Introduction
Since prehistoric times, human beings from different cultures have used plants as medicinal sources. Until the 19th century, physicians had used them as the main source of pain relief for their patients. Today, the World Health Organization estimates that about 80 % of people in developing countries still use traditional medicine. Medicinal plants have shown numerous unique and interesting pharmacological properties, including antimicrobial properties[1] [2]. Plants synthesize a wide variety of metabolites to protect themselves and maintain homeostasis in their environment. Often, these secondary metabolites differ among plant species in their quantity, diversity, and biological activities[3].
Rosemary (Rosmarinus officinalis L. syn Salvia rosmarinus Spenn.) is a plant that grows wild in the Mediterranean basin. It is a perennial and aromatic plant belonging to the Lamiaceae family and recently merged with the genus Salvia, so it is now known as Salvia Rosmarinus[2] [4]. It can reach 150 cm in height and is a lush, branched, and evergreen shrub. S. rosmarinus is traditionally used as a culinary species to modify or improve food flavors and other organoleptic properties. It is also used in traditional and folk medicine, being a highly valued medicinal herb. About 20 types or varieties of S. rosmarinus can be distinguished according to morphological descriptors; however, the infraspecific systematics are confusing and uncertain[5] [6].
Currently, S. rosmarinus is one of the most important major sources of naturally occurring biologically active compounds in the functional food industry[7] [8]. However, this plant possesses many pharmacological activities such as hepatoprotective, antimicrobial, antiulcerogenic, antidiabetic, diuretic, anti-inflammatory, anticarcinogenic, and antioxidant properties. Most of these activities are related to the phenolic content in this shrub. Significantly, the potent antioxidant activity is primarily due to phenolic diterpenes, such as carnosol, carnosic acid, rosmadial, or rosmanol, among others[9].
Botanical description
S. rosmarinus. taxonomic series No.: 32677 (ITIS, 2023), belongs to the Lamiaceae family, formerly called Labiatae, and is known by the popular name Rosemary in English, Alecrim in Portuguese, and Romero in Spanish. It is a xeromorphic shrub that grows spontaneously in stony places, sand, cliffs, and sea fences in different parts of the world, such as Europe, Africa, America, and Asia. The shrub is fragrant, with leaves characterized by being strongly curved, linear, and aromatic. The upper part of the leaf has a very intense green hue, while the lower area tends to be gray, with a width of about 4 cm, an average size between 1.0-2.5 cm long, and a thickness ranging from 1-3 mm. The flowers are small and arranged in axillary pauciflorus whorls, light blue, or lilac, both having an intense and fragrant aroma. This aroma is due to the volatile oil accumulated in various parts of the flower, such as the glandular trichomes, petals, and capitate. In Figure 1, the plant of S. rosmarinus is presented[10] [11].
Chemical composition
The components that confer pharmacological properties to S. rosmarinus are classified into flavonoids, terpenoids (sesquiterpenes, triterpenes, diterpenes, monoterpenes), and hydroxycinnamic derivatives[9]. It has been reported that S. rosmarinus extracts contain about 24 % of volatile molecules belonging to terpenes, while in the non-volatile fraction, flavonoids appear along with non-volatile terpenoids and phenolic acids. Phytochemical studies have indicated that the concentration of non-volatile compounds varies between 1.8 % and 2.5 %, depending on the region. The predominant constituents are α-pinene, 1,8-cineole, limonene, camphor, and borneol. These differences arise due to the form of cultivation, the growing cycle, and the aging that the plant undergoes at the time of cutting[12] [13].
On the other hand, among the most reported chemical compounds in S. rosmarinus extracts are rosmarinic acid (RA), chlorogenic acid, caffeic acid, ρ-coumaric acid, carnosic acid (CA), oleanolic acid, betulinic acid, ursolic acid, rosmanol, and carnosol (CR), among other di- and triterpenoids. In such a way, the most involved compounds in various investigations are CA, CR, and AR. This is because these chemical compounds are the main ones responsible for the medicinal activities of the extracts[5] [14] [15].
The chemical composition of the essential oil of S. rosmarinus has been described in a general way by various researchers who have reported the identification of various chemical molecules such as camphene, α-pinene, and β-pinene, as well as some terpenes like camphor, 1,8-cineole, verbinol, borneol, linalool, rosmanol, terpineol, carnosol, isorosmanol, α-amyrin, β-amyrin, and β-caryophyllene. Additionally, vanillic, chlorogenic, caffeic, ursolic, rosmarinic, carnosic, butylinic, oleanolic, betulinic acids are also identified and different chemical molecules such as botulin, bornyl acetate, 3-octanone, isobanylacetate[16].
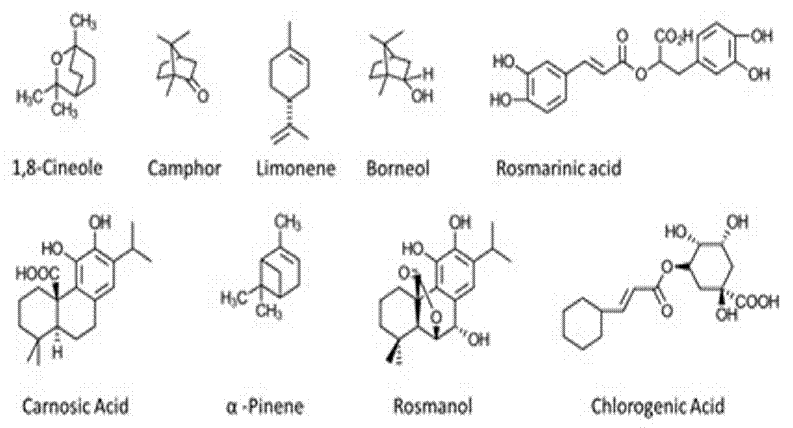
The different fractions, both volatile and non-volatile, vary greatly depending on the plant due to various factors such as climatic changes, age, region of procurement, and season of the year. Similarly, the concentration of these compounds is not evenly distributed throughout the plant. For example, CA and CR can be found mainly in the photosynthetic tissues of the plant, such as sepals and petals. Several fractions are also significantly affected by the type of processing for the extraction of bioactive compounds. Therefore, the conditions for obtaining these bioactive compounds are crucial, including temperature, type of solvent or extracting agent, and time[9]. Figure 2 shows the structures of some of the chemical compounds present in S. rosmarinus.
