruits, such as apple, kiwifruit, papaya, pear, quince, stone fruits, and soft fruits, often contain substantial amounts of quinic acid (Arfaioli & Bosetto, 1993; Kalt & McDonald, 1996; Moing, Svanella, Gaudillere, Gaudillere, & Monet, 1999; González- Aguilar, Buta, & Wang, 2003; Wu et al., 2007; García-Mariño, de la Torre, & Matilla, 2008; Nishiyama, Fukuda, Shimohashi, & Oota, 2008; Ayaz, Kadioglu, Bertoft, Acar, & Turna, 2001; Bae, Yun, Jun, Yoon, Nam, & Kwon 2014; Szychowski, Munera-Picazo, Szumny, Carbonell-Barrachina, & Hernández, 2014). For a given type of fruit, which organic acids are accumulated in the flesh, and when this accumulation occurs during development, are often characteristic features. Table 1 summarizes the main organic acid(s) that are accumulated in large amounts during development and/or contained in the flesh of ripe fruits of different shrubby and woody species.
In the flesh of many fruits, such as grape, cherry and several soft fruits, the concentration of malate and/ or citrate (mg∙g-1 FW) increases until the beginning of ripening and then decreases (Ruffner, 1982a; Famiani et al., 2005, 2014a, 2014b; Walker et al., 2011a). The bulk of the volume of the flesh of most fruits consists of parenchyma cells, and in turn the bulk of the volume of these cells consists of a large vacuole. Most of the stored Krebs cycle acids are contained in these vacuoles (Ruffner, 1982a, 1982b; Etienne et al., 2013).
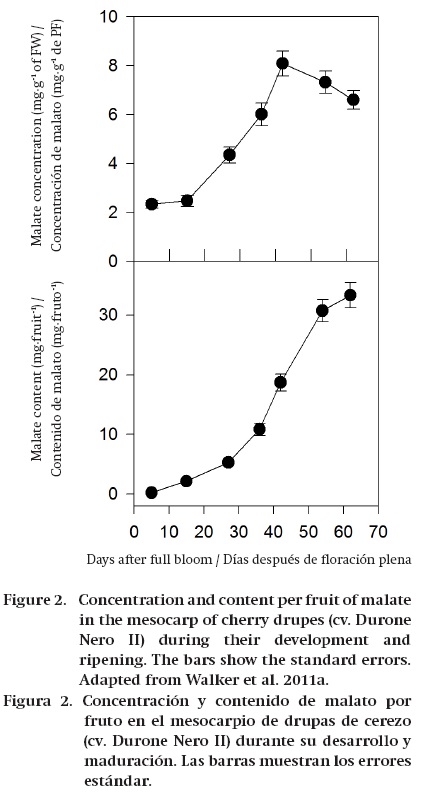
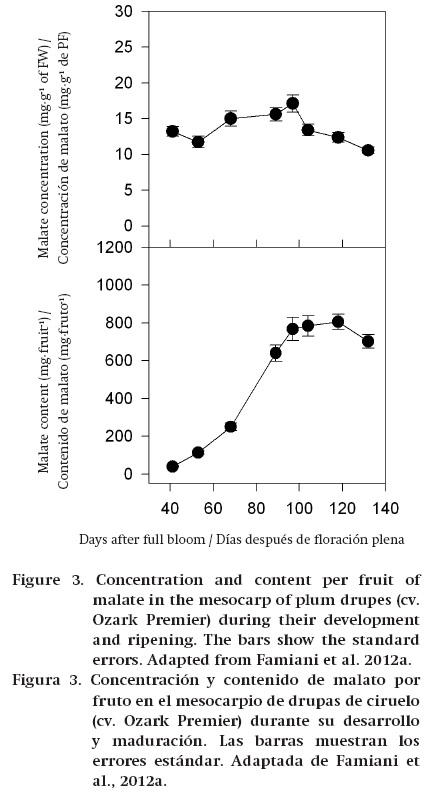
During the ripening of some fruits, such as grape, tomato and some soft fruits, the amount of malate/ citrate in terms of both concentration (mg∙g-1 FW) and content per fruit (mg∙fruit-1) decreases, and this shows that stored organic acids are dissimilated/metabolized (Ruffner, 1982b; Goodenough, Prosser, & Young, 1985; Famiani et al., 2005, 2009, 2014a, 2014b; Famiani & Walker, 2009; Sweetman et al., 2009) (Figure 1). In other fruits, such as mango, strawberry and some cherries, the concentration of citrate/malate (mg∙g-1 FW) decreases during ripening; however, the amount of these compounds per fruit (mg∙fruit-1) increases up to commercial harvest (Figure 2). Therefore, in these fruits there is no net dissimilation of malate/citrate, and the decrease in their concentration (mg∙g-1 FW) is only a dilution effect due to the increase in the size of the fruits, which is brought about largely by vacuolar expansion (Selvaraj & Kumar, 1989; Famiani et al., 2005; Walker et al., 2011a). Also, in kiwifruit no net dissimilation was observed up to harvesting (Walton & de Jong, 1990). In some plums the concentration (mg∙g-1 FW) of malate decreases during ripening. By contrast, the amount of malate per fruit (mg∙fruit-1) increases or remains constant for most of ripening, and decreases only at a very advanced stage of ripening (Famiani et al., 2012a) (Figure 3). Finally, in banana, feijoa and lemon both the organic acid concentration (mg∙g-1 FW) and content per fruit (mg∙fruit-1) increase throughout ripening (Bartholomew & Sinclair, 1951; Harman, 1987; Agravante, Matsui, & Kitagawa, 1991). Therefore, during ripening there can be either a net dissimilation or synthesis of stored Krebs cycle acids, and which occurs is dependent on which fruit is considered. However, a complicating factor is that superimposed on any net synthesis or dissimilation of malate/citrate that occurs over a period of days, there is a cycle of synthesis and dissimilation (Walker et al., 2011a and references therein). The function of this turnover is uncertain; however, it could be associated with the coordination of the import and utilisation of nitrogenous compounds and associated pH regulation (Walker et al., 2011a; Famiani et al., 2012a).
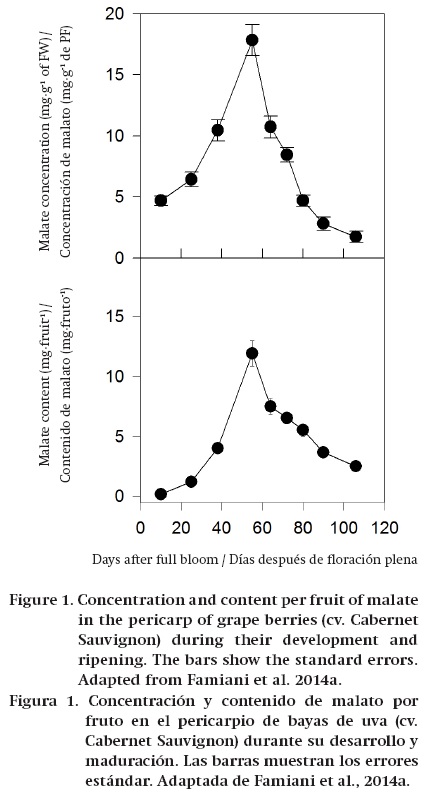
The studies outlined above have dealt with changes in organic acid content up to commercial ripeness. In many fruits that are over-ripe there is a decline in organic acid content (e.g. sour cherry, plum and grape, unpublished data). If fruits are stored, the storage conditions can affect the organic acid content. In apple after storage under normal air for 3-6 months the malic acid concentration is lower than at harvest (Ackermann, Fischer, & Amad, 1992; Róth et al., 2007). However, this decrease can be reduced if the atmosphere surrounding the apples is modified (Róth et al., 2007). Also, in kiwifruit a slight reduction of citric and malic acid contents was observed after about five months of storage under normal air (temperature = 0° ± 0.5 °C, relative humidity > 90 %) (unpublished data).
Metabolism of Krebs cycle acids in fleshy fruits
Synthesis. The Krebs cycle acids that accumulate within the flesh of fruits do not appear to be imported, but rather they are synthesized within the flesh from imported sugars (Ruffner, 1982b; Law & Plaxton, 1995). Sugars can also derive from photosynthesis within the fruit, however, these sugars account for only a very small proportion of the sugars present in the fruit (Kanellis & Roubelakis-Angelakis, 1993; Palliotti, Silvestroni, & Petoumenou, 2010).
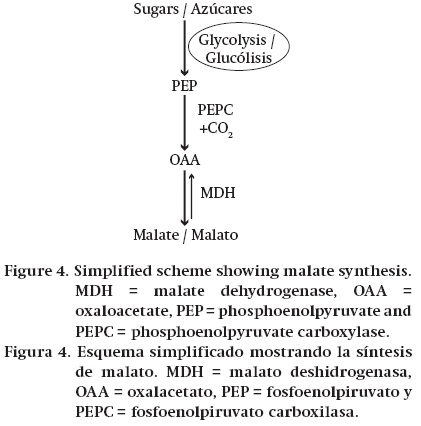
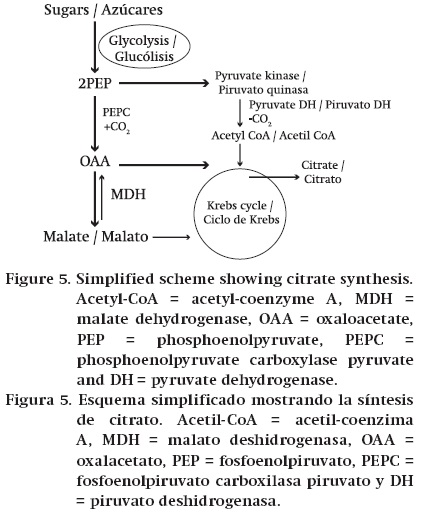
The bulk of Krebs cycle acids present in fruits is synthesized from sugars in the following way. Products from the metabolism of sugars enter the glycolytic pathway and this converts them to phosphoenolpyruvate (PEP). For example, imported sucrose is often hydrolysed to glucose and fructose by one of the invertases. These sugars are then phosphorylated by either glucokinase or fructokinase and enter glycolysis. In plants, the cytosolic enzyme phosphoenolpyruvate carboxylase (PEPC) is necessary for the synthesis of the Krebs cycle acids from sugars. It catalyses the conversion of PEP to oxaloacetate (OAA) (Ruffner, 1982b; Law & Plaxton, 1995). If the fruit is accumulating malate, OAA is converted to malate by cytosolic malate dehydrogenase (NAD-MDH), and malate is transported across the tonoplast into the vacuole in which it is stored (Ruffner, 1982b; Terrier & Romieu, 2001) (Figure 4). If citrate is being accumulated one molecule of PEP is converted to OAA by PEPC (Figure 5). A second PEP is converted to acetyl CoA by the sequential actions of pyruvate kinase and pyruvate dehydrogenase. Aceytl CoA and OAA are then combined by citrate synthase in the mitochondrion to give citrate. Citrate is then transported across the tonoplast into the vacuole in which it is stored. The amount of acids that are stored in the vacuole is the major determinant of the content of Krebs cycle acids in fruits. In the case of malate, it is thought that transport processes at the tonoplast are the key factor in determining the vacuolar content (Ruffner, 1982b; Terrier & Romieu, 2001; Etienne et al., 2013).
Utilization of organic acids. During the ripening of some fruits, such as grape, tomato and some soft fruits, the amount of malate/citrate in terms of both concentration (mg∙g-1 FW) and content per fruit (mg∙ fruit-1) decreases and this means that organic acids are metabolized (Ruffner, 1982b; Goodenough et al., 1985; Famiani et al., 2005, 2009, 2014a, 2014b; Famiani & Walker, 2009; Sweetman et al., 2009). There are a number of possible fates for this citrate/malate, and these are oxidation by the Krebs cycle (respiration), gluconeogenesis, amino acid synthesis/ transformations, synthesis of secondary products and/ or the production of ethanol by fermentation (Farineau & Laval-Martin, 1977; Ruffner, 1982b; Famiani et al., 2000, 2005, 2014a).
