Original articles
Does the antidepressant-like effect of mirtazapine and venlafaxine differ between male and female rats?
¿El efecto de tipo antidepresivo de la mirtazapina y la venlafaxina difiere entre ratas macho y hembra?
-
Publication dates-
June 05, 2020
January , 2020
- Article in PDF
- Article in XML
- Automatic translation
- Send this article by e-mail
- Share this article +
Abstract
Introduction
Depression is a global health problem with nearly 350 million people affected, mainly women. However, nowadays a rising amount of men are being diagnosed. This makes necessary the screening of new treatment options that are effective in women as well as in men.
Objective
To analyze if the administration of mirtazapine and venlafaxine to male and female rats shows a sex-related antidepressant-like effect, and the possible associated neurochemical mechanisms.
Method
Mirtazapine (40 mg/kg) or venlafaxine (60 mg/kg) were administered subchronically to young adult male and female (ovariectomized and steroid-primed) rats, and their antidepressant-like effects were evaluated using the forced swim test (FST). The active behaviors, swimming and climbing, were also analyzed.
Results
a) mirtazapine and venlafaxine reduced immobility in the FST in males and females; b) both antidepressants increased climbing and swimming in male rats; c) in female rats, mirtazapine and venlafaxine only increased swimming.
Discussion and conclusion
In males, the effects of mirtazapine and venlafaxine seem to be produced by the activation of the serotonergic and noradrenergic systems. Conversely, estradiol might be modulating the mechanisms of action of both antidepressants in females producing only an increased swimming and suggesting the participation of the serotonergic system.
Keywords::
Mirtazapine, venlafaxine, antidepressants, sex differences, forced swim test
Introduction
Depression is a common affective disorder which affects approximately 4% of the world population (World Health Organization [WHO], 2018). It is characterized by low mood and anhedonia, and in the worst cases it can lead to suicide. Although most studies report that the incidence of depression is greater in women than in men (by nearly 2:1), there is evidence that this gap might be narrowing (Addis, 2008). Also, some of the most severe consequences of depression, as drug abuse and suicide, are far more frequent in men than in women (Cochran & Rabinowitz, 2000).
-
World Health Organization [WHO], 2018Depresión, 2018
-
Addis, 2008Gender and depression in men. Clinical PsychologyScience and Practice, 2008
-
Cochran & Rabinowitz, 2000Men and depression: Clinical and empirical perspectives, 2000
Currently, multiple therapeutic options for depression are available. However, many studies have shown that there is a gender-related difference in the response to some antidepressants (Berlanga & Flores-Ramos, 2006; Kornstein et al., 2000; Šagud, Hotujac, Milhaljević-Pelleš, & Jakovljević 2002). For example, women seem to tolerate and respond better to selective serotonin reuptake inhibitors (SSRIs) (Martenyi, Dossenbach, Mraz, & Metcalfe, 2001; Sramek, Murphy, & Cutler, 2016) than to noradrenaline reuptake inhibitors (NRIs). On the other hand, men show improvement with both SSRIs and NRIs (Berlanga & Flores Ramos, 2006), but also respond well to tricyclic antidepressants (Kornstein et al., 2000).
-
Berlanga & Flores-Ramos, 2006Different gender responses to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetineJournal of Affective Disorders, 2006
-
Kornstein et al., 2000Gender differences in treatment response to sertraline versus imipramine in chronic depressionAmerican Journal of Psychiatry, 2000
-
Šagud, Hotujac, Milhaljević-Pelleš, & Jakovljević 2002Gender differences in depressionCollegium Antropologicum, 2002
-
Martenyi, Dossenbach, Mraz, & Metcalfe, 2001Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profileEuropean Neuropsychopharmacology, 2001
-
Sramek, Murphy, & Cutler, 2016Sex differences in the psychopharmacological treatment of depressionDialogues in Clinical Neuroscience, 2016
-
Berlanga & Flores Ramos, 2006Different gender responses to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetineJournal of Affective Disorders, 2006
-
Kornstein et al., 2000Gender differences in treatment response to sertraline versus imipramine in chronic depressionAmerican Journal of Psychiatry, 2000
Some antidepressants like venlafaxine and the atypical antidepressant, mirtazapine, exert their actions on the noradrenergic and serotonergic systems. Venlafaxine is a serotonin and noradrenaline reuptake inhibitor (SNRI) (Holliday & Benfield, 1995). Mirtazapine’s main mechanism of action is through the antagonism of α2-adrenergic auto- and heteroreceptors, as well as the blockade of 5-HT2 and 5-HT3 serotonin receptors, which enhance the release of serotonin and noradrenaline, and favors the 5-HT1A-mediated serotonergic neurotransmission (Anttila & Leinonen, 2001). The dual action of these antidepressants makes them suitable for the treatment of depression in both, men and women.