Culinary uses
Generally, this plant finds use in Mediterranean cuisine as a condiment contributing flavor to food. The two fundamental ways in which fresh or dried leaves are utilized in the kitchen involve imparting a bitter, astringent, and highly aromatic flavor. Different preparations, such as pork, fish, meat, poultry, soups, stews, dressings, sauces, and various preserves, often see the addition of S. rosmarinus. Due to the drying of the leaves, some attributes, like the fresh aroma, are lost. However, the dried leaves gradually lose their quality of life, rendering them highly favorable for storage and transportation. This characteristic transforms them into a profitable product in the market. As a result, the S. rosmarinus plant has been employed in the food industry to enhance the shelf life of foods, add value, and improve their quality[14] [15].
Food industry
Since ancient times, S. rosmarinus has maintained great popularity within the food industry because it contains several bioactive compounds that provide food with antioxidant activity. Some of the compounds responsible include rosmarinic acid, carnosic acid, carnosol, rosmariquinone, rosmanol, and rosmaridiphenol, which can undergo reactions with free radicals formed in oxidation processes. Nowadays, foods containing these types of compounds are of great interest because an increasing number of consumers are opting for foods containing natural antioxidants, considering them to be healthy or "non-chemical"[16] [17].
In 2015, the extension of the shelf life and quality of refrigerated fillets of Nile tilapia (Orechromis niloticus) by immersing them in a methanolic extract of S. rosmarinus at 1.5 % was reported by Khalafalla et al., The result was efficient antioxidant activity with a clear reduction in the value of TBA-RS (a reagent composed of 2-thiobarbituric acid and glacial acetic acid), prolonging the shelf life of these fillets for up to 9 days more than the control[18].
In 2016 in Portugal, a study was reported on the antimicrobial effect provided by the essential oil of S. rosmarinus and thyme in foods packaged using the Sous vide cook-chill (SVCC) technology. This technology is characterized by vacuum packaging of raw or partially prepared foods before pasteurization, followed by rapid cooling and storage below 3 °C. In 2016, Gouveia et al., mentioned that at 2 °C, the samples containing thyme essential oil presented a reduction in the population of L. monocytogenes; however, it was lower than that observed in S. rosmarinus oil. Likewise, the latter managed to show inhibition towards L. monocytogenes at a temperature of 8 °C for up to a maximum of 14 days. Furthermore, they highlighted that this dangerous pathogen is found in some "Ready-to-eat" foods and depends on the number of additives present in the food, as well as the temperatures used for storage[19].
In 2016, the antimicrobial Activity against C. perfringens of six essential oils usually used as condiments in Brazil (rosemary, basil, marjoram, mint, thyme, and anise) was described by Radaelli. It was pointed out that the use of such oils from commonly employed spices clearly offers an alternative to chemical preservatives for the inactivation and control of pathogens in food. It was also reported that their results suggest that oxygenated compounds, especially monoterpenes and phenylpropanoids, may be responsible for carrying out the antimicrobial Activity. However, a synergistic effect of these chemical compounds with other constituents present in smaller amounts in the essential oil is also considered[20]. The main objective of this research is to provide an overview of S. rosmarinus, its main applications, and the implementation of ultrasound extraction for obtaining bioactive compounds present in this plant. This positions this technique as a green and alternative methodology for the extraction of phytochemical compounds.
Materials and methods
The present research was carried out based on a literature search in various databases such as: Scifinder, PubMed, Scopus, ScienceDirect, looking for articles related to S. rosmarinus. The search criteria were keywords such as: Rosemary, S. rosmarinus, ultrasound, green extraction, secondary metabolite.
Results and discussion
Bioactive compounds of plant origin
In nature, unlike animals, plants are stationary and are not exempt from aggressive environmental changes. They are also exposed to harmful microorganisms that become aggressive pathogens, insect pests, parasitic plants, and weeds. This is not to mention temperature changes, mineral and nutrient deficiencies, osmotic imbalance, and contamination by heavy metals. Therefore, plants have a wide range of cellular, molecular, and biochemical protective mechanisms. The metabolic pathways of plants synthesize two types of organic compounds of natural origin or metabolites: (i) primary plant metabolites (PPM), and (ii) secondary plant metabolites (SPM). PPM are produced in large quantities and are responsible for maintaining various vital processes in plants, such as photosynthesis, respiration, growth, and development. Some prominent PPMs include carbohydrates, lipids, proteins, and hormones. They also participate in the primary response by regulating these biomolecules against pathogen infection[12]
In contrast, SPM are produced in minimal quantities, however, they have fundamental functions in plant adaptation to unfavorable conditions. They include various groups of chemical compounds such as alkaloids, flavonoids, anthocyanins, lignans, quinones, peptides, phenols, terpenoids, and amines. These compounds are primarily used in some pesticides, agrochemicals, and food additives. Moreover, they are directly or indirectly related to taste, color, and aroma. Secondary metabolites are synthesized by various pathways in plants and are directly involved in the plant's defensive reaction to an external stimulus[13].
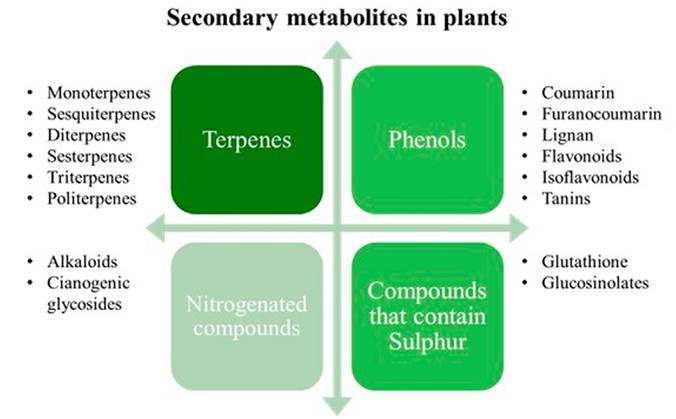
At present, about 200,000 secondary metabolites (MSPs) have been identified, which are divided into four main groups: terpenes, phenols, and nitrogen/sulfur-containing substances. The most important functions of secondary metabolites lie in plant protection[21]. In Figure 3, a classification of the four main groups of secondary metabolites in plants is presented. In Table 1. Main types of the most reported secondary metabolites in the literature for S. rosmarinus. as well as their biological activity are presented.