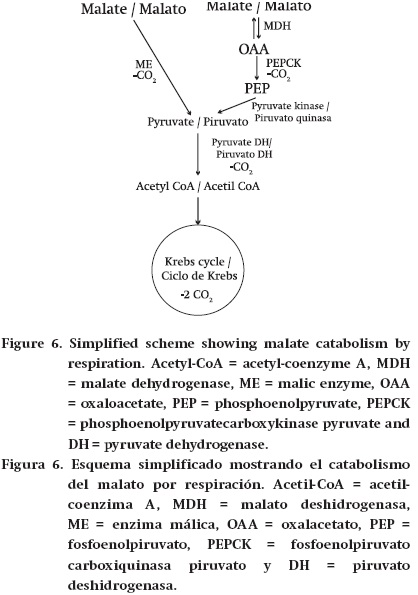
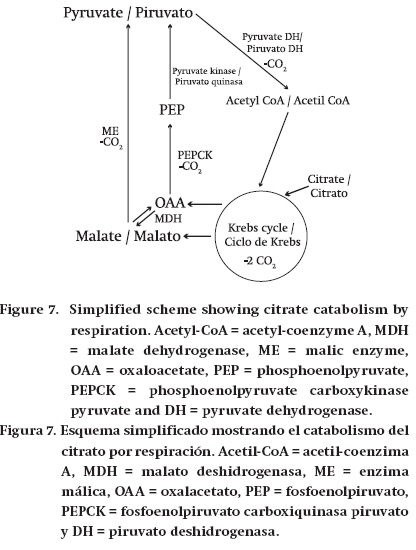
Respiration. The catabolism of malate and citrate through respiration implies the following reactions. Malate is transformed into pyruvate, by ME or through the combined action of MDH, PEPCK and pyruvate kinase (Figure 6). Pyruvate is then metabolized by pyruvate dehydrogenase before entering the Krebs cycle. In addition, both malate and citrate can enter the Krebs cycle directly (Figure 7). Then the Krebs cycle either partially or completely oxidizes these compounds to CO2. Cytosolic NADP-ME is important in the dissimilation of Krebs cycle acids in several fruits (Dilley, 1962; Goodenough et al., 1985; Chen et al., 2009; Sweetman et al., 2009). Moreover, citrate can be converted by cytosolic forms of the Krebs cycle enzymes aconitase and isocitrate dehydrogenase to 2-oxoglutarate, which can then enter the Krebs cycle. In addition, citrate can potentially be converted to OAA and acetyl CoA by cytosolic ATP-citrate lyase. However, there is uncertainty as to which of the above reactions is used in many fruits: these could be dependent on the fruit considered and the stage of development.
While all fruits carry out respiration there are marked differences in both the rates and patterns of changes in respiration between fruits of different plant species. All fruits show a high respiration rate per gram of FW at the beginning of their development and this then decreases (largely as a result of the increase in the size of the vacuole which reduces the amount of cytoplasm per gram of FW). However, some fruits show a sharp increase in respiration during ripening, and this is called the climacteric. Fruits can be divided into two types based on this: climacteric and non-climateric fruits. Climateric fruits include apple, apricot, avocado, chinene (Persea schiedeana Nees), kiwifruit, mamey sapote, pear, peach, plum, soursop, and tomato; non- climacteric fruits include cherry, strawberry, citrus, grape, and pineapple (Tucker, 1993; Kader, 2002; del Angel-Coronel, Cruz-Castillo, de la Cruz-Medina, & Famiani, 2010). Organic acids may be utilized by respiration during the climacteric. An increase in the abundance of NADP-ME together with a dissimilation of malate occurs in some fruits, such as tomato and loquat, during the climacteric (Hirai, 1982; Goodenough et al., 1985; Amorós, Zapata, Pretel, Botella, & Serrano, 2003). However, in the flesh of the non-climacteric fruit grape there is a net dissimilation of malate during ripening (Famiani et al., 2007, 2014a, 2014b), and, in at least one variety of the non-climacteric cherry there is no net dissimilation of stored organic acids in its flesh during ripening (Walker et al., 2011a). Therefore, there does not appear to be a correlation between the net dissimilation of Krebs cycle acids in the flesh of fruits during ripening and the occurrence of a respiratory climacteric.
For many years it was thought that the increase in the respiratory quotient (RQ = CO2 evolution/O2 consumption) during the ripening of grapes was a result of the use of organic acids as a respiratory substrate (Peynaud & Ribéreau-Gayon, 1971; Ruffner, 1982b; Kanellis & Roubelakis-Angelakis, 1993). However, recent studies have shown that this increase in RQ is largely brought about by other processes such as fermentation and the use of NADH in biosynthesis rather than oxidative phosphorylation (Famiani et al., 2014a; Famiani, Farinelli, Palliotti, Battistelli, Moscatello, & Walker, 2015).
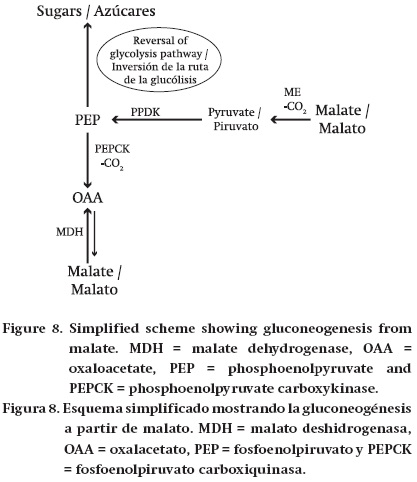
Gluconeogenesis. There is evidence for gluconeogenesis from malate in the flesh of grape, cherry and tomato during ripening (Ruffner, Koblet, & Rast, 1975; Ruffner & Kliewer, 1975; Farinea & Laval-Martin, 1977; Leegood & Walker, 1999; Osorio et al., 2013). The first steps in gluconeogenesis from malate involve its conversion to PEP. In plants, this conversion requires either MDH and PEPCK or ME together with pyruvate, orthophosphate dikinase (PPDK) (Walker & Chen, 2002; Leegood & Walker, 2003). In the case of PEPCK malate is converted to OAA by malate dehydrogenase, and OAA is then converted to PEP by PEPCK (Figure 8). In the case of malic enzyme and PPDK, malate is converted to pyruvate by ME and pyruvate is converted to PEP by PPDK (Figure 8). In both cases the PEP so produced is then converted to sugars by a reversal of the reactions of glycolysis (Plaxton, 1996). In the flesh of grape, plum, cherry and soft fruits, PPDK appears to be either not present or at very low abundance (Famiani et al., 2005, 2009, 2014b; Famiani & Walker, 2009). This suggests that the majority of any gluconeogenic flux in the flesh of these fruits utilizes the PEPCK pathway. PPDK was detected in the fruit of cactus pear (Opuntia ficus-indica) (Walker, Famiani, Baldicchi, Cruz-Castillo, & Inglese, 2011b), but this plant is a Crassulacean acid metabolism (CAM) plant and PPDK functions in its photosynthesis. Although the contribution of malate and citrate to the amount of stored sugars in ripe fruits can potentially be up to 20 % (Famiani et al., 2009), in reality it will be much lower. This is because the vast majority of the dissimilated malate and citrate is used by catabolic processes such as the Krebs cycle (Ruffner et al., 1975; Famiani et al., 2015). In grape this contribution is likely to be less than 1 % (Famiani et al., 2015).
For many years it was thought that glycolysis was inhibited in ripening grape and that malate provided the bulk of the substrate used by metabolism. Gluconeogenesis was thought to occur because there was an excess of malate (Ruffner, 1982b; Sweetman et al., 2009). However, recent work has shown that this is untrue and that sugars provide the bulk of the substrate used by metabolism (Famiani et al., 2014a, 2015). One explanation for the occurrence of gluconeogenesis is that malate flux from the vacuole is not constant during ripening, and there are times when there is an enhanced efflux of malate which results in an excess of malate in the cytosol (Famiani et al., 2015).
Amino acid and secondary product synthesis. Protein synthesis occurs in the flesh of fruits during ripening, as does the conversion of either stored or newly imported amino acids into those amino acids that accumulate within the ripe fruit (Kanellis & Roubelakis-Angelakis, 1993). Moreover, secondary products, such as pigments that give the fruit its color, are synthesized during ripening. Stored Krebs cycle acids may provide precursors for such amino acids and secondary product synthesis in the flesh of fruits in which Krebs cycle acids are dissimilated during ripening (Famiani et al., 2005, 2009, 2014a, 2014b; Pereira et al., 2006; Famiani & Walker, 2009). Amino acids can be divided into families on the basis of their biosynthetic pathway, with each family having a different precursor. Aspartate, glutamate, PEP and pyruvate are the precursors for the synthesis of many amino acids (Lea, 1993). The carbon skeletons of glutamate and aspartate are the organic acids 2-oxoglutarate and OAA respectively, which are intermediates of the Krebs cycle. OAA can also be obtained from malate through the action of cytosolic MDH. PEP can be synthesized from malate through the action of MDH and PEPCK or ME and PPDK. Pyruvate can be obtained through the combined action of MDH, PEPCK and pyruvate kinase or through the action of ME. Hence, the metabolism of many organic and amino acids is closely linked. PEP is also a substrate for the synthesis of secondary products (e.g. phenolic compounds and pigments). Acetyl CoA is used as a precursor for the synthesis of flavonoids and isoprenoids, and acetyl CoA can derive from either pyruvate by the action of pyruvate dehydrogenase, or citrate by the action of ATP isocitrate lyase (Etienne et al., 2013).
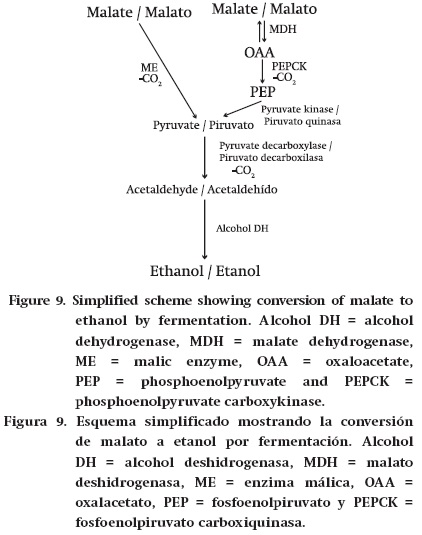
Fermentation. Krebs cycle acids can also be converted to ethanol. There is evidence for the occurrence of aerobic fermentation to ethanol in the grape pericarp, and this may be of common occurrence in the vineyard (Romieu, Tesniere, Thanham, Flanzy, & Robin, 1992; Tesnière, Romieu, Dugelay, Nicol, Flanzy, & Robin, 1994; Terrier & Romieu, 2001). Similarly, in the juice sacs of citrus during ripening there can be an accumulation of ethanol and decreased aerobic respiration, and this also occurs during the storage of harvested fruits (Roe, Davis, & Bruemmer, 1984; Baldwin, 1993). Krebs cycle acids are converted into ethanol as follows (Figure 9). Citrate is converted to malate by reactions catalysed by Krebs cycle enzymes (or in some cases their cytosolic form). Malate is converted into pyruvate by either ME or the combined actions of MDH, PEPCK and pyruvate kinase. This pyruvate is then converted to ethanol by the sequential actions of pyruvate decarboxylase and alcohol dehydrogenase.
Glyoxylate cycle. This effectively enables acetyl- CoA to be converted into malate. This cycle has been suggested to function in malate synthesis in young grape and banana fruits and also to provide substrates for gluconeogenesis during post-harvest ripening of banana (Surendranathan & Nair, 1976; Pua, Chandramouli, Han, & Liu, 2003; Liu, Yang, Murayama, Taira, & Fukushima, 2004; Terrier et al., 2005). However, the enzyme isocitrate lyase (ICL), which is necessary for the cycle to work, was not detected in either the pericarp of several soft fruits or grape berries at several stages of development (Famiani et al., 2005; 2014b). Hence, our view is that the occurrence of ICL (potentially as a component of the glyoxylate cycle) in many fruit at certain stages of development or under certain conditions remains uncertain and further investigations are required. However, these studies should determine the abundance of the enzyme and not just its transcript.
Vacuolar storage of organic acids
In grape and other fruits, a widespread view is that malate content is largely determined by its compartmentation in the vacuole. That is, most of the malate is located in the vacuole where it is inaccessible to the enzymes that metabolize it. The main factor that is thought to determine this compartmentation is transport of malate across the membrane that separates the vacuole from the cytosol (i.e. the tonoplast) (Ruffner, 1982b; Etienne et al., 2013). Embedded in the tonoplast there are a number of proteins involved in malate/ citrate transport. Those involved in malate transport likely include several different malate transporters, and in addition there are malate channels. One transporter is AttDT, which appears to be regulated by cytosolic pH. That is, if the pH of the cytosol falls, an increase in malate transport to the cytosol occurs (Hu et al., 2005). Less is known about citrate transport processes (Hurth et al., 2005; Etienne et al., 2013). However, for both malate and citrate, the contributions of the different proteins involved in their transport across the tonoplast are poorly understood. In the case of citrate it is thought that a combination of metabolism and transport processes at the tonoplast may control its vacuolar content (Etienne et al., 2013); however, this is not certain. Neither is it certain that in all fruits transport processes at the tonoplast are the predominant factor in determining vacuolar malate content.
Functions of Krebs cycle acids in fleshy fruits
In all fruits, as in all plant tissues, Krebs cycle acid anions are intermediates of many metabolic pathways. However, in many fruits large amounts of Krebs cycle acids accumulate in the vacuole, and these stored acids have other functions. First, in many fruits they are likely to contribute to making the fruit unpalatable until its seed(s) have developed. When the seed(s) are approaching maturity the fruit starts to ripen and its concentration of Krebs cycle acids (mg∙g-1 FW) decreases. If there is a net dissimilation of these acids during ripening they can be used as substrates for metabolism (see above). Second, recent studies have suggested that a key function of the Krebs cycle acids in fruits may be in the coordination of the import and utilisation of nitrogenous compounds and associated pH regulation (Walker et al., 2011a; Famiani et al., 2012a).