Table 1 Secondary metabolites for S. rosmarinus.
| Structure | Compound | Biological Activity | Ref. |
|---|---|---|---|
|
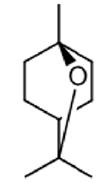
1,8-cineole | Antimicrobial Activity, Proliferative and antioxidant activity | [22] [23] [24] [25] |
|
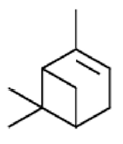
α-pinene | Antiproliferative Activity, Antimicrobial Activity, Antioxidant activity, Green pesticide and anticarcinogenic Activity | [26] [27] [23] [28] [29] [24] |
|
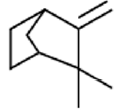
Camphene | Fumigant activity, Anticarcinogenic activity | [30] [31] |
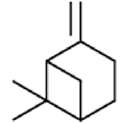
|
β-pinene | Antioxidant activity, Photoprotective activity, Insecticidal and repellent activity | [24] [29] [32] |
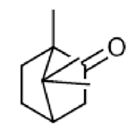
|
Camphor | Anticancer Activity, Antimicrobial Activity, Antimicrobial Activity, Antimicrobial Activity, Antioxidant activity, Food preservative | [33] [34] |
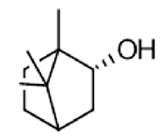
|
Borneo | Anticancer Activity, Antioxidant activity, Anti-inflammatory Activity, Analgesic Activity | [35] [2] |
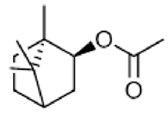
|
Bornyl acetate | Immunomodulatory Activity, Anti-inflammatory Activity, Sedative Activity | [36] |
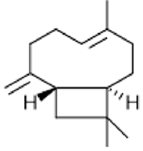
|
β-caryophyllene | Anticoccidial Activity, pesticide, Proliferative Activity, Activity against cardiac hypertrophy | [37] [38] [25] |
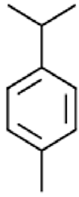
|
P-cymene | Antioxidant activity, Anti-inflammatory Activity, Antiparathyroid Activity, Antiproliferative Activity and Antidiabetic Activity. | [39] |
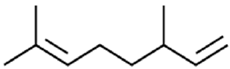
|
β-myrcene | Chemoprophylactic Activity | [40] |

|
Limonene | Antiproliferative Activity, Photoprotective Activity, Anticancer Activity, Anticancer Activity, Antioxidant Activity | [41] [32] |

|
γ-terpinene | Antioxidant Activity, Photoprotective Activity | [41] [42] |

|
Geraniol | Bacterial Activity, Antimicrobial Activity, Antimicrobial Activity, Fungicidal Activity, Anti-inflammatory Activity | [43] |

|
Carnosol | Anticarcinogenic, antimicrobial, antiinflammatory | [44] [45] |
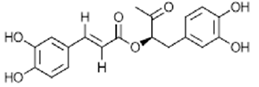
|
Rosmarinic Acid | Antioxidant, anti-inflammatory, antimicrobial | [46] [47] [48] |
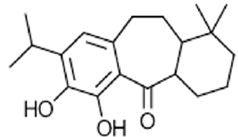
|
Rosmaridiphenol | Anti-tumor, anti-inflammatory, neuroprotective | [49] |
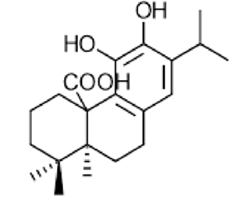
|
Carnosic acid | Antioxidant, anti-inflammatory, antimicrobial | [46] [47] [48] [50] |
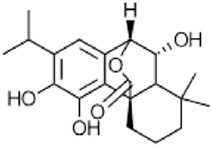
|
Isorosmanol | Antimicrobial, anti-inflammatory, anticarcinogenic | [51] |

|
Elenolic acid | Antioxidant, anti-inflammatory, antimicrobial | [52] |

|
Rosmanol | Anticarcinogenic, antimicrobial, anti-inflammatory | [53] |

|
Ursolic acid | Antioxidant, anti-inflammatory, anticarcinogenic | [54] |

|
Oleanolic acid | Anti-tumor, anti-inflammatory, neuroprotective | [55] |
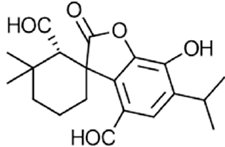
|
Rosmadial | Antioxidant, anti-inflammatory, antimicrobial | [56] [57] [48] |
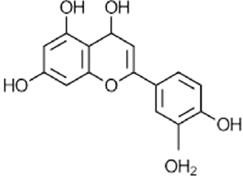
|
Luteolin | Antioxidant, anti-inflammatory, anticarcinogenic | [56] |
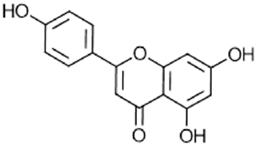
|
Apigenin | Anticarcinogenic, antimicrobial, antiinflammatory | [56] |
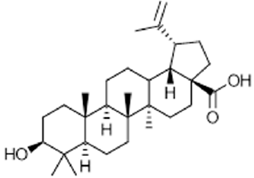
|
Betulinic acid | Anti-tumor, anti-inflammatory, neuroprotective | [58] [59] |

|
Quercetin | Anticarcinogenic, antimicrobial, antiinflammatory | [60] [61] |

|
Caffeic acid | Anticarcinogenic, antimicrobial, anti-inflammatory | [46] [57] |

|
Genkwanin | Antioxidant, anti-inflammatory, anticarcinogenic | [56] |

|
Colorogenic acid | Anticarcinogenic, antimicrobial, anti-inflammatory | [62] [63] |
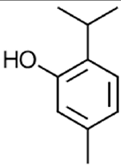
|
Thymol | Antioxidant, antimicrobial, anti-inflammatory, anticarcinogenic | [64] |
Anticarcinogenic activity
The constitution of the human diet is one of the most substantial factors influencing the risk of developing cancer; the components of the diet can contribute either positively or negatively to the likelihood of contracting such a condition. Chemoprevention is the long-term pharmacological control of the risk of cancer, for which a myriad of plants and their components have been analyzed for their potential anticarcinogenic Activity. Around 60 % of the drugs used today in cancer treatment are derived from natural products[65] [66].
As is well known, the S. rosmarinus plant exhibits significant antioxidant activity, inhibiting genotoxicity and providing protection against carcinogens or toxic agents. However, the side effects of therapeutic methods largely hinder their effectiveness, increasing the demand for new research on more efficient methods and treatments in the fight against cancer[67].
Polyphenols are chemical compounds capable of inducing cell differentiation and modulating growth, causing interference in tumor development and progression. Since S. rosmarinus is rich in various phenolic compounds, numerous studies focus on the anticarcinogenic Activity that this plant may exhibit. Some diterpenic polyphenols, such as carnosol and carnosic acid, are chemical compounds present in the dried leaves of S. rosmarinus at a 5 % concentration. It is reported that these compounds are largely responsible for the anticarcinogenic Activity[25] [68].
Antioxidant capacity
Natural plant-derived antioxidants are becoming increasingly important, not only around food preservation and stability but also in preventive medicine. The Lamiaceae family has been highly relevant in research on antioxidant compounds, thanks to the high concentration of polyphenols. Antioxidants play a crucial role in the prevention and treatment of diseases associated with oxidative damage, such as cancer, cardiovascular diseases, and neurodegenerative diseases. Free radicals, reactive oxygen species, and hydrogen peroxide are inevitably produced by living organisms in metabolic processes. Continuous exposure to free radicals causes functional and structural damage, such as aging and cell death[69].
The antioxidant capacity of S. rosmarinus is due to the phenolic compounds present in the plant, which can inhibit the production of reactive species. The accumulation of these species has a negative effect on various organs and even systems of the human body[70]. Various studies have demonstrated that this property is directly related to the concentration of phenolic diterpenes. However, the content of these antioxidant molecules depends on different factors, including seasonal variations, environmental influences, species, origin, and plant growth. As mentioned earlier, antioxidants are chemical compounds that can inhibit and delay the oxidation of different lipids and biomolecules. They prevent the initiation of a chain reaction by radicals or propagation, which can cause functional damage in the human body, such as cancer or cardiovascular diseases. An antioxidant can prevent such processes due to its redox properties, such as hydrogen donation, reducing behavior, or quenching of singlet molecular oxygen[71] [72].
In 2008, Wang et al., examined the in vitro antioxidant activity of the essential oil of S. rosmarinus obtained through steam distillation, compared to three of its main components (α-pinene, 1,8-cineole, β-pinene). The oil and components were subjected to evaluation of their antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, with ascorbic acid used as a positive control. The inhibition percentages of the radical were 62.45 %, 42.7 %, 45.61 %, and 46.21 % for the essential oil of S. rosmarinus, α-pinene, 1,8-cineole, and β-pinene, respectively. In general, the essential oil showed higher antioxidant activity than its components[73].
In 2015, Chen et al., used the ABTS (2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)) and FRAP (ferric ion reduction) techniques to assess the antioxidant activity of aqueous extracts of S. rosmarinus[72]. In 2023, Perales and collaborators demonstrated the antioxidant capacity of S. rosmarinus using the DPPH technique. The plant was collected from Iturbide, Nuevo León. They obtained the extracts through ultrasound-assisted maceration at 40 KHz for 60 minutes at room temperature, with a ratio of 1:12.5, solid: liquid. Antioxidant capacity was measured at 519 nm using a spectrophotometer, and ascorbic acid was used as a positive control. Subsequently, serial dilutions were performed, and the extracts were evaluated in triplicate. The authors successfully demonstrated an antioxidant capacity of 31.653 (IC50(μg/mL)), even higher than that of the ascorbic acid control[68].
Antioxidant capacity
Most plants produce secondary metabolites with antimicrobial Activity in response to the attack of pathogens or their normal course of growth and development. The increasing use of essential oils or aqueous extracts represents a new way to combat the proliferation of microorganisms. The escalating use of antibiotics in medicine, agriculture, and livestock has contributed to the growing resistance of various microorganisms to drugs. Drug resistance has been categorized as a global health problem, prompting an increasing number of researchers to delve into the investigation of new bioactive antimicrobial compounds. Several studies have examined the antimicrobial capacity of the S. rosmarinus plant against a broad spectrum of gram-positive bacteria (S. aureus, S. pyogenes, S. pneumoniae) and gram-negative bacteria (E. coli, S. typhi)[74] [75].
Its antimicrobial Activity is primarily attributed to carnosol, carnosic acid, and rosmarinic acid. S. rosmarinus extracts have bioactive properties and antimicrobial Activity, stimulating anticarcinogenic Activity and phagocytosis by inactivating adhesins, enzymes, and transport proteins in bacteria. Flavonoids tend to form soluble protein complexes in the bacterial wall, which destroy it in response to microbial infection. On the other hand, essential oils exhibit antiseptic and antimicrobial capabilities attributed to bioactive compounds present in this plant, such as camphor, α-pinene, 1,8-cineole, eucalyptol, verbenone, limonene, borneol, and camphene. The mechanism of action of diterpenes involves lysing the bacterial cell membrane due to their lipophilic compounds, including some triterpene alcohols like alpha and beta-amyrin, as well as acids such as oleanolic acid and ursolic acid[76].
The S. rosmarinus extract acts at the cellular membrane level, increasing permeability and causing distortion of the cell wall in both Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria. The following are descriptions of some in vitro studies demonstrating the antimicrobial capacity of the S. rosmarinus plant. In 2010, Castaño et al., demonstrated the antimicrobial activity of the essential oil and ethanolic extract of S. rosmarinus, obtained through steam distillation and maceration, respectively, against Gram-positive and Gram-negative bacteria, including S. aureus, E. coli, S. sonnei, S. typhimurium, P. aeruginosa, and L. monocytogenes, using the minimum inhibitory concentration (MIC) technique through microdilution colorimetry in broth. For the ethanolic extract, they subjected dried S. rosmarinus leaves to 95 % ethanol for 48 hours, followed by preconcentration using a rotary evaporator and finally lyophilization. Meanwhile, for the oil, dehydrated leaves were used, and the oil was obtained by steam distillation. The inoculum was obtained from an exponentially growing culture, and an aliquot of the inoculum was adjusted to 0.5 McFarland (1.5 x 108 CFU/mL). The essential oil exhibited antimicrobial Activity against a wide variety of Gram-positive and Gram-negative bacteria. The growth inhibition of Escherichia coli was achieved at a concentration of 4096 ppm, while for Shigella sonnei and Staphylococcus aureus, it was at 512 ppm. On the other hand, in the ethanolic extract, concentrations of 1024 ppm were obtained for S. sonnei, S. typhimurium, and L. monocytogenes. Greater sensitivity was observed in Gramnegative bacteria compared to Gram-positive ones, and the compound with the broadest inhibition spectrum was the essential oil, attributed to increased permeability and changes in the structure of the cell membrane[77].