Effects of both environmental conditions and agronomical practices on the content of Krebs cycle acids in fruits
Temperature. The temperature at which fruits are grown affects both their titratable acidity and content of stored organic acids. For both grape and kiwifruit higher temperatures before ripening increase their malate content (Klenert, Rapp, & Alleweldt, 1978; Richardson et al., 2004), whereas higher temperatures during ripening decrease their content (Kliewer, 1970; Buttrose, Hale, & Kliewer, 1971; Hale & Buttrose, 1974; Ruffner, Hawker, & Hale, 1976; Lakso & Kliewer, 1978; Alleweldt, During, & Jung, 1984; Richardson et al., 2004; Lobit, Génard, Soing, & Habib, 2006). Similarly, elevated temperatures during ripening decrease titratable acidity (Kliewer, 1973; Wang & Camp, 2000; Gautier, Rocci, Buret, Grassely, & Causse, 2005; Trad et al., 2013). Furthermore, at least in grape before ripening, the temperature difference between the day and the night has an effect: cool nights and warm days increase organic acid content (especially malic) more than warm days and warm nights (Kliewer, 1973). Recently it was shown that in grape an increase in maximum temperature (4 - 10 °C above controls) before ripening resulted in a higher malate content (this effect was enhanced by warmer nights); during veraison and ripening the effect was to reduce malate content. This was unless the minimum temperatures (during the night) were also raised by 4 - 6 °C, because in this case malate content was not reduced, suggesting that the regulation of malate metabolism differs during day and night (Sweetman, Sadras, Hancock, Soole, & Ford, 2014). Increased temperature of fruits during ripening almost certainly decreases their content of Krebs cycle acids by increasing the rate of many metabolic processes. In fruits, as in all plant tissues, an increase in temperature increases the rate of their metabolism, and hence the consumption of compounds such as sugars and stored Krebs cycle acids that serve as metabolic substrates.
Temperature affects the flux through diverse pathways, for example, respiration and secondary metabolite synthesis (Ruffner, 1982b; Pereira et al., 2006). Moreover, it has been proposed that an increase in temperature could alter transport of malate/citrate at the tonoplast resulting in a decrease in their vacuolar content, and as a result more malate/citrate would be available in the cytosol for use in metabolism (Etienne et al., 2013).
An increase in the rate of respiration of fruits as temperature increases has been shown in many studies (Hansen, 1942; Ruffner et al., 1976; Ruffner, 1982b). The results of Taureilles-Saurel, Romieu, Robin, and Flanzy (1995a, 1995b) support an increase in flux through the Krebs cycle in response to higher temperatures. Transcript levels of several mitochondrial dicarboxylate/ tricarboxylate transporters increased with warming (Rienth et al., 2014), suggesting/supporting an increased import of malate into the mitochondria for respiration (Sweetman et al., 2014).
Temperature affects the metabolic fate of dissimilated malate in ripening grape berries. At higher temperatures, the ratio of the amount of malate utilized by respiration to that used by gluconeogenesis is greater than at lower temperatures; however, at all temperatures the proportion of malate utilized by gluconeogenesis is small (Ruffner, 1982b; Famiani et al., 2015). At lower temperatures there is a lower rate of respiration and hence more malate is likely used in gluconeogenesis. Furthermore, temperature also increases the amount of ethanol produced by fermentation(Terrier& Romieu, 2001). Theexplanation for this is likely that an increase in temperature increases the rate of metabolic processes, and an increase in respiration provides ATP and NAD(P)H for these. However, a high respiratory activity at elevated temperatures consumes a large amount of O2, and ethanol fermentation that produces ATP without consuming O2 increases. It has been concluded that ethanol production is likely to be very common in grapes grown in the vineyard, as bunches can be subjected to temperatures as high as 50 °C (Romieu et al., 1992). Indeed, the direct exposure of fruit to light can increase their temperature well above air temperature (Smart & Sinclair, 1976).
Light. Several environmental (latitude and orientation of the field if it is on a hill) and cultural factors (training system and pruning) affect light availability for both fruits and leaves. However, the effect of light on fruit composition is difficult to interpret. One reason is that solar irradiance also increases fruit temperature (Smart & Sinclair, 1976), and hence there is an interaction between light and temperature. A second reason is that shade can delay ripening, and this can cause a higher content of organic acids at harvest (Jackson & Lombard, 1993; Tombesi, Antognozzi, & Palliotti, 1993).
In grape, de Bolt, Ristic, Iland, and Ford (2008) varied light intensity at constant fruit temperature. A much lower amount of malate accumulated during stage I of berry development and a lower decrease in malate content occurred during stage III (ripening) in bunches enclosed in boxes compared to those fully exposed to light. The final content of malate was higher in the berries enclosed by boxes. The final soluble solids content (which is an important parameter in defining the ripening stage) was similar in the two treatments. In addition, Hummel and Ferree (1998) showed that in grape increased illumination (compared to a control) decreased total titratable acidity (TTA) and malate content, and this was observed when grapes were harvested at a similar soluble solids concentration or at the same date. Yang, Zhu, Bu, Hu, Wang, and Huang (2009) studied the effects of bagging on development and quality of longan, and they found that the amount of light transmittance affected malic acid content. Photoperiod can also affect fruit composition (including organic acid content) in some fruits such as raspberry (Mazur et al., 2014). Overall these studies suggest that light can alter the organic acid content of fruits directly, that is by a means that is not dependent on increasing fruit temperature.
Cultivar. The different cultivars of one species can have very different organic acids contents (in terms of either the type of acid present or its content) (Kliewer, Howarth, & Omori, 1967; Arfaioli & Bosetto, 1993; Moing et al., 1998; Gurrieri et al., 2001; Albertini, Carcouet, Pailly, Gambotti, Luro, & Berti, 2006; Saradhuldhat & Paull, 2007; Wu et al., 2007; Muñoz-Robredo, Robledo, Manríquez, Molina, & Defilippi, 2011). For example, for some fruits, such as grape, citrus and pineapple, there are either high or low acidity cultivars (Diakou et al., 1997; Sadka, Dahan, Cohen, & Marsh, 2000; Sadka, Dahan, Or, Roose, Marsh, & Cohen, 2001; Saradhuldhat & Paull, 2007). Hence, cultivar can be one of the main factors affecting organic acid content. However, the differences in metabolism between these cultivars that are responsible for these different organic acid contents are not known with any certainty. It should be noted that cultivars differ in their vegetative characteristics (i.e. vigor and foliage density), and that this too could indirectly affect the organic acid content of the fruit.
There have been many studies that have tried to link organic acid contents to metabolism in the cytoplasm. In peach the activities of PEPC, NAD-MDH and NADP- ME could not explain the difference in malate and citrate content between low- and high-acid cultivars (Moing et al., 1998, 2000). Similarly, PEPC cannot account for differences in malate content of low- and high-acid cultivars in apple and loquat (Chen et al., 2009; Yao et al., 2009). In both plum and cherry, there was no correlation between the abundance of PEPCK and malate/citrate dissimilation (Walker et al., 2011a; Famiani et al., 2012a). The abundance of the enzyme citrate synthase does not appear to be responsible for the differences in the citrate content of fruits of low- and high-acid varieties of several fruits (e.g. citrus, melon, peach and pineapple) (Canel, Bailey-Serres, & Roose, 1996; Sadka et al., 2001; Etienne, Moing, Dirlewanger, Raymond, Monet, & Rothan, 2002; Saradhuldhat & Paull, 2007; Tang, Bie, Wu, Yi, & Feng, 2010). On the other hand, it has been suggested that NADP-ME may play a role in determining the low malate content of low acid cultivars of apple and loquat (Chen et al., 2009; Yao et al., 2009). A comparison of low- and high-acid peach varieties showed that there was a difference in the expression of vacuolar proton pumps and it is possible that this could be related to the ability of the vacuole to store malate and citrate (Etienne et al., 2002).In tomato, altered organic acids levels were found in lines which contained sequences for i) PEPCK, ii) PEPC, numerous membrane transporters and a fructokinase-like protein, or iii) an alcohol dehydrogenase and an NADP-linked malic enzyme (Eshed & Zamir, 1994; Causse et al., 2004), indicating that it is likely that some of these proteins are involved in altering the Krebs cycle acid content of fruits. In citrus and pineapple, studies in which low- and high-acid genotypes were compared led to the suggestion that a reduction of aconitase activity in high acidity genotypes played a role in determining citric acid content (Bogin & Wallace, 1966; Sadka et al., 2000; Saradhuldhat & Paull, 2007).
Taken together the above studies suggest that there is not just one mechanism, but different ones that may use a range of metabolic pathways that determine cultivar- dependent differences in fruit organic acid content.
Rootstock. Fruit of 'Rangpur' (Citrus limonia Osb.) and sweet lemon (Citrus limettioides Tan.), high-acid and low- acid species, respectively, were reciprocally grafted. The fruit content of organic acids was unchanged, and in this situation the rootstock had very little effect (Castle, 1995). However, there are studies which show that rootstock can affect the organic acid contents of fruits, such as cherry, citrus, grape, kiwifruit, peach, etc. (Caruso, Giovannini, & Liverani, 1996; Boyes, Strübi, & Marsh, 1997; Cantín, Pinochet, Gogorcena, & Moreno, 2010; Gong, Blackmore, & Walker, 2010; Usenik, Fajt, Mikulic-Petkovsek, Slatnar, Stampar, & Veberic, 2010; Orazem, Stampar, & Hudina, 2011a, 2011b; Legua et al., 2014). In addition to a possible direct influence on the organic acid content of the fruit, the rootstocks may have indirect effects, that is by altering, for example, vegetative vigor, density of the canopy (hence amount of light reaching the fruit), source/sink ratio, water and mineral salt uptake, and these are known to affect the organic acid content of fruits.
Mineral nutrition. In the leaves of many plants nitrogen metabolism is the major factor affecting the content of Krebs cycle acids and large amounts of these can accumulate (Smith & Raven, 1979). This is because the assimilation of nitrate or ammonium into amino acids is not a proton neutral process. Malate metabolism is associated with the pH regulation necessary for this nitrogen assimilation. For example, the assimilation of nitrate into amino acids in leaves consumes protons. Malic acid is synthesized in the leaf and malate is transported to the root. This leaves protons in the leaf to restore the pH (Raven and Smith, 1976). In contrast to leaves, fruits import amides such as glutamine and asparagine as their main source of nitrogen, and the assimilation of these into compounds such as amino acids is proton neutral. Hence, there is no requirement for the synthesis of large amounts of malate in response to their assimilation (Walker & Chen, 2002).
Most studies of the effect of mineral nutrition on fruit quality have dealt with nitrogen and potassium, and for both their effects vary in different studies. Nitrogen supply affects plant vigor and the leaf/fruit ratio, which can then affect both fruit ripening and humidity and sunlight penetration inside the canopy. Therefore, it is often difficult to determine whether the effect of nitrogen is direct or indirect.
In grape, a low nitrogen status of the plant reduces vine vigor, and berries from such plants have both a high soluble solids content and pH together with a low malic acid content (Reynard, Zufferey, Nicol, & Murisier, 2011). In apricot a relatively low N supply (80 kg·ha-1, as opposed to a higher N supply of 150 kg·ha-1) resulted in fruits with a slightly higher sugar concentration and lower acid concentration (Radi, Mahrouz, Jaouad, & Amiot, 2003). In peach and tomato a higher nitrogen supply resulted in higher values of titratable acidity and an increase in organic acid contents (Davies & Winsor, 1967; Davies, 1964; Jia et al., 1999). In orange a higher nitrogen supply resulted in a higher titratable acidity (Reitz & Koo, 1960). On the other hand, nitrogen supply caused a reduction in titratable acidity in pineapple (Spironello, Quaggio, Teixeira, Furlani, & Sigrist, 2004), and had no significant effect in peach and kiwifruit (Cummings & Reeves, 1971; Pacheco et al., 2008). In addition, the form in which nitrogen is supplied affects the organic acid content of fruits. Hydroponically-cultivated tomato plants fed with NH4+ had lower citrate and malate contents in ripe fruits than plants fed with NO-3 or NO-3 + NH4+ (Xin-Juan, Qing-Yu, Xiao-Hui, Shen, & Dong, 2012). Nevertheless, the differences in malate/citrate content observed in these fruits were much less than the differences in content in leaves found in comparable studies (Walker & Chen, 2002). However, in this study (Xin-Juan et al., 2012) no significant correlations were found between the different organic acid contents in the fruits of plants grown using different forms of nitrogen and the content of the enzymes studied (PEPC, NAD- MDH and CS), suggesting that other factors, such as compartmentation, might be involved in determining differences in organic acid content (Gutierréz-Granda & Morrison, 1992).