Solano and Zambrano, in 2016, demonstrated the inhibition of the oil and aqueous phase extracts of S. rosmarinus against S. mutans. They used dried leaves of S. rosmarinus, which were macerated with distilled water for three days with continuous agitation at room temperature. Subsequently, the extract was filtered, and the water was evaporated for 18 hours at 70 °C. On the other hand, the oily extract was obtained through steam distillation. To establish the concentration of the extracts, the broth dilution method was performed to obtain the minimum bactericidal concentration (MBC). They observed that the aqueous extracts showed no inhibition against Streptococcus mutans, while the oily extracts exhibited inhibitions of 11.93 mm[78].
Montero et al., in 2017, demonstrated the antimicrobial Activity of the oily extract of the S. rosmarinus plant against E. coli, obtaining inhibition zones of 10.90 mm at a concentration of 80 %[79].
On the other hand, Karadag et al., in 2019, for the antimicrobial tests, ground S. rosmarinus leaves with methanol for 24 hours, filtered and evaporated, to obtain subfractions. These subfractions were prepared using liquid-liquid extraction with hexane and ethyl acetate, respectively. These authors demonstrated that the hexane fractions are susceptible to S. aureus, obtaining a minimum bactericidal concentration of 500 μg/mL[2].
Pharmacological and therapeutic studies
Medications and plant-based treatments for the management of various diseases are complementary approaches in medicine due to their few side effects[80]. Filiptsova et al., in 2018, conducted a study to observe the effects of lavender and S. rosmarinus essential oils, obtained through steam distillation, on short-term human memory. They obtained the oils using steam distillation. The study involved 79 high school students (34 boys and 45 girls) aged 13 to 17 years, residing in the Ukrainian metropolis. Participants were divided into 3 groups: the control group (not exposed to any oil), the group sprayed with lavender oil, and the S. rosmarinus group. A standard Petri dish was placed in each corner of the room, filled from the bottom with tap water at room temperature in a volume of 15 mL, and 10 drops of each essential oil were added. Statistically significant differences were found in the short-term memory productivity of participants in different groups. Therefore, S. rosmarinus and lavender essential oils significantly increased image memory compared to the control group. Inhalation of S. rosmarinus essential oil increased the memorization of numbers, while inhalation of lavender oil weakened the process[81].
El-Desouky et al., in 2019, demonstrated the nephroprotective effect of green tea extract (GTE), rosmarinic acid (RA), and S. rosmarinus (RE) on acute renal toxicity initiated by N-nitrosodiethylamine (DEN) and promoted by ferric nitrilotriacetate (Fe-NTA) in Wistar rats. S. rosmarinus extract countered the initiation of diethylnitrosamine and ferric nitriloacetate-induced nephrotoxicity in rats. Rats were classified into 5 groups: Group 1 included healthy rats, Group 2 received DEN+Fe-NTA, Group 3 received 200 mg/kg body weight of RE-DEN-Fe-NTA, Group 4 received 1 g/kg of GTE-DEN-Fe-NTA, and Group 5 received 50 mg/kg of RA+DEN+Fe-NTA. RE, GTE and RA were administered orally for 14 days before a single intraperitoneal administration of DEN (160 mg/kg) until the end of the experiment. Eighteen days after DEN, a single intraperitoneal dose of Fe-NTA (5 mg Fe/kg) was administered to promote nephrotoxicity in rats. The kidneys of each group were histopathologically examined at intervals. GTE, RA, and RE exerted a protective effect against renal toxicity, with GTE showing a more pronounced effect on renal function parameters, while RA showed the best protective results[82].
Jaglanian and Tsiani, in 2020, demonstrated the inhibition of proliferation and survival of prostate cancer cells by acting on Akt (protein kinase B) and mTOR (protein). They used a methanolic extract of S. rosmarinus obtained using an ultrasonic probe. They found a significant inhibition of survival and proliferation of androgen-sensitive PC-3 prostate cancer cells, while normal prostatic epithelial cells PNT1A were not affected. Furthermore, treatment with S. rosmarinus induced apoptosis and reduced the migration of PC-3 prostate cancer cells[8].
Makaremi et al., in 2021, inhibited tumor growth in mice with colorectal cancer CT-26 using alcoholic extracts of S. rosmarinus obtained through ultrasound-assisted extraction. These authors conducted the measurement of cell cytotoxicity through the MTT colorimetric assay, showing a concentrationdependent increase in cytotoxicity in the CT-26 cell group. Cell treatment with half the inhibitory concentration (IC50) of S. rosmarinus, turmeric, and the combination induced apoptosis. In vivo studies revealed that the combined treatment (S. rosmarinus with turmeric) inhibited tumor growth in mice transplanted with CT-26 cells without side effects, such as weight loss or renal and hepatic functional changes. Additionally, mice treated with the plant mixture exhibited a significant increase in the proportion of cytotoxic T lymphocytes. In conclusion, the results showed that the combination of S. rosmarinus with turmeric has the potential to inhibit the growth of the CT-26 cancer cell line, reducing tumor growth in mice with colorectal cancer[83].
Cytotoxicity of S. rosmarinus
It has been reported that the main bioactive compounds contained in the plant, such as rosmarinic acid (RA), chlorogenic, caffeic, and ρ-coumaric acids, carnosic acid (CA), oleanolic acid, betulinic acid, ursolic acid, rosmanol, and carnosol (CR), exhibit no toxicity. However, some individuals sensitive to the bioactive compounds in this plant may experience allergies or contact dermatitis. Similarly, the use of this plant is not recommended for individuals with conditions such as gallstones without prior consultation with a doctor. While intoxication from S. rosmarinus infusions is not common, an overdose could lead to consequences such as vomiting, hemorrhages, uterine gastroenteritis, renal irritation, and abdominal spasms. Regarding the essential oil of S. rosmarinus, in much higher concentrations, it can be toxic to the central nervous system and may induce seizures[5] [84].
Extraction technologies
In recent years, a wide variety of methods have been used for obtaining bioactive compounds from different plants, such as conventional heating extraction, aqueous alkaline extraction, solid-liquid extraction, extraction with conventional or traditional solvents, extraction with vegetable oils, and extraction using emerging technologies such as microwave and ultrasound, among others. However, extraction methodologies that are more environmentally friendly and do not compromise bioactive compounds during the extraction process have been preferred[85]. To achieve this, a possible solution could involve the use of emerging techniques and the implementation of green solvents. Table 2 shows the advantages and disadvantages of conventional methods compared to ultrasound.
Table 2 Advantages and disadvantages of conventional methods compared to ultrasound.
| Extraction | Advantages | Disadvantages |
|---|---|---|
| Soxhlet extraction | High extraction efficiencies, no filtration or centrifugation required. | Use of non-polar organic solvents, thermal degradation of bioactive compounds, solvent boiling temperatures. |
| Maceration | Variety of solvents, simplicity of operation. | Long extraction times (hours weeks), requires filtration, high extraction temperatures. |
| Infusion | Extraction of soluble and volatile compounds, variety of temperatures, shorter extraction times compared to maceration. | Long extraction times (hours), concentration of the extract obtained and filtration. |
| Decoction | Implementation of hard samples (seeds and stems). | Limited to thermostable and water-soluble bioactive compounds, high temperatures (boiling). |
| Conventional heating | Extraction of oils and bioactive compounds, rapid extraction. | High temperatures, use of toxic solvents, high energy requirements and decomposition of thermally sensitive components. |
| Ultrasonic extraction | Use of safe solvents, room temperature, low energy requirement, high extraction efficiencies, short extraction times (minutes). | Liquid samples, solvent required, difficult extraction process for viscous samples. |
The extraction of bioactive and phytochemical compounds from plants is relevant due to the range of applications in the therapeutic, pharmaceutical, and food industries. This leads to the search for more efficient techniques that aid in the extraction of purer compounds with higher extraction yields. Several factors have been reported to limit or favor extraction techniques, such as the source or raw material, temperature, and solvent used. The current interest is in developing efficient and environmentally friendly techniques. Therefore, there is a notable focus on green or emerging techniques, including ultrasound-assisted extractions, supercritical fluids, pressurized liquids, microwaves, and cold plasma. These techniques, whether used individually or in combination, offer significant advantages over conventional methods, such as reducing high concentrations of solvents, shorter extraction times, lower temperatures, and higher quantitative and qualitative yields[86] [87]. As described by Chemat, "Green extraction is based on extraction methodologies and processes that reduce energy consumption, allow the use of alternative solvents, and produce renewable natural products, ensuring a safe and highquality extract or product."[88], Ultrasound extraction addresses some of the limitations of conventional techniques and incorporates key points of "green extraction"[89].
Ultrasound
Richards and Loomis first studied the effects of ultrasound in 1927, successfully solubilizing dimethyl sulfate in an alkaline solution. The case was forgotten for about 60 years; however, around the 1980s, sonochemistry experienced a revival and was highly implemented in different areas. Most modern devices rely on transducers (converters of electrical or mechanical energy into sound energy) for the generation of ultrasonic energy, which are made up of piezoelectric materials. If ultrasound is applied to a system, chemical changes can occur due to the generation and implosion of cavitation bubbles[90] [91].
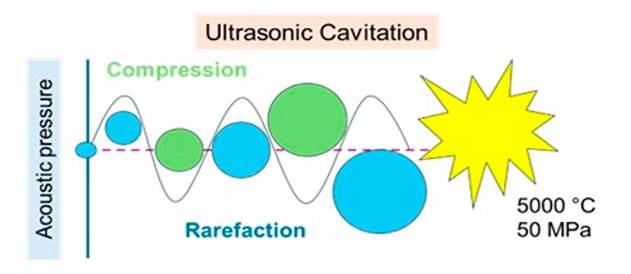
The cavitation phenomenon was first identified and reported in 1895 by Thorneycroft and Barnaby. It is based on the formation of cavitation bubbles, which grow until they collapse due to the pressure formed by their surroundings, as shown in Figure 4. Cavitation bubbles generate local heating and high pressure, known as a hot spot[92].
Ultrasound is defined as sound of high frequency, which is above the limit at which the human ear can respond. The range of human hearing is generally considered to be between 16 Hz and 18 kHz, while ultrasound is typically considered to have frequencies between 20 kHz and 100 MHz. Sonochemistry usually operates in the range of around 20 to 40 kHz, although current sonochemical research explores broader ranges[93].
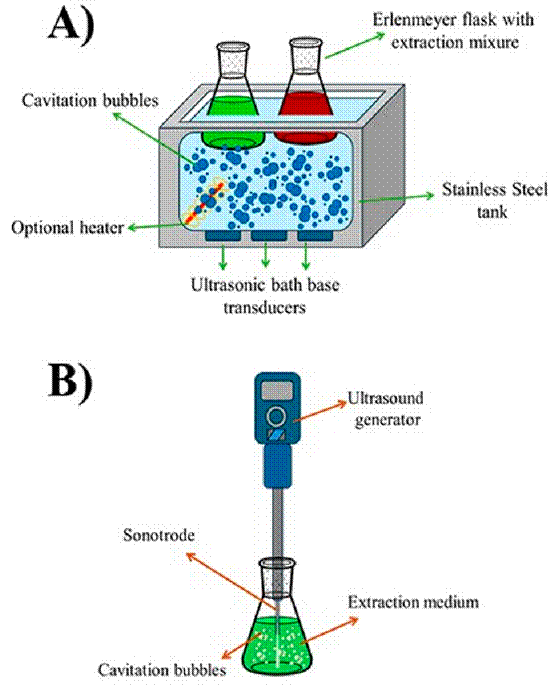
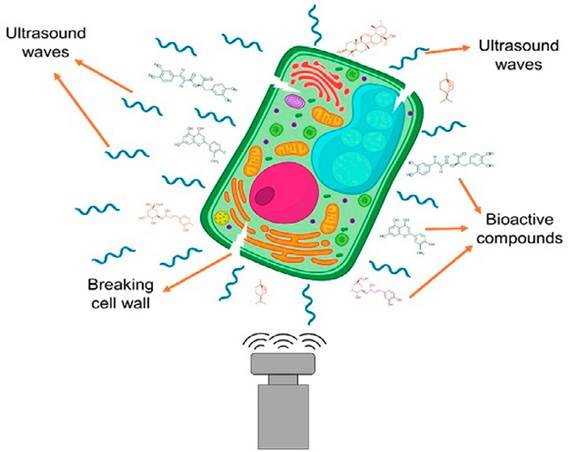
The ultrasound equipment was designed based on a piezoelectric transducer to treat solid materials in a liquid medium, such as ultrasound baths and ultrasound probes, as shown in Figure 5. Ultrasound baths have been widely used in industries for cleaning and extracting bioactive compounds, such as in the pharmaceutical, cosmetic, and ornamental industries. They consist of a transducer, a tank, a timer probe, and a heater. Indirect sonication is more effective as it is non-invasive, does not affect the degradation of bioactive compounds, and eliminates foam formation. On the other hand, direct sonication using an ultrasound probe is the most commonly used method, as it directly transfers ultrasound intensity to the sample, resulting in higher extraction efficiency and significantly reducing the time compared to the bath[94] [95].