In several studies, an increased potassium supply to the plant has been found to increase the titratable acidity of its fruits (Reitz & Koo, 1960; Embleton, Jones, Pallares, & Platt, 1978; du Preez, 1985; Ruhl, 1989; Spironello et al., 2004; Alva, Mattos, Paramasivam, Patil, Dou, & Sajwan, 2006). However, in other cases an increased potassium supply led to either a reduction of fruit titratable acidity (Vadivel & Shanmugavelu, 1978; Ramesh-Kumar & Kumar, 2007) or had no effect on titratable acidity and organic acid contents (Cummings & Reeves, 1971; Pacheco et al., 2008; Etienne, Génard, Bancel, Benoit, Lemire, & Bugaud, 2014). In grape, musts which contain high amounts of potassium also tend to have high pH and malate content (Ruhl, 1989; Jackson & Lombard, 1993; Mpelasoka, Schachtman, Treeby, & Thomas, 2003). Failla, Scienza, and Brancadoro (1996) found a relationship between potassium and malic acid at veraison which, however, was not evident in the ripe berry. The positive correlation between malate and potassium contents is also demonstrated by the positive correlation between malate content and ash alkalinity (that is strongly related to potassium content) found in ripe fruits (Genevois & Peynaud, 1947a, 1947b; Souty, Perret, & Andre, 1967; Morrison & Noble, 1990). It has been suggested that a possible explanation for the relationship between potassium and organic acid contents in some fruits is that potassium may have a role in balancing the charge present on organic acid anions in the vacuole (Lang, 1983; Lobit, Genard, Wu, Soing, & Habib, 2003).
Water availability. Excess water supply, resulting from either excessive rainfall or irrigation, can affect fruit composition. In over-watered grapevines it appears that a higher organic acid content of the fruit is brought about by both a delay in the ripening of the fruit and excessive growth of the leaves that shade the berries (Smart & Coombe, 1983; Jackson & Lombard, 1993). In grape, especially if grown in dry climatic regions, irrigation can increase titratable acidity and organic acid content (Esteban, Villanueva, & Lissargue, 1999; des Gachons, Leeuwen, Tominaga, Soyer, Gaudillere, & Dubourdieu, 2004; de la Hera-Orts, Martinez-Cutillas, Lopez-Roca, & Gomez-Plaza, 2005).
When fruits of two cultivars of nectarines from well- watered and under-watered trees were compared, it was found that fruits from under-watered plants had a lower amount of both malic and total acids per fruit (Thakur & Singh, 2011). However, in most other studies an increased supply of water decreased the titratable acidity and organic acid content of ripe fruits. In different citrus species (orange, clementine, satsuma mandarin) cultivated using different amounts of water supply, it was found that a lower availability of water resulted in fruits with a higher titratable acidity (Yakushiji, Morinaga, & Nonami, 1998; González-Altozano & Castel, 1999; Brandon, Hockema, & Etxeberria, 2001; Kallsen, Sanden, & Arpaia, 2011). Apple and tomato fruits from well-watered plants had a lower titratable acidity (Mills, Behboudian, & Clothier, 1996; Veit-Köhler, Krumbein, & Kosegarten 1999). In strawberry, fruits from well-watered plants and under-watered plants showed differences in organic acid content, both when expressed on a fresh and dry weight basis; however, these effects were not uniform but cultivar dependent (Bordonaba & Terry, 2010). In peach, irrigation did not result in significant differences in the contents of malic and citric acids, and did not affect the seasonal pattern of changes in the content of these acids (Wu, Genard, Lescourret, Gomez, & Li, 2002).
Hence, the effect of water availability on the organic acid content of fruits is complex, and this is because of the interactions with other factors, such as climatic conditions, cultivar and the time during fruit development when water stress occurs. In addition, water supply can increase vegetative growth, which can have an effect, and increase yield (as result of larger berries) and slows/delays ripening (Jackson & Lombard, 1993). The situation is further complicated because water availability can affect the volume of the fruits and this causes complications when expressing data from experiments. For example, a fruit whose volume has increased as a result of additional water import will have a lower organic acid content per FW but its content per DW will be unaltered.
The use of saline water for the irrigation of tomato resulted in a reduction in fruit growth and yield; however, the fruit had increased contents of organic acids, sugars and soluble solids. In addition, there was an increase in the transcription of a PEPCK gene (Incerti, Navari-Izzo, Pardossi, Mensual, & Izzo, 2007; Saito et al., 2008).
Fruit load (source/sink ratio). A different fruit load is the result of fruit-set, which can be affected by both environmental and cultivation practices. Fruit thinning and pruning (including defoliation) are important tools to regulate fruit load and the source/sink ratio. An increase in fruit load modifies fruit composition directly by reducing the amount of sugars and other nutrients imported into a fruit in a given period of time. It also changes fruit composition indirectly by slowing fruit development and ripening (Jackson & Lombard, 1993; Poiroux-Gonord, Fanciullino, Bert, & Urban, 2012). In grape, both a higher juice pH and soluble solids content together with a lower content of organic acids are often found in grape berries from plants with a low fruit load (Jackson & Lombard, 1993; Palliotti & Poni, 2011). However, the results of such studies are dependent on when the fruit is sampled. That is whether they are harvested at the same date or when they have a similar soluble solids content (similar ripening stage). When berries were harvested at a similar soluble solids concentration, it was found that an increase in fruit load delayed the time at which berries were suitable for harvesting, and in these berries titratable acidity was reduced, as were tartaric and malic acid contents (Hummel & Ferree, 1998). These results suggest that an increase in crop load decreases the amount of nutrients imported into a given berry, and this then reduces the amount of organic acids synthesized in each berry. Similarly, in kiwifruit yield is negatively correlated with total titratable acidity at harvest (Famiani, Casulli, Baldicchi, Battistelli, Moscatello, & Walker, 2012b). In peach and mango a different behaviour of citric and malic acids was observed.
At the beginning of growth, fruits with high leaf/ fruit ratio had lower malate and higher citrate concentrations, whereas near maturity, greater assimilate supply was related to high malate and low citrate concentrations Souty, Génard, Reich, & Albagnac, 1999; Wu et al., 2002; Léchaudel, Joas, Caro, Génard, & Jannoyer, 2005). In these studies the comparisons were done on samples collected on the same date and not at similar stages of development and ripening; the pattern of changes in organic acid contents indicate a slower development and late ripening of fruits growing on shoots with a low leaf/ fruit ratios. However, it appears that fruits from plants with a higher leaf/fruit ratio often have a higher organic acid content, and this may be a result of an increased import of photoassimilates. In banana, fruit load had no effects on the accumulation of organic acids in the fruits (Etienne et al., 2014).
Source-sink relationships can also be altered by applying compounds that increase the sink strength of the fruit. For example, the application of growth regulators which promote fruit growth (such as the cytokinin-like compounds CPPU and Thidiazuron) alter the acidity of kiwifruits at harvest (Famiani, Battistelli, Moscatello, Boco, & Antognozzi, 1999). However, this change in acidity appears to be a result of earlier ripening, and the effects of these plant growth regulators are dependent on both the time of their application and the concentration that is used (Cruz-Castillo et al., 2014).
CONCLUSIONS
The aims of this review were to give a clear overview of the occurrence, metabolism and functions of organic acids in fruits, together with an outline of how different environmental conditions and cultivation practices can alter their contents. The following points can be emphasized.
The observation that organic acid concentration (mg∙g-1 FW) decreases during ripening can be misleading. This is because in many fruits this decrease is not brought about by the metabolism of the organic acid but by an increase in the weight of the fruit caused by its expansion. If there is an actual dissimilation of malate/ citrate during ripening, their metabolic fate can be respiration, gluconeogenesis, amino acid synthesis/ transformations, synthesis of secondary products and/or the production of ethanol by fermentation. If gluconeogenesis occurs, then it appears that in most fruits the PEPCK and not the PPDK pathway is utilized.
Although much progress has been made in understanding the metabolism of malate and citrate metabolism in fruits, many questions remain to be answered. The reason why gluconeogenesis occurs in fruits is one such question. To further understand this requires a combination of biochemical and molecular approaches. For example, metabolic studies in tomato plants with reduced amounts of PEPCK in their fruits have provided useful information on the pathways of gluconeogenesis in tomato fruit (Osorio et al., 2013). In fruits both PEPCK and PEPC are present in the cytosol of the same cells (Famiani et al., 2005). PEPC functions in the conversion of PEP to malate and PEPCK functions in the reverse reaction. To understand how the activity of these enzymes are coordinated will require biochemical approaches as was used to study the regulation of these enzymes in CAM plants (Walker & Chen, 2002). Another example of an important gap in our knowledge of malate metabolism in fruits is how malate transport across the tonoplast is regulated. Molecular approaches have identified homologues of malate transporters in fruits (Etienne et al., 2013). However, to understand how the activity of these transporters is regulated will require biochemical studies using, for example, assays of their activity in systems in which the transporters are reconstituted into lipid vesicles. Such studies could investigate the effects of metabolite effectors and phosphorylation of the transporter. There have been no such studies in fruits.
In several cases how environmental and agronomical factors bring about changes in the organic acid content of fruits is not simple. This may be due in part to the fact that these factors can often change conditions other than the one which is being studied (e.g. an increase in light exposure also increases temperature; a high fruit load can delay ripening). However, the effects of temperature are understood in a number of situations. The light likely has a direct effect on organic acids. For a given type of fruit the cultivar has a large and reproducible effect on organic acid content. However, in different fruits it appears that different mechanisms (e.g. metabolic pathways or transport processes at the tonoplast) are responsible for these differences. Further studies, using a combination of physiological, biochemical and molecular approaches, are necessary to further understand the mechanisms responsible for these differences. An understanding of these mechanisms would be of assistance in both genetic improvement programs and in the optimization of cultural practices. For many species/genotypes, an increased fruit load decreases the organic acid content of the fruits. The direct effects of cultural practices such as irrigation and the application of fertilizers are complex. Often their effects are variable, and this is because there are interactions with other environmental and cultural factors. Therefore, detailed studies are required to define the effects of a given cultural practice under different environmental and cultural conditions.
ACKNOWLEDGEMENTS
The work was partially funded by the "Fondazione Cassa di Risparmio di Perugia e Codice Progetto 2010.011.0470".
REFERENCES
Ackermann, J., Fischer, M., & Amad, R. (1992). Changes in sugars, acids, and amino acids during ripening and storage of apples (cv. Glockenapfel). Journal of Agricultural and Food Chemistry, 40(7), 1131-1134. doi: 10.1021/jf00019a008 [ Links ]
Adamczak, A., Buchwald, W., & Kozłowski, J. (2011). Variation in the content of flavonols and main organic acids in the fruit of European cranberry (Oxycoccus palustris Pers.) growing in peatlands of North-Western Poland. Herba Polonica, 57(4), 5-15. [ Links ]
Agravante, J. U., Matsui, T., & Kitagawa, H. (1991). Sugars and organic acids in ethanol-treated and ethylene-treated banana fruits. Japanese Society for Food Science and Technology, 38, 441-444. doi: 10.3136/nskkk1962.38.441 [ Links ]
Albertini, M. V., Carcouet, E., Pailly, O., Gambotti, C., Luro, F., & Berti, L. (2006). Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. Journal of Agricultural and Food Chemistry, 54(21), 8335-8339. doi: 10.1021/jf061648j [ Links ]
Alique, R., & Zamorano, J. P. (2000). Influence of harvest date within the season and cold storage on cherimoya fruit ripening. Journal of Agricultural and Food Chemistry, 48(9), 4209-4216. doi: 10.1021/jf9913561 [ Links ]
Alleweldt, G., During, H., & Jung, K. H. (1984). Zum einfluss des klimas auf beerentwicklung, ertrag, und qualitt bei reben: ergebnisse einer siebenjahringen factorenanalyse. Vitis, 23, 27-142. Recuperado de http://www.vitis-vea.de/admin/volltext/e022738.pdf [ Links ]
Alva, A. K., Mattos, D. Jr., Paramasivam, S., Patil, B., Dou, H., & Sajwan, K. S. (2006). Potassium management for optimizing citrus production and quality. International Journal of Fruit Science, 6(1), 3-43. doi: 10.1300/J492v06n01_02 [ Links ]
Amorós, A., Zapata, P., Pretel, M. T., Botella, M. A., & Serrano, M. (2003). Physico-chemical and physiological changes during fruit development and ripening of five Loquat (Eriobotrya Japonica Lindl.). Food Science & Technology, 9(1), 43-51. doi: 10.1177/1082013203009001007 [ Links ]
Arenas-Ocampo, M. L., Evangelista-Lozano, S., Errasquin- Arana, R., Jiménez-Aparicio, A., & Dávila-Ortíz, G. (2007). Softening and biochemical changes of sapote mamey fruit Pouteria sapota at different development and ripening stages. Journal of Food Biochemistry, 27(2): 91-107. doi: 10.1111/j.1745-4514.2003.tb00269.x [ Links ]
Arfaioli, P., & Bosetto, M. (1993). Time changes of free organic acid contents in seven Italian pear (Pyrus communis) varieties with different ripening times. Agricoltura Mediterranea, 123(3), 224-230. [ Links ]
Ayaz, F. A., Kadioglu, A., Bertoft, E., Acar, C., & Turna, I. (2001). Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos and V. myrtillus) native to Turkey. New Zealand Journal of Crop and Horticultural Science, 29(2), 137-141. doi: 10.1080/01140671.2001.9514171 [ Links ]
Bae, H., Yun, S. K., Jun, J. H., Yoon, I. K., Nam, E. Y., & Kwon, J. H. (2014). Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. Journal of Applied Botany and Food Quality, 87, 24-29. doi: 10.5073/JABFQ.2014.087.004 [ Links ]
Baldicchi, A., Farinelli, D., Micheli, M., Di Vaio, C., Moscatello, S., Battistelli, A., Walker, R. P., & Famiani, F. (2015). Analysis of seed growth, fruit growth and composition and phospoenolpyruvate carboxykinase (PEPCK) occurrence in apricot (Prunus armeniaca L.). Scientia Horticulturae, 186, 38-46. doi: 10.1016/j.scienta.2015.01.025 [ Links ]
Baldwin, E. A. (1993). Citrus fruit. En: Seymou, G. B., Taylor, J. E., & Tucker, G. A. (Eds.), Biochemistry of Fruit Ripening (pp. 107-149). Chapman and Hall, London. [ Links ]
Barbieri, C., Bignami, C., Cristofori, V., Paolocci, M., & Bertazza, G. (2011). Characterization and exploitation of minor pome fruits in Italy. Acta Horticulturae, 918, 953-960. doi: 10.17660/ActaHortic.2011.918.125 [ Links ]
Bartholomew, E. T., & Sinclair, W. B. (1951). The lemon fruit: its composition, physiology, and products. University of California Press, Berkeley. [ Links ]
Bartolomé, A. P., Rupérez, P., & Fúster, C. (1995). Pineapple fruit: morphological characteristics, chemical composition and sensory analysis of Red Spanish and Smooth Cayenne cultivars. Food Chemistry, 53(1), 75-79. doi: 10.1016/0308-8146(95)95790-D [ Links ]
Bogin, E., & Wallace, A. (1966). The inhibition of lemon citrate-condensing enzyme by ATP. Biochimica et Biophysica Acta, 128(1), 190-192. doi:10.1016/0926-6593(66)90158-5 [ Links ]
Bolivar-Fernández, N., Saucedo-Veloz, C., Solís-Pereira, S., & Sauri-Duch, E. (2009). Maduración de frutos de saramuyo (Annona squamosa L.) desarrollados en Yucatán, México. Agrociencia, 43(2), 133-141. [ Links ]
Bordonaba, G. J., & Terry, L. A. (2010). Manipulating the taste- related composition of strawberry fruits (Fragaria x ananassa) from different cultivars using deficit irrigation. Food Chemistry, 122(4), 1020-1026. [ Links ]
Boyes, S., Strübi, P., & Marsh, H. (1997). Sugar and organic acid analysis of Actinidia arguta and rootstock–scion combinations of Actinidia arguta. Journal of Food Science, 30(4): 390-397. doi: 10.1006/fstl.1996.0201 [ Links ]
Brandon, R., Hockema, R., & Etxeberria, E. D. (2001). Metabolic contributors to drought-enhanced accumulation of sugars and acids in oranges. Journal of the American Society for Horticultural Science, 126(5), 599-605. Recuperado de http://www.crec.ifas.ufl.edu/academics/faculty/Etxeberria/PDF/Hockema%20and%20Etxeberria.pdf [ Links ]
Buttrose, M. S., Hale, C. R., & Kliewer, W. M. (1971). Effect of temperature on the composition of 'Cabernet sauvignon' berries. American Journal of Enology Viticulture, 22(2), 71-75. [ Links ]
Canel, C., Bailey-Serres, J. N., & Roose, M. L. (1996). Molecular characterization of the mitochondrial citrate synthase gene of an acidless pummelo (Citrus maxima). Plant Molecular Biology, 31(1), 143–147. doi: 10.1007/BF00020613 [ Links ]
Cantín, C. M., Pinochet, J., Gogorcena, Y., & Moreno, M. A. (2010). Growth; yield and fruit quality of 'Van' and 'Stark Hardy Giant' sweet cherry cultivars as influenced by grafting on different rootstocks. Scientia Horticulturae, 123(3), 329-335. doi: 10.1016/j.scienta.2009.09.016 [ Links ]
Caruso, T., Giovannini, D., & Liverani, A. (1996). Rootstock influences the fruit mineral; sugar and organic acid content of a very early ripening peach cultivar. Journal Horticultural Science and Biotechnology, 71(6), 931-937. [ Links ]
Castle, W. S. (1995). Rootstock as a fruit quality factor in citrus and deciduous tree crops. New Zealand Journal of Crop and Horticultural Science, 23(4), 383-394. doi: 10.1080/01140671.1995.9513914 [ Links ]
Causse, M., Duffe, P., Gomez, M. C., Buret, M., Damidaux, R., Zamir, D., Gur, A., Chevalier, C., Lemaire-Chamley, M., & Rothan, C. (2004). A genetic map of candidate genes and QTLs involved in tomato fruit size and composition. Journal Experimental Botany, 55(403), 1671-1685. doi: 10.1093/jxb/erh207 [ Links ]
Çelik, H., Özgen, M., Serçe, S., & Kaya, C. (2008). Phytochemical accumulation and antioxidant capacity at four maturity stages of cranberry fruit. Scientia Horticulturae, 117 (4), 345-348. doi: 10.1016/j.scienta.2008.05.005 [ Links ]
Chen, F. X., Liu, X. H., & Chen, L. S. (2009). Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chemistry, 114(2), 657-664. doi: 10.1016/j.foodchem.2008.10.003 [ Links ]
Clements, R. L. (1964). Organic acids in citrus fruits. I. Varietal differences. Journal of Food Science, 29(3), 276-280. doi: 10.1111/j.1365-2621.1964.tb01731.x [ Links ]
Cruz-Castillo, J. G., Baldicchi, A., Frioni, T., Marocchi, F., Moscatello, S., Proietti, S., Battistelli, A., & Famiani, F. (2014). Pre-anthesis CPPU low dosage application increases 'Hayward' kiwifruit weight without affecting the other qualitative and nutritional characteristics. Food Chemistry, 158, 224-228. doi: 10.1016/j.foodchem.2014.01.131 [ Links ]
Cummings, G. A., & Reeves, J. (1971). Factors influencing chemical characteristics of peaches. Journal of the American Society for Horticultural Science, 96, 320-322. [ Links ]
Daito, H., & Sato, V. (2007). Changes in the sugar and organic acid components of satsuma mandarin fruit during maturation. Journal of the Japanese Society Horticultural Science, 54(2), 155-162. doi: 10.2503/jjshs.54.155 [ Links ]
Daood, H. G., Biacs, P., Czinkotai, B., & Hoschke, Á. (1992). Chromatographic investigation of carotenoids, sugars and organic acids from Diospyros kaki fruits. Food Chemistry, 45(2), 151-153. doi: 10.1016/0308-8146(92)90027-Y [ Links ]
Davies, J. N. (1964). Effect of nitrogen, phosphorus and potassium fertilisers on the non-volatile organic acids of tomato fruit. Journal of the Science of Food and Agriculture, 15(10), 665-673. doi: 10.1002/jsfa.2740151002 [ Links ]
Davies, J. N., & Winsor, G. W. (1967). Effect of nitrogen, phosphorus, potassium, magnesium and liming on the composition of tomato fruit. Journal of the Science of Food and Agriculture, 18(10), 459-466. doi: 10.1002/jsfa.2740181005 [ Links ]
de Bolt, S., Ristic, R., Iland, P. G., & Ford, C. M. (2008). Altered light interception reduces grape berry weight and modulates organic acid biosynthesis during development. HortScience, 43(3), 957-961. Recuperado de http://hortsci.ashspublications.org/content/43/3/957.full.pdf+html [ Links ]
de la Hera-Orts, M., Martinez-Cutillas, A., Lopez-Roca, J., & Gomez-Plaza, E. (2005). Effect of moderate irrigation on grape composition during ripening. Spanish Journal of Agricultural Research, 3(3), 352–361. doi: 10.5424/sjar/2005033-158 [ Links ]
del Angel-Coronel, O. A., Cruz-Castillo, J. G., de la Cruz-Medina, J., & Famiani, F. (2010). Ripening and physiological changes in the fruit of Persea schiedeana Nees during the postharvest period. HortScience, 45(1), 172-175. [ Links ]
des Gachons, C. P., Leeuwen, C. V., Tominaga, T., Soyer, J. P., Gaudillere, J. P., & Dubourdieu, D. (2004). Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L cv Sauvignon blanc in field conditions. Journal of the Science of Food and Agriculture, 85(1), 73-85. doi: 10.1002/jsfa.1919 [ Links ]
Diakou, P., Moing, A., Svanella, L., Ollat, N., Rolin, D. B., Gaudillere, M., & Gaudillere, J. P. (1997). Biochemical comparison of two grape varieties differing in juice acidity. Australian Journal of Grape and Wine Research, 3(3), 1-10. doi: 10.1111/j.1755-0238.1997.tb00122.x [ Links ]
Dilley, D. R. (1962). Malic enzyme activity in apple fruit. Nature, 196, 387-388. doi: 10.1038/196387a0 [ Links ]
du Preez, M. (1985). Effect of fertilisation on fruit quality. Deciduous Fruit Grower, April: 138-140.
Embleton, T., Jones, W., Pallares, C., & Platt, R. G. (1978). Effects of fertilization of citrus on fruit quality and ground water nitrate-pollution potential. International citrus congress, Sidney, Australia, 280-285. [ Links ]
Eshed, Y., & Zamir, D. A. (1994). A genomic library of Lycopersicon pennellii in L. esculentum: a tool for fine mapping of genes. Euphytica, 79(3), 175-179. doi: 10.1007/BF00022516 [ Links ]
Esteban, M. A., Villanueva, M. J., & Lissargue, J. R. (1999). Effect of irrigation on changes in berry composition of Tempranillo during maturation. Sugars; organic acids; and mineral elements. American Journal of Enology and Viticulture, 50(4), 418-434. [ Links ]
Etienne, A., Génard, M., Bancel, D., Benoit, S., Lemire, G., & Bugaud, C. (2014). Citrate and malate accumulation in banana fruit (Musa sp. AA) is highly affected by genotype and fruit age, but not by cultural practices. Scientia Horticulturae, 169(16), 99-110. doi:10.1016/j.scienta.2014.02.013 [ Links ]
Etienne, A., Génard, M., Lobit, P., Mbeguié-A-Mbéguié, D., & Bugaud, C. (2013). What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. Journal of Experimental Botany, 64(6), 1451-1469. doi: 10.1093/jxb/ert035 [ Links ]
Etienne, C., Moing, A., Dirlewanger, E., Raymond, P., Monet, R., & Rothan, C. (2002). Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: involvement in regulating peach fruit acidity. Physiologia Plantarum, 114(2), 259-270. doi: 10.1034/j.1399-3054.2002.1140212.x [ Links ]
Failla, O., Scienza, A., & Brancadoro, L. (1996). Effects of nutrient spray applications on malic and tartaric acid levels in grapevine berry. Journal of Plant Nutrition, 19(1): 45-50. doi: 10.1080/01904169609365105 [ Links ]
Famiani, F., Farinelli, D., Palliotti, A., Moscatello, S., Battistelli, A., & Walker, R. P. (2014a). Is stored malate the quantitatively most important substrate utilised by respiration and ethanolic fermentation in grape berry pericarp during ripening? Plant Physiology and Biochemistry, 76, 52-57. doi:10.1016/j.plaphy.2013.12.017 [ Links ]
Famiani, F., Baldicchi, A., Battistelli, A., Moscatello, S., & Walker, R. P. (2009). Soluble sugar and organic acid contents and the occurrence and potential role of phosphoenolpyruvate carboxykinase (PEPCK) in gooseberry (Ribes grossularia L.). Journal of Horticultura Science and Biotechnology, 84(3), 249-254. [ Links ]
Famiani, F., Baldicchi, A., Farinelli, D., Cruz-Castillo, J. G., Marocchi, F., Mastroleo, M., Moscatello, S., Proietti, S., & Battistelli, A. (2012a). Yield affects qualitative kiwifruit characteristics and dry matter content may be an indicator of both quality and storability. Scientia Horticulturae, 146, 124-130. doi:10.1016/j.scienta.2012.08.009 [ Links ]
Famiani, F., Battistelli, A., Moscatello, S., Boco, M., & Antognozzi, E. (1999). Thidiazuron affects fruit growth, ripening and quality of Actinidia deliciosa. J. Hortic. Sci. Biotechnol. 74: 375-380. [ Links ]
Famiani, F., Casulli, V., Baldicchi, A., Battistelli, A., Moscatello, S., & Walker, R. P. (2012b). Development and metabolism of the fruit and seed of the japanese plum Ozark Premier (Rosaceae). Journal of Plant Physiology, 169(6), 551-560. doi:10.1016/j.jplph.2011.11.020 [ Links ]
Famiani, F., Casulli, V., Proietti, P., Walker, R. P., & Battistelli, A. (2007). Organic acid metabolism in grape: role of phosphoenolpyruvate carboxykinase. Acta Horticulturae, 754, 599-602. doi: 10.17660/ActaHortic.2007.754.80 [ Links ]
Famiani, F., Cultrera, N., Battistelli, A., Casulli, V., Proietti, P., Standardi, A., Chen, Z. H., Leegood, R. C., & Walker, R. P. (2005). Phosphoenolpyruvate carboxykinase and its potential role in the catabolism of organic acids in the flesh of soft fruit during ripening. Journal of Experimental Botany, 56 (421), 2959-2969. doi: 10.1093/jxb/eri293 [ Links ]
Famiani, F., Farinelli, D., Palliotti, A., Battistelli, A., Moscatello, S., & Walker, R. P. (2015). Malate as a substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biologia Plantarum (in press).