Some of the most important factors to consider when implementing this technique for the extraction of bioactive compounds include considering the equipment's power, also expressed as the percentage of amplitude ranging from 0 to 100 %. This amplitude is linear to power. It has been reported that the power used for each selected plant sample depends entirely on the type of sample. However, working with powers in the range of 20 to 70 W and amplitudes of 30 to 80 %, the extraction yield increases with the increase in power and subsequently decreases after reaching a peak. This is explained by the increase in violent cavitation effects, which increases with the power. The size of the resonant bubble is directly proportional to the power, and as it increases, the size of the bubble and its implosion also intensify. This leads to greater sample fragmentation, pore formation, and higher extraction yields[96].
Other factors to consider include the equipment frequency, duty cycles, relaxation, extraction time, as well as the solvent-solid ratio and temperature. However, these factors show a trend where, as time, solvent-solid ratio, or frequency increases, the extraction percentages also increase until reaching a peak, after which they start to decrease. Therefore, optimal extraction design by varying these factors is important to obtain good extraction yields, which directly depend on the type of material used[97] [98]. In addition to selecting the appropriate parameters for extraction, it is crucial to consider energy consumption. Ultrasound equipment requires electrical energy for operation. Taking this into account, it is essential to measure the energy consumption during extraction. Nowadays, some researchers use a wattmeter to calculate energy expenditure. These calculations are based on the assumption that 1 kWh of energy generates 800 g of CO 2 during the combustion of fossil fuels, which is released into the atmosphere[99].
The choice of the appropriate solvent for the extraction of bioactive compounds depends on physical properties such as surface tension, viscosity, and vapor pressure. These physical characteristics of solvents directly affect the cavitation phenomenon. Cavitation has been observed in solvents with high vapor pressure, low viscosity, and low surface tension. However, high cavitation intensity has also been observed in solvents with low vapor pressure, high viscosity, and high surface tension. Various solvents have been used for the extraction of bioactive compounds, with the most popular ones being methanol, ethanol, and different water ratios. Currently, there is an increasing focus on research into the implementation of deep eutectic solvents, particularly environmentally friendly ones[94]. Table 3 shows the main parameters governing the extraction of bioactive compounds.
Table 3 Advantages and disadvantages of conventional methods compared to ultrasound.
| Plant Matter | Bioactive Compounds | Ultrasonic Parameters | Solvent Used | Ref. |
|---|---|---|---|---|
| Tradescantia zebrina leaves | Phenolics and anthocyanins | 6.25 min, 60 oC and 20% amplitude. | Water | [102] |
| Papaya pulp and skin | Antioxidants | 30 oC, 19.70-16.46 min. | Ethanol-water (60:30) | [103] |
| Hypnea flageliformis | Antioxidants and phenolics | Power 80%, time 30 min. | Chlorine chloride and lactic acid (1:2) with 20% water | [104] |
| Fresh Eucalyptus globulus leaves | Terpenes and phenolic compounds | Terpenes: pH 4, 80 W, 40 oC, 15 min. Polyphenols: pH 4, 120 W, 50 oC, 15 min. | Ethanol-water (60:30) | [105] |
| Byrsonima crassifolia | Antioxidant compounds | 0.01 W/cm3, 105 min. 50 oC | Ethanol-water (70:30) | [106] |
| Solanum torvum Sw | Polyphenols and flavonoids | 50-60 W cm-2, 30-45 oC | Methanol-water (55:65) | [107] |
| Rubus idaeus L. | Anthocyanins and phenolic compounds | 60 min. | Eutectic solvent-water (1:1) (Acetic acid, formic acid, lactic acid, maltose, and glucose) | [108] |
The advantages derived from the use of extracts and the growing interest in research have led to the development of various technologies that can enable better extraction without compromising the final product. One emerging technology that has shown substantial advantages over traditional maceration, conventional heating, and Soxhlet extraction is the use of ultrasound-assisted extraction, which is characterized as a high-efficiency, low-cost, environmentally friendly extraction method with flexibility to integrate with other treatment processes[100].
Extraction of bioactive compounds using ultrasound
Ultrasound-assisted extraction is carried out by creating cavitation bubbles that generate thermal and mechanical points within plant cells, as show in Figure 6. This leads to the rupture of the cell wall and the release of bioactive compounds into a solvent through diffusion. The extraction mechanism involves unique and combined mechanisms such as cellular alteration and mass transfer. It has been reported that the cavitation phenomenon creates pores, microchannels, and cavities in plant material, facilitating the penetration of solvent molecules and promoting extraction. Additionally, studies indicate that wet plant material is more effective. On the other hand, research suggests that 20 kHz ultrasound can break hydrogen bond and glycosidic linkages, change amide functional groups to carboxyls, and enhance extraction[101].
Albu et al., in 2004, reported the use of ultrasound to improve the extraction processes of carnosic acid from S. rosmarinus using butanone, ethyl acetate, and ethanol as solvents, both from dry and fresh leaves. They found that sonication improved the yields of carnosic acid for all three solvents and shortened the extraction times. They also mentioned that sonication improved the choice of less aggressive solvents, such as ethanol, because ethanol is a poor solvent under conventional conditions (high pressures and temperatures) and achieved a similar extraction efficiency compared to the other two solvents used. Extraction from dry herb with ethanol proved to be more efficient than that from fresh material, which they attributed to the water present in fresh leaves[109].
On the other hand, Paniwnyk et al., in 2009, studied the effect of ultrasound on the extraction of antioxidants from S. rosmarinus using solvents compared to conventional heating. They found that ultrasound provides more efficient extraction by increasing the extraction by 66 % of rosmarinic acid (mg/g) at temperatures of 35 °C and with less dependence on the extraction solvent used compared to the conventional heating method. They also reported that the application of this technique is possible for the extraction of bioactive compounds with antioxidant activity[110].