Famiani, F., Moscatello, S., Ferradini, N., Gardi, T., Battistelli, A., & Walker, R. P. (2014b). Occurrence of a number of enzymes involved in either gluconeogenesis or other processes in the pericarp of three cultivars of grape (Vitis vinifera L.) during development. Plant Physiol Biochem, 84, 261-270. doi: 10.1016/j.plaphy.2014.10.003 [ Links ]
Famiani, F., & Walker, R. P. (2009). Changes in abundance of enzymes involved in organic acid, amino acid and sugar metabolism, and photosynthesis during the ripening of blackberry fruit. Journal of the American Society for Horticultural Science, 134(2), 167-75. Recuperado de http://journal.ashspublications.org/content/134/2/167.full.pdf+html [ Links ]
Famiani, F., Walker, R. P., Tecsi, L., Chen, Z. H., Proietti, P., & Leegood, R. C. (2000). An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. Journal of Experimental Botany, 51(345), 675-683. doi: 10.1093/jexbot/51.345.675 [ Links ]
Farineau, J., & Laval-Martin, D. (1977). Light versus dark carbon metabolism in cherry tomato fruits. II. Relationship between malate metabolism and photosynthetic activity. Plant Physiology, 60(6), 877-880. doi: 10.1104/pp.60.6.877 [ Links ]
Ford, C. M. (2012). The biochemistry of organic acids in the grape, pp. 67-88. In: The Biochemistry of the grape berry. Geros, H., Chaves, M. M., & Delrot, S. (eds.). Bentham Science Publishers, Illinois. [ Links ]
Füzfai, Z., Katona, Z. F., Kovács, E., & Molnár-Perl, I. (2004). Simultaneous identification and quantification of the sugar, sugar alcohol, and carboxylic acid contents of sour cherry, apple, and ber fruits, as their trimethylsilyl derivatives, by gas chromatography- mass spectrometry. Journal of Agricultural and Food Chemistry, 52(25), 7444-7452. doi: 10.1021/jf040118p [ Links ]
Gao, Q. H., Wu, C. S., Yu, J. G., Wang, M., Ma, Y. J., & Li, C. L. (2012). Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. Journal of Food Science, 77(11), C1218-C1225. doi: 10.1111/j.1750-3841.2012.02946.x [ Links ]
García-Mariño, N., de la Torre, F., & Matilla, A. J. (2008). Organic acids and soluble sugars in edible and nonedible parts of damson plum (Prunus domestica L. subsp. Insititia cv. Syriaca) fruits during development and ripening. Food Science and Technology International, 14(2), 187-93. doi: 10.1177/1082013208092150 [ Links ]
Gautier, H., Rocci, A., Buret, M., Grassely, D., & Causse, M. (2005). Fruit load or fruit position alters response to temperature and subsequently cherry tomato quality. Journal of the Science of Food and Agriculture, 85(6), 1009-1016. doi: 10.1002/jsfa.2060 [ Links ]
Genevois, L., & Peynaud, E. (1947a). Composition de 16 variétés de pêches. Revue Horticole, 30, 295-298. [ Links ]
Genevois, L., & Peynaud, E. (1947b). Composition de neuf variétés de prunes. Revue Horticole, 30, 317-318. [ Links ]
Glew, R. H., Ayaz, F. A., Sanz, C., Vanderjagt, D. J., Huang, H. S., Chuang, L. T., & Strnad M. (2003a). Changes in sugars, organic acids and amino acids in medlar (Mespilus germanica L.) during fruit development and maturation. Food Chemistry, 83(3), 363-369. doi: 10.1016/S0308-8146(03)00097-9 [ Links ]
Glew, R. H., Ayaz, F. A., Sanz, C., Vanderjagt, D. J., Huang, H. S., Chuang, L. T., & Strnad, M. (2003b). Effect of postharvest period on sugars, organic acids and fatty acids composition in commercially sold medlar (Mespilus germanica 'Dutch') fruit. European Food Research and Technology, 216(5), 390-394. doi: 1007/s00217-002-0654-3 [ Links ]
Gong, H., Blackmore, D. H., & Walker, R. R. (2010). Organic and inorganic anions in Shiraz and Chardonnay grape berries and wine as affected by rootstock under saline conditions. Australian Journal of Grape and Wine Research, 16(1), 227-236. doi: 10.1111/j.1755-0238.2009.00070.x [ Links ]
González-Altozano, P., & Castel, J. R. (1999). Regulated deficit irrigation in 'Clementina De Nules' citrus trees. I. Yield and fruit quality effects. Journal of Horticultural Science and Biotechnology, 74(4), 706-713. Recuperado de http://www.researchgate.net/profile/Juan_Castel/publication/259935904_RDI_Clementina_de_Nules_trees._II._Veg_growth/links/0c96052ea430780e60000000.pdf [ Links ]
González-Aguilar, G. A., Buta, J. G., & Wang, C. Y. (2003). Methyl jasmonate and modified atmosphere packaging (MAP) reduce decay and maintain postharvest quality of papaya 'Sunrise'. Postharvest Biology and Technology, 28(3), 361-370. doi: 10.1016/S0925-5214(02)00200-4 [ Links ]
Goodenough, P. W., Prosser, I. M., & Young, K. (1985). NADP- linked malic enzyme and malate metabolism in ageing tomato fruit. Phytochemistry, 24(6), 1157-62. doi: 10.1016/S0031-9422(00)81093-6 [ Links ]
Gundogdu, M., Muradoglu, F., Gazioglu-Sensoy, R. I., & Yilmaz, H. (2011). Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Scientia Horticulturae, 132(5), 37-41. doi:10.1016/j.scienta.2011.09.035 [ Links ]
Gurrieri, F., Audergon, J. M., Albagnac, G., & Reich, M. (2001). Soluble sugars and carboxylic acids in ripe apricot fruit as parameters for distinguishing different cultivars. Euphytica, 117(3), 183-189. doi: 10.1023/A:1026595528044 [ Links ]
Gutierréz-Granda, M. J., & Morrison, J. C. (1992). Solute distribution and malic enzyme activity in developing grape berries. American Journal of Enology and Viticulture, 43(4), 323-328. [ Links ]
Hale, C. R., & Buttrose, M. S. (1974). Effect of temperature on ontogeny of berries of Vitis vinifera L. cv Cabernet Sauvignon. Journal of the American Society for Hotricultural Science, 99(5), 390-394. [ Links ]
Hansen, E. (1942). Quantitative study of ethylene production in relation to respiration of pears. Botanical Gazette, 103(3), 543-558. [ Links ]
Harman, J. E. (1987). Feijoa fruit: growth and chemical composition during development. New Zealand Journal of Experimental Agriculture, 15(2), 209-215. doi: 10.1080/03015521.1987.10425561 [ Links ]
Herrmann, K. M. (1995). The shikimate pathway: early steps in the biosynthesis of aromatic compounds. The Plant Cell, 7, 907-919. Recuperado de http://www.plantcell.org/content/7/7/907.full.pdf+html [ Links ]
Hirai, M. (1982). Accelerated sugar accumulation and ripening of loquat fruit by exogenously applied ethylene. Journal of the Japanese Society Horticultural Science, 51, 159–64. doi: 10.2503/jjshs.51.159 [ Links ]
Hu, Z., Wang, H., & Hu, G. (2005). Measurements of sugars, organic acids and vitamin C in litchi fruit by high performance liquid chromatography. Journal of Fruit Science, 22(5), 582-585. [ Links ]
Hulme, A. C. (1971). The biochemistry of fruits and their products, vol. 2. Academic Press, London and New York. [ Links ]
Hummel, A. K., & Ferree, D. C. (1998). Interaction of crop level and fruit cluster exposure on 'Seyval Blanc' fruit composition. Journal of the American Society for Horticultural Science, 123(5), 755-761. Recuperado de http://journal.ashspublications.org/content/123/5/755.full.pdf+html [ Links ]
Hurth, M. A., Suh, S. J., Kretzschmar, T., Geis, T., Bregante, M., Gambale, F., Martinoia, E., & Neuhaus, H. E. (2005). Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast. Plant Physiology, 137, 901-910. doi: 10.1104/pp.104.058453 [ Links ]
Incerti, A., Navari-Izzo, F., Pardossi, A., Mensual, A., & Izzo, R. (2007). Effect of sea water on biochemical properties of fruit of tomato (Lycopersicon esculentum Mill.) genotypes differing for ethylene production. Journal of the Science of Food and Agriculture, 87(13), 2528-2537. doi: 10.1002/jsfa.3020 [ Links ]
Jackson, D. I., & Lombard, P. B. (1993). Environmental and management practices affecting grape composition and wine quality - a review. American Journal of Enology and Viticulture, 44(4), 409-430. [ Links ]
Jia, H., Hirano, K., & Okamoto, G. (1999). Effects of fertilizer levels on tree growth and fruit quality of 'Hakuho' peaches (Prunus persica). Journal of the Japanese Society for Horticultural Science, 68(3), 487-493. doi: 10.2503/jjshs.68.487 [ Links ]
Johanningsmeiner, S. D., Mcfeeters, R. F., & Drake, M. (2005). A hypothesis for the chemical basis for perception of sour taste. Journal for the Food Science, 70(2), 44-48. doi: 10.1111/j.1365-2621.2005.tb07111.x [ Links ]
Kader, A. A. (2002). Post-harvest technology of horticultural crops. Oakland University of California; Division of Agriculture and Natural Resources, Publication: 3311-3535. [ Links ]
Kallsen, C. E., Sanden, B., & Arpaia, M. L. (2011). Early navel orange fruit yield, quality, and maturity in response to late-season water stress. HortScience, 46(8), 1163-1169. Recuperado de http://hortsci.ashspublications.org/content/46/8/1163.full.pdf+html [ Links ]
Kalt, W., & Mcdonald, J. E. (1996). Chemical composition of lowbush blueberry cultivars. Journal of the American Society for Horticultural Science, 121(1), 142-146. Recuperado de http://journal.ashspublications.org/content/121/1/142.full.pdf+html [ Links ]
Kanellis, A. K., & Roubelakis-Angelakis, K. A. (1993). Grape. En Seymour, G. B., Taylor, J. E. & Tucker, G.A. (eds.), Biochemistry of fruit ripening (pp.189-234). Chapman and Hall, London. [ Links ]
Klenert, M., Rapp, A., & Alleweldt, G. (1978). Einfluss des traubentemperatur auf beerenwachstum and beerenreife der rebsorte silvaner. Vitis, 17, 350-360. Recuperado de http://www.vitis-vea.de/admin/volltext/e002252.pdf [ Links ]
Kliewer, W., Howarth, L., & Omori, M. (1967). Concentrations of tartaric acid and malic acids and their salts in Vitis vinifera grapes. American Journal of Enology and Viticulture, 18(1), 42-54. [ Links ]
Kliewer, W. M. (1970). Effect of day temperature and light intensity on coloration and composition of Vitis vinifera L. grapes. Journal of the American Society for Horticultural Science, 95(6), 693-697. [ Links ]
Kliewer, W. M. (1973). Berry composition of Vitis vinifera cultivars as influenced by photo-and nycto- temperatures during maturation. Journal of the American Society for Horticultural Science, 98, 153-159. [ Links ]
Lakso, A. N., & Kliewer, W. M. (1978). The influence of temperature on malic acid metabolism in grape berries. II. Temperature responses of net dark CO2 fixation and malic acid pools. American Journal of Enology and Viticulture, 29(3), 145-149. [ Links ]
Lang, A. (1983). Turgor-related translocation. Plant Cell and Environment, 6(9), 683-689. doi: 10.1111/1365-3040.ep11589312 [ Links ]
Law, R. D., & Plaxton, W. C. (1995). Purification and characterization of a novel phosphoenolpyruvate carboxylase from banana fruit. Biochemical Journal, 307, 807–816. Recuperado de http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1136721/pdf/biochemj00064-0189.pdf [ Links ]
Lea, P. J. (1993). Nitrogen metabolism. En: Lea, P. J. & Leegood, R. C. (Eds.), Plant biochemistry and molecular biology (pp. 155-180). John Wiley, New York. [ Links ]
Léchaudel, M., Joas, J., Caro, Y., Génard, M., & Jannoyer, M. (2005). Leaf:fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. Journal of the Science Food and Agriculture, 85(2), 251-260. doi: 10.1002/jsfa.1968 [ Links ]
Leegood, R. C., & Walker, R. P. (2003). Regulation and roles of phosphoenolpyruvate carboxykinase in plants. Archives of Biochemistry and Biophysics, 414(2), 204-210. doi: 10.1016/S0003-9861(03)00093-6 [ Links ]
Leegood, R. C., & Walker, R. P. (1999). Phosphoenolpyruvate carboxykinase in plants: its role and regulation. En: Bryant, J. A., Burrell, M. M. & Kruger, N. J. (Eds.), Plant carbohydrate biochemistry (pp. 201-211). BIOS Scientific Publishers Ltd, Oxford. [ Links ]
Legua, P., Forner, J. B., Hernández, F., & Forner-Giner, M. A. (2014). Total phenolics, organic acids, sugars and antioxidant activity of mandarin (Citrus clementina Hort. ex Tan.): Variation from rootstock. Scientia Horticulturae, 174(22), 60-64. doi: 10.1016/j.scienta.2014.05.004 [ Links ]
Liu, S., Yang, Y., Murayama, H., Taira, S.,& Fukushima, T. (2004). Effects of CO2 on respiratory metabolism in ripening banana fruit. Postharvest Biology and Technology, 33(1), 27-34. doi: 10.1016/j.postharvbio.2004.01.006 [ Links ]
Lobit, P., Génard, M., Soing, P., & Habib, R. (2006). Modelling malic acid accumulation in fruits: relationships with organic acids, potassium and temperature. Journal of Experimental Botany, 57(6), 1471-1483. doi: 10.1093/jxb/erj128 [ Links ]
Lobit, P., Genard, M., Wu, B. H., Soing, P., & Habib, R. (2003). Modelling citrate metabolism in fruits: responses to growth and temperature. Journal of Experimental Botany, 54(392), 2489-2501. doi: 10.1093/jxb/erg264 [ Links ]
Mazur, S. P., Sønsteby, A., Wold, A. B., Foito, A., Freitag, S., Verrall, S., Conner, S., Stewart, D., & Heide, O. M. (2014). Post-flowering photoperiod has marked effects on fruit chemical composition in red raspberry (Rubus idaeus). Annals of Applied Biology, 165(3), 454-465. doi: 10.1111/aab.12153 [ Links ]
Medlicott, A. P., & Thompson, A. K. (1985). Analysis of sugars and organic acids in ripening mango fruits (Mangifera indica L. var Keitt) by high performance liquid chromatography. Journal of the Science and Food Agricultural, 36(7), 561-566. doi: 10.1002/jsfa.2740360707 [ Links ]
Melgarejo, P., Salazar, D. M., & Artéz, F. (2000). Organic acids and sugars composition of harvested pomegranate fruits. European Food Research and Technology, 211(3), 185-190. doi: 10.1007/s002170050021 [ Links ]
Mikulic-Petkovsek, M., Schmitzer, V., Slatnar, A., Stampar, F., & Veberic, R. (2012). Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. Journal of Food Science, 77(10), C1064-C1070. doi: 10.1111/j.1750-3841.2012.02896.x [ Links ]
Mills, T. M., Behboudian, M. H., & Clothier, B. E. (1996). Water relations; growth; and the composition of 'Braeburn' apple fruit under deficit irrigation. Journal of the American Society for Horticultural Science, 121(2), 286-291. Recuperado de http://journal.ashspublications.org/content/121/2/286.full.pdf+html [ Links ]
Moing, A., Rothan, C., Svanella, L., Just, D., Diakou, P., Raymond, P., Gaudillere, J. P., & Monet, R. (2000). Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development. Physiologia Plantarum, 108(1), 1-10. doi: 10.1034/j.1399-3054.2000.108001001.x [ Links ]
Moing, A., Rothan, C., Svanella, L., Just, D., Diakou, P., Rolin, D., Gaudillere, J. P., Monet, R. (1998). Organic acid metabolism during the development of two peach cultivars. Acta Horticulturae, 465, 425-432. doi: 10.17660/ActaHortic.1998.465.53 [ Links ]
Moing, A., Svanella, L., Gaudillere, M., Gaudillere, J. P., & Monet, R. (1999). Organic acid concentration is little controlled by phosphoenolpyruvate carboxylase activity in peach fruit. Australian Journal of Plant Physiology, 26(6), 579-585. doi: 10.1071/PP98164 [ Links ]
Morrison, J. C., & Noble, A. C. (1990). The effects of leaf and cluster shading on the composition of Cabernet sauvignon grapes and on fruit and wine sensory properties. American Journal of Enology and Viticulture, 41(3), 193-200. [ Links ]
Morvai, M., & Molnar-Perl, I. (1992). Simultaneous gas chromatographic quantitation of sugars and acids in citrus fruits, pears, bananas, grapes, apples and tomatoes. Chromatographia, 34(9-10), 502-504. doi: 10.1007/BF02290244 [ Links ]
Mpelasoka, B. S., Schachtman, D. P., Treeby, M. T., & Thomas, M. R. (2003). A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Australian Journal of Grape and Wine Research, 9(3), 154-168. doi: 10.1111/j.1755-0238.2003.tb00265.x [ Links ]
Muñoz-Robredo, P., Robledo, P., Manríquez, D., Molina, R., & Defilippi, B. G. (2011). Characterization of sugars and organic acids in commercial varieties of table grapes. Chilean Journal of Agricultural Research, 71(3), 452-458. Recuperado de http://www.readcube.com/articles/10.4067%2Fs0718-58392011000300017 [ Links ]
Neri, F., Pratella, G. C., & Brigati, S. (2003). Gli indici di maturazione per ottimizzare la qualità organolettica della frutta. Rivista di Frutticoltura e di Ortofloricoltura, 5, 20-29. [ Links ]
Nishiyama, I., Fukuda, T., Shimohashi, A., & Oota, T. (2008). Sugar and organic acid composition in the fruit juice of different Actinidia varieties. Food Science Technology Research, 14(1), 67-73. doi: 10.3136/fstr.14.67 [ Links ]
Nour, V., Trandafir, I., & Ionica, M. E. (2010). HPLC organic acid analysis in different citrus juices under reversed phase conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 38(1), 44-48. [ Links ]
Orazem, P., Stampar, F., & Hudina, M. (2011a). Fruit quality of Redhaven and Royal Glory peach cultivars on seven different rootstocks. Journal of Agricultural and Food Chemistry, 59(17), 9394-9401. doi: 10.1021/jf2009588 [ Links ]
Orazem, P., Stampar, F., & Hudina, M. (2011b). Quality analysis of 'Redhaven' peach fruit grafted on 11 rootstocks of different genetic origin in a replant soil. Food Chemistry, 124(4), 1691-1698. doi:10.1016/j.foodchem.2010.07.078 [ Links ]
Osorio, S., Vallarino, J. G., Szecowka, M., Ufaz, S., Tzin, V., Angelovici, R., Galili, G., & Fernie, A. R. (2013). Alteration of the interconversion of pyruvate and malate in the plastid or cytosol of ripening tomato fruit invokes diverse consequences on sugar but similar effects on cellular organic acid, metabolism, and transitory starch accumulation. Plant Physiol, 161(2), 628-643. doi: 10.1104/pp.112.211094 [ Links ]
Pacheco, C., Calouro, F., Vieira, S., Santos, F., Neves, N., Curado, F., Franco, J., Rodrigues, S., & Antunes, D. (2008). Effect of nitrogen and potassium fertilization on yield and fruit quality in kiwifruit. 4th Proceedings "IASME/ WSEAS International Conference on "Energy, environment, ecosystems and sustainable development (EEESD'08)", Algarve, Portugal, June 11-13 2008. [ Links ]
Palliotti, A., Silvestroni, O., & Petoumenou, D. (2010). Seasonal patterns of growth rate and morphophysiological features in green organs of Cabernet sauvignon grapevines. American Journal of Enology and Viticulture, 61(1), 74-82. [ Links ]
Palliotti, A., & Poni, S. (2011). Traditional and innovative summer pruning techniques for vineyard management. Advances in Horticultural Science, 25(3), 151-163. [ Links ]
Pereira, G. E., Gaudillere, J. P., Pieri, P., Hilbert, G., Maucourt, M., Deborde, C., Moing, A., & Rolin, D. (2006). Microclimate influence on mineral and metabolic profiles of grape berries. Journal of Agricultural and Food Chemistry, 54(18), 6765–6775. doi: 10.1021/jf061013k [ Links ]
Peynaud, E., & Ribéreau-Gayon, G. P. (1971). The grape. En: Hulme, A. C. (Ed.), The biochemistry of fruits and their products, vol. 2 (pp. 179-205). Academic Press, London. [ Links ]
Plaxton, W. C. (1996). The organization and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology, 47, 185-214. doi: 10.1146/annurev.arplant.47.1.185 [ Links ]
Poiroux-Gonord, F., Fanciullino, A. L., Bert, L., & Urban, L. (2012). Effect of fruit load on maturity and carotenoid content of clementine (Citrus clementina Hort. ex Tan.) fruits. Journal of the Science of Food and Agriculture, 92(10), 2076-2083. doi: 10.1002/jsfa.5584 [ Links ]
Pua, E. C., Chandramouli, S., Han, P., & Liu, P. (2003). Malate synthase gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Williams). Journal of Experimental Botany, 54(381), 309-316. doi: 10.1093/jxb/erg030 [ Links ]
Radi, M., Mahrouz, M., Jaouad, A., & Amiot, M. J. (2003). Influence of mineral fertilization (NPK) on the quality of apricot fruit (cv. Canino). The effect of the mode of nitrogen supply. Agronomie, 23(8), 737-745. doi: 10.1051/agro:2003052 [ Links ]
Ramesh-Kumar, A., & Kumar, N. (2007). Sulfate of potash foliar spray effects on yield, quality, and post-harvest life of banana. Better Crops, 91, 22-24. [ Links ]
Raven, J. A., & Smith, F. A. (1976). Nitrogen assimilation and transport in vascular land plants in relation to intracellular ph regulation. New Phytologist, 76(3), 415-431. doi: 10.1111/j.1469-8137.1976.tb01477.x [ Links ]
Reitz, H. J., & Koo, R. C. J. (1960). Effect of nitrogen and potassium fertilization on yield, fruit quality, and leaf analysis of Valencia orange. American Society for Horticultural Science, 75, 244-252. [ Links ]
Reynard, J. S., Zufferey, V., Nicol, G. C., & Murisier, F. (2011). Soil parameters impact the vine-fruit-wine continuum by altering vine nitrogen status. Journal International des Science Vigne et du Vin, 45(4), 211-221. [ Links ]
Richardson, A. C., Marsh, K. B., Boldingh, H. L., Pickering, A. H., Bulley, S. M., Frearson, N. J., Ferguson, A. R., Thornber, S. E., Bolitho, K. M., & Marcrae, E. A. (2004). High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant, Cell & Environment, 27(4), 423-435. doi: 10.1111/j.1365-3040.2003.01161.x [ Links ]
Rienth, M., Torregrosa, L., Luchaire, N., Chatbanyong, R., Lecourieux, D., Kelly, M. T., & Romieu, C. (2014). Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biology, 14, 108. doi: 10.1186/1471-2229-14-108 [ Links ]
Roe, B., Davis, P. L., & Bruemmer, J. H. (1984). Pyruvate metabolism during maturation of Hamlin oranges. Phytochemistry, 23(4), 713-717. doi: 10.1016/S0031-9422(00)85010-4 [ Links ]
Romero-Rodriguez, M. A., Vazquez-Oderiz, M. L., López-Hernández, J., & Simal-Lozano, J. (1992). Determination of vitamin C and organic acids in various fruits by HPLC. Journal of Chromatographic Science, 30(11), 433-437. doi: 10.1093/chromsci/30.11.433 [ Links ]
Romieu, C., Tesniere, C., Thanham, L., Flanzy, C., & Robin, J. P. (1992). An examination of the importance of anaerobiosis and ethanol in causing injury to grape mitochondria. American Journal of Enology and Viticulture, 43(2), 129-133. [ Links ]
Róth, E., Berna, A., Beullens, K., Yarramraju, S., Lammertyna, J., Schenk, A., & Nicolaï, B. (2007). Postharvest quality of integrated and organically produced apple fruit. Postharvest Biology and Technology, 45(1), 11-19. doi: 10.1016/j.postharvbio.2007.01.006 [ Links ]
Ruffner, H. P. (1982a). Metabolism of tartaric and malic acid in Vitis: a review. Parte A. Vitis, 21, 241-249. [ Links ]
Ruffner, H. P. (1982b). Metabolism of tartaric and malic acid in Vitis: a review. Parte B. Vitis, 21, 346-358. [ Links ]
Ruffner, H. P., Koblet, W., & Rast, D. (1975). Gluconeogenese in reifenden Beeren von Vitis vinifera. Vitis, 13, 319- 328. [ Links ]
Ruffner, H. P., Kliewer, W. M. (1975). Phosphoenolpyruvate carboxykinase activity in grape berries. Plant Physiology, 56(1), 67-71. doi: 10.1104/pp.56.1.67 [ Links ]