In 2016, a study was conducted on the antioxidant activity and rosmarinic acid content in ethanolic extracts of six medicinal plants (L. angustifolia, H. perforatum, S. officinalis, M. sylvestris, M. officinalis, S. rosmarinus) using ultrasound technique with a frequency of 35 kHz for 15 minutes. The study reported that all plants showed antioxidant activity greater than 70 % (0.1 g/ml), except for L. angustifolia and M. sylvestris. They also reported that all plants contain rosmarinic acid. In conclusion, they found that ultrasound is a fast and effective technique for preserving antioxidant activity and rosmarinic acid content in a matter of minutes[111].
Zhong et al., in 2021, reported the extraction of polar extracts from S. rosmarinus using ethanol. They found two new compounds and six already known compounds, with most of the isolated compounds showing significant antimicrobial properties, with minimum concentration values ranging from 2 to 128 μg/mL. However, these inhibitions were weaker than those obtained with the polar fraction. Additionally, the polar fraction was reported as a promising food additive due to its higher antimicrobial activity than the essential oil[112]. Table 4 shows some references of extraction of bioactive compounds from S. rosmarinus. using ultrasound as an extraction method.
Table 4 Advantages and disadvantages of conventional methods compared to ultrasound.
| Plant Matter | Bioactive Compounds | Extraction Efficiency | Results | Ref. |
|---|---|---|---|---|
| S. rosmarinus | Carnosic acid and rosmarinic acid | 24.0 ± 0.3 mgCA*g-1 Biomass 8.4 ± 0.3 mgRA*g-1 Biomass | Ultrasonic extraction is more effective in only 10 min compared to 48 h of agitation. | [113] |
| S. rosmarinus | Rosmarinic acid | Extraction yield 6.10% | The best extraction yield was obtained with a natural deep eutectic solvent | [114] |
| S. rosmarinus | Rosmarinic acid and carnosol | C: 5.61 ± 1.96 mg/g RA: 3.61 ± 0.94 mg/g | A yield increase of 11% was obtained with eutectic solvents compared to organic solvents. | [115] |
| S. rosmarinus | Rosmarinic acid and carnosol | Extraction yield 13.73% and C+CA 17.63 mg/100g | High C+CA concentrations were obtained with short extraction times (10 min) and relatively low temperatures (30 oC). | [116] |
| M. officinalis | Rosmarinic acid | 86.3 ± 4.1 mgRA/gplantaseca | The ultrasound technique proved to be more efficient than microwave and conventional heating, in addition to reducing the extraction temperature and time. | [117] |
| S. rosmarinus | Ursolic acid and carnosic acid | Extraction yield 18.8 ± 2.2% CA: 15.4 ±0.0 mg/grosmary UA: 35.3 ±0.0 mg/grosmary | The ultrasound technique proves to be more efficient than Conventional Solvent Extraction, Controlled Instant Pressure Drop Deodorization, Pressurized Liquid Extraction, Microwaves, Subcritical Extraction with Water, Supercritical Fluid Extraction, and Ionic Liquids. Additionally, it is the technique with the lowest carbon emissions and the lowest energy consumption. | [118] |
| S. rosmarinus | Flavonoids and rosmarinic acid | Extraction yield 22% F: 10.3 mg/g RA: 4.8 mg/g | The ultrasound technique proved to be the most suitable for the extraction of bioactive compounds compared to the microwave technique and conventional methods. | [119] |
| P. scutellarioides | Rosmarinic acid | 118 mg/g | The best extraction conditions were obtained using ultrasound for a duration of 45 minutes and a frequency of 30 kHz. | [120] |
| S. rosmarinus | Carnosic acid and rosmarinic acid | Extraction yield 100% | It was demonstrated that the proposed approach has higher efficiency, a shorter extraction time, and is a new alternative for the extraction of carnosic acid and rosmarinic acid compared to traditional reference methods, in addition to the use of ionic liquids. | [121] |
| S. officinalis | Carnosic acid and carnosol | CA: 147.5 mg/100g C: 42.7 mg/100g | They report ultrasound-assisted extraction as a green, simple, and safe technique for extracting bioactive compounds. They also report higher concentrations of analytes compared to the maceration technique. | [122] |
| S. rosmarinus | Carnosic and rosmarinic acids | CA: 62.19 ± 0.903 RA: 54.14 ± 0.786 | The ultrasound technique shows great potential as an ecological method to obtain S. rosmarinus, extract rich in antioxidants with higher concentrations of polyphenolic constituents compared to the supercritical extraction method. | [123] |
* CA: Carnosic acid, RA: Rosmarinic acid, C: Carnosol, F: Glavonoids, UA: Ursolic acid.
Conclusions
The S. rosmarinus plant has been used since ancient times, from home remedies to therapeutic applications. Currently, it has gained significant importance in various fields of research due to its culinary, antioxidant, antimicrobial, and therapeutic properties resulting from its secondary metabolites, such as rosmarinic acid, carnosol, and carnosic acid, among others. Although conventional methodologies used for extracting bioactive compounds from S. rosmarinus are widely employed today, there is still a search for more environmentally sustainable methods that allow a reduction in solvents, high temperatures, and waste generation.
The use of non-conventional technologies such as ultrasound has successfully improved extraction percentages and the selectivity of S. rosmarinus components compared to thermal and hydrothermal methods, with enhanced extraction yields when using poor solvents like ethanol, which is a less effective solvent in conventional extractions. Furthermore, emerging technologies enable the industrial scaling of extraction and open new possibilities for implementing various metabolites from natural resources in a wide range of applications, from culinary to therapeutic areas.
Declaration of conflict of interest
All authors declare that there is no personal or financial relationship with other individuals or organizations that could inappropriately influence or bias the work.
Contributions of the authors
J. J. C. P. conceptualized the project, wrote, reviewed and edited the different versions of the manuscript, carried out analysis, designed and developed the methodology. W. Y. V. L. participated in the investigation, carried out analyses, wrote, reviewed and edited different versions of the manuscript. A. O. C. F. carried out analysis and oversaw the project. S. C. E. G. carried out analyses and reviewed and edited different versions of the manuscript. E. M. M. R. carried out analyses and reviewed and edited the manuscript. A. S. G. conceptualized the project, designed and developed the methodology, wrote, reviewed and edited the different versions of the manuscript, funding acquisition, wrote, reviewed and edited the different versions of the manuscript. All authors reviewed and approved the final version of the manuscript.











 nueva página del texto (beta)
nueva página del texto (beta)