Ruffner, H. P., Hawker, J. S., & Hale, C. R. (1976). Temperature and enzymic control of malate metabolism in berries of Vitis vinifera. Phytochemistry, 15(12), 1877-1880. doi: 10.1016/S0031-9422(00)88835-4 [ Links ]
Ruhl, E. H. (1989). Effect of potassium and nitrogen supply on the distribution of minerals and organic acids and the composition of grape juice of Sultana vines. Australian Journal of Experimental Agriculture, 29(1), 133- 137. doi: 10.1071/EA9890133 [ Links ]
Ruiz-Rodríguez, B. M., Morales, P., Fernández-Ruiz, V., Sánchez-Mata, M. C., Cámara, M., Díez-Marqués, C., Pardo-De-Santayana, M., Molina, M., & Tardío, J. (2011). Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Research International, 44(5), 1244-1253. doi: 10.1016/j.foodres.2010.11.015 [ Links ]
Sadka, A., Dahan, E., Cohen, L., & Marsh, K. B. (2000). Aconitase activity and expression during the development of lemon fruit. Physiologia Plantarum, 108(3), 255-262. doi: 10.1034/j.1399-3054.2000.108003255.x [ Links ]
Sadka, A., Dahan, E., Or, E., Roose, M. L., Marsh, K. B., & Cohen, L. (2001). Comparative analysis of mitochondrial citrate synthase gene structure; transcript level and enzymatic activity in acidless and acid-containing citrus varieties. Functional Plant Biology, 28(5), 383-390. doi:10.1071/PP00136 [ Links ]
Saito, T., Matsukura, C., Ban, Y., Shoji, K., Sugiyama, M., Fukuda, N., & Nishimura, S. (2008). Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). Journal of the Japanese Society for Horticultural Science, 77(1), 61-68. Recuperado de www.jstage.jst.go.jp/article/jjshs1/77/1/77_1_61/_pdf [ Links ]
Saradhuldhat, P., & Paull, R. E. (2007). Pineapple organic acid metabolism and accumulation during fruit development. Scientia Horticulturae, 112(3), 297-303. doi: 10.1016/j.scienta.2006.12.031 [ Links ]
Selcuk, N., & Erkan, M. (2015). The effects of 1-MCP treatment on fruit quality of medlar fruit (Mespilus germanica L. cv. Istanbul) during long term storage in the palliflex storage system. Postharvest Biology and Technology, 100, 81-90. doi: 10.1016/j.postharvbio.2014.09.018 [ Links ]
Selvaraj, Y., & Kumar, R. (1989). Pyruvate kinase activity in ripening mango (Mangifera indica L.) fruit. Journal of Food Science and Technology, 26(4), 228-229. [ Links ]
Smart, R., & Coombe, B. G. (1983). Water relations of grapevines. En: Kozlowski, T. T. (Ed.), Water deficits and plant growth, vol. VII: Additional woody crop plants (pp. 137-196). Academic Press, New York. [ Links ]
Smart, R. E., & Sinclair, T. R. (1976). Solar heating of grape berries and other spherical fruits. Agricultural Meteorology, 17(4), 241-259. doi: 10.1016/0002-1571(76)90029-7 [ Links ]
Smith, F. A., & Raven, J. A. (1979). Intracellular pH and its regulation. Annual Reviews of Plant Physiology, 30, 289- 311. doi: 10.1146/annurev.pp.30.060179.001445 [ Links ]
Soares, F. D., Pereira, T., Marques, M. O. M., & Monteiro, A. R. (2007). Volatile and non-volatile chemical composition of the white guava fruit (Psidium guajava) at different stages of maturity. Food Chemistry, 100(1), 15-21. doi: 10.1016/j.foodchem.2005.07.061 [ Links ]
Souty, M., Perret, A., & Andre, P. (1967). Premières observations sur quelques variétés de pêches destinées à la conserve. Annual Technology Agricultural, 16, 55-68. [ Links ]
Souty, M., Génard, M., Reich, M., & Albagnac, G. (1999). Effect of assimilate supply on peach fruit maturation and quality. Canadian Journal of Plant Science, 79, 259-268. [ Links ]
Spironello, A., Quaggio, J. A., Teixeira, L. A. J., Furlani, P. R., & Sigrist, J. M. M. (2004). Pineapple yield and fruit quality effected by NPK fertilization in a tropical soil. Revista Brasileira de Fruticultura, 26(1), 155-159. doi: 10.1590/S0100-29452004000100041 [ Links ]
Surendranathan, K. K., & Nair, P. M. (1976). Stimulation of the glyoxylate shunt in gamma-irradiated banana. Phytochemistry, 15(3), 371-373. doi: 10.1016/S0031-9422(00)86825-9 [ Links ]
Szychowski, P. J., Munera-Picazo, S., Szumny, A., Carbonell- Barrachina, Á. A., & Hernández, F. (2014). Quality parameters, bio-compounds, antioxidant activity and sensory attributes of Spanish quinces (Cydonia oblonga Miller). Scientia Horticulturae, 165(22), 163-170. doi: 10.1016/j.scienta.2013.11.028 [ Links ]
Sweetman, C., Deluc, L. G., Cramer, G. R., Ford, C. M., & Soole, K. L. (2009). Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry, 70(11-12), 1329-1344. doi: 10.1016/j.phytochem.2009.08.006 [ Links ]
Sweetman, C., Sadras, V. O., Hancock, R. D., Soole, K. L., & Ford, C. M. (2014). Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. Journal of Experimental Botany, 65(20), 5975–5988. [ Links ]
Thakur, A., & Singh, Z. (2011). Responses of 'Spring Bright' and 'Summer Bright' nectarines to deficit irrigation: fruit growth and concentration of sugars and organic acids. Scientia Horticulturae, 135(24), 112-119. doi: 10.1016/j.scienta.2011.12.013 [ Links ]
Tang, M., Bie, Z. L., Wu, M. Z., Yi, H. P., & Feng, J. X. (2010). Changes in organic acids and organic acids metabolism enzymes in melon fruit during development. Scientia Horticulturae, 123(3), 360-365. doi: 10.1016/j.scienta.2009.11.001 [ Links ]
Taureilles-Saurel, C., Romieu, C. G., Robin, J. P., & Flanzy, C. (1995a). Grape (Vitis vinifera L.) malate dehydrogenase. I. Interacellular compartmentation of the isoforms. American Journal of Enology and Viticulture, 46(1), 22-28. [ Links ]
Taureilles-Saurel, C., Romieu, C. G., Robin, J. P., & Flanzy, C. (1995b). Grape (Vitis vinifera L.) Malate dehydrogenase. II. Characterization of the major mitochondrial and cytosolic isoforms and their role in ripening. American Journal of Enology and Viticulture, 46(1), 29-36. [ Links ]
Terrier, N., & Romieu, C. (2001). Grape berry acidity. En: Roubelakis-Angelakis, K. A. (Ed.), Molecular biology and biotechnology of the grapevine (pp. 35-57). Springer, Dordrecht. [ Links ]
Terrier, N., Glissant, D., Grimplet, J., Barrieu, F., Abbal, P., Couture, C., Ageorges, A., Atanassova, R., Léon, C., Renaudin, J. P., Dédaldéchamp, F., Romieu, C., Delrot, S., & Hamdi, S. (2005). Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta, 222(5), 832-847. doi: 10.1007/s00425-005-0017-y [ Links ]
Tesnière, C., Romieu, C., Dugelay, I., Nicol, M. Z., Flanzy, C., & Robin, J. P. (1994). Partial recovery of grape energy- metabolism upon aeration following anaerobic stress. Journal of Experimental Botany, 45(1), 145-151. doi: 10.1093/jxb/45.1.145 [ Links ]
Tombesi, A., Antognozzi, E., & Palliotti, A., (1993). Influence of light exposure on characteristics and storage life of kiwifruit. New Zealand Journal of Crop and Horticultural Science,21(1),85-90.doi:10.1080/01140671.1993.9513750 [ Links ]
Trad, M., Gaaliche, B., Renard, C. M. G. C., & Mars, M. (2013). Inter- and intra-tree variability in quality of figs. Influence of altitude, leaf area and fruit position in the canopy. Scientia Horticulturae, 162, 49-54. doi: 10.1016/j.scienta.2013.07.032 [ Links ]
Tucker, G. A. (1993). Introduction. En: Seymour, G. B., Taylor, J. E. & Tucker, G. A. (Eds.), Biochemistry of fruit ripening (pp. 1-51). Chapman and Hall, London. [ Links ]
Ulrich, R. (1971). Organic acids. En: Hulme, A. C. (Ed.), The biochemistry of fruits and their products, vol. 1. (pp. 98-118). Academic Press, London, New York. [ Links ]
Usenik, V., Fajt, N., Mikulic-Petkovsek, M., Slatnar, A., Stampar, F., & Veberic, R. (2010). Sweet cherry pomological and biochemical characteristics influenced by rootstock. Journal of Agricultural and Food Chemistry, 58(8), 4928-4933. doi: 10.1021/jf903755b [ Links ]
Vadivel, E., & Shanmugavelu, K. G. (1978). Effect of increasing rates of potash on banana quality cv. Robusta. Revue de la Potasse, 24, 1-4. [ Links ]
Veit-Köhler, U., Krumbein, A., & Kosegarten, H. (1999). Effect of different water supply on plant growth and fruit quality of Lycopersicon esculentum. Journal of Plant Nutrition and Soil Science, 162(6), 583-588. doi: 10.1002/(SICI)1522-2624(199912)162:6<583::AID-JPLN583>3.0.CO;2-P [ Links ]
Walker, R. P., & Chen, Z. H. (2002). Phosphoenolpyruvate carboxykinase: structure function and regulation. Advances in Botanical Research, 38, 93-189. doi: 10.1016/S0065-2296(02)38029-7 [ Links ]
Walker, R. P., Battistelli, A., Moscatello, S., Chen, Z. H., Leegood, R. C., & Famiani, F. (2011a). Phosphoenolpyruvate carboxykinase in cherry (Prunus avium L.) fruit during development. Journal of Experimental Botany, 62(15), 5357-65. doi: 10.1093/jxb/err189 [ Links ]
Walker, R. P., Famiani, F., Baldicchi, A., Cruz-Castillo, J. G., & Inglese, P. (2011b). Changes in enzymes involved in photosynthesis and other metabolic processes in the fruit of Opuntia ficus-indica during growth and ripening. Scientia Horticulturare, 128(3), 213-219. doi: 10.1016/j.scienta.2011.01.017 [ Links ]
Walton, E. F., & de Jong, T. M. (1990). Estimating the bioenergetic cost of a developing kiwifruit berry and its growth and maintenance respiration components. Annals of Botany, 66(4), 417-424. [ Links ]
Wang, S. Y., & Camp, M. J. (2000). Temperature after bloom affects plant growth and fruit quality of strawberry. Scientia Horticulturare, 85(3), 183-199. doi: 10.1016/ S0304-4238(99)00143-0 [ Links ]
Whiting, G. C. (1958). The non-volatile organic acids of some berry fruits. Journal of the Science of Food and Agriculture, 9(4), 244-248. doi: 10.1002/jsfa.2740090411 [ Links ]
Widodo, S. E., Shiraishi, M., & Shiraishi, S. (1995). Organic acids in the juice of acid lemon and Japanese acid citrus by gas chromatography. Journal of the Faculty of Agriculture, Kyushu University, 40(1-2), 39-44. [ Links ]
Wu, B. H., Genard, M., Lescourret, F., Gomez, L., & Li, S. H. (2002). Influence of assimilate and water supply on seasonal variation of acids in peach (cv Suncrest). Journal of the Science of Food and Agriculture, 82(15), 1829-1836. doi: 10.1002/jsfa.1267 [ Links ]
Wu, J., Gao, H., Zhao, L., Liao, X., Chen, F., Wang, Z., & Hu, X. (2007). Chemical compositional characterization of some apple cultivars. Food Chemistry, 103(1), 88-93. doi: 10.1016/j.foodchem.2006.07.030 [ Links ]
Xin-Juan, X., Qing-Yu, L., Xiao-Hui, S., Shen, Q. R., & Dong, C. X. (2012). Dynamic regulation of nitrogen and organic acid metabolism of cherry tomato fruit as affected by different nitrogen forms. Pedosphere, 22(1), 67-78. doi: 10.1016/S1002-0160(11)60192-6 [ Links ]
Yakushiji, H., Morinaga, K., & Nonami, H. (1998). Sugar accumulation and partitioning in Satsuma mandarin tree tissues and fruit in response to drought stress. Journal of the American Society for Horticultural Science, 123(4), 719-726. [ Links ]
Yang, W. H., Zhu, X. C., Bu, J. H., Hu, G. B., Wang, H. C., & Huang, X. M. (2009). Effects of bagging on fruit development and quality in cross-winter off-season longan. Scientia Horticulturae, 120(2), 194-200. doi: 10.1016/j.scienta.2008.10.009 [ Links ]
Yao, Y. X., Li, M., Liu, Z., You, C. X., Wang, D. M., Zhai, H., & Hao, Y. J. (2009). Molecular cloning of three malic acid related genes MdPEPC, MdVHA-A, MdcyME and their expression analysis in apple fruits. Scientia Horticulturae, 122(3), 404-408. doi: 10.1016/j.scienta.2009.05.033 [ Links ]