-
Holliday & Benfield, 1995Venlafaxine: A review of its pharmacology and therapeutic potential in depressionDrugs, 1995
-
Anttila & Leinonen, 2001A review of the pharmacological and clinical profile of mirtazapineCNS Drug Reviews, 2001
Previous studies have reported that venlafaxine has a greater effect in male than in female rats in the chronic mild stress model of depression, through the measurement of sucrose consumption (Xing et al., 2013), and in the open field test, that evaluates experimental anxiety (Gray & Hughes, 2015). However, there are no reports on putative sex differences using the forced swim test (FST), which is a common test for the screening of drugs with antidepressant properties and permits to suggest putative mechanisms of action. Thus, in the FST, the administration of noradrenergic or dopaminergic drugs reduce immobility at an expense of enhancing climbing, while drugs that promote serotonergic neurotransmission also reduce immobility but increase swimming behavior (Bogdanova, Kanekar, D’Ancy, & Renshaw, 2013; Detke, Rickels, & Lucki, 1995).
-
Xing et al., 2013Gender differences in CMS and the effect of antidepressant venlafaxine in ratsNeurochemistry International, 2013
-
Gray & Hughes, 2015Drug-, dose- and sex-dependent effects of chronic fluoxetine, reboxetine and venlafaxine on open-field behavior and spatial memory in ratsBehavioural Brain Research, 2015
-
Bogdanova, Kanekar, D’Ancy, & Renshaw, 2013Factors influencing behavior in the forced swim testPhysiology and Behavior,, 2013
-
Detke, Rickels, & Lucki, 1995Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressantsPsychopharmacology, 1995
In this study, intact male and ovariectomized steroid-primed female rats were used. This design was purposed to ensure that all females were in the same hormonal state (induced proestrus) during the antidepressant treatment, and to observe the potential influence of gonadal hormones on the antidepressant-like effect, given that male rats were intact. In the FST, under basal conditions, females in proestrus exhibit less immobility than those in metestrus-diestrus (Estrada-Camarena, López-Rubalcava, Hernández-Aragón, Mejía-Mauries, & Picazo, 2011) or ovariectomized ones (Vega-Rivera, Fernández-Guasti, Ramírez-Rodríguez, & Estrada-Camarena, 2013a). Furthermore, the antidepressant responses also vary depending on the phase of the rat’s estrous cycle (Barros & Ferigolo, 1998).
-
Estrada-Camarena, López-Rubalcava, Hernández-Aragón, Mejía-Mauries, & Picazo, 2011Long-term ovariectomy modulates the antidepressant-like action of estrogens, but not of antidepressantsJournal of Psychopharmacology, 2011
-
Vega-Rivera, Fernández-Guasti, Ramírez-Rodríguez, & Estrada-Camarena, 2013aAcute stress further decreases the effect of ovariectomy on immobility behavior and hippocampal cell survival in ratsPsychoneuroendocrinology, 2013
-
Barros & Ferigolo, 1998Ethopharmacology of imipramine in the forced swimming test: gender differencesNeuroscience & Biobehavioral Reviews, 1998
Method
Design of the study
A quantitative, preclinical cross-sectional, controlled, and comparative study was performed.
Subjects
Young adult male (250-350 g) and female (180-250 g) Wistar rats were used in this study. All animals were provided by the vivarium of our research center (CINVESTAV), and kept in controlled conditions of temperature (23° C) on a 12-h/12-h inverted light-dark cycle, with lights on at 22:00 hrs. The animals were kept in groups of seven-eight per cage (each cage measuring 44 cm width × 33 cm length × 20 cm height), with ad libitum access to water and commercial rat chow.
Female rats were anesthetized with tribromoethanol 2% at a dose of 1 ml/kg, and bilaterally ovariectomized (OVX). After surgery, the rats were kept for recovery for thirteen days. Past these days, the rats were hormonally primed with 10 μg estradiol benzoate, and 20 hours later with 3 mg progesterone (Hernández-Munive, Rebolledo-Solleiro, Ventura-Aquino, & Fernández-Guasti, 2018). Hormones were dissolved in corn oil and administered subcutaneously in a volume of .3 ml/rat. The tests were made 24 hours after the administration of estradiol benzoate, and four hours after the administration of progesterone.
-
Hernández-Munive, Rebolledo-Solleiro, Ventura-Aquino, & Fernández-Guasti, 2018Reduced lordosis and enhanced aggression in paced and non-paced mating in diabetic female ratsThe Journal of Sexual Medicine, 2018
Measurements
To compare the antidepressant-like effect of mirtazapine and venlafaxine among male and female rats, the same antidepressants doses were used in both sexes. Mirtazapine was administered at 40 mg/kg, while the dose of venlafaxine was 60 mg/kg. These doses were chosen according to previous experiments (Álvarez-Silva & Fernández-Guasti, 2019). Motor coordination and general activity were evaluated prior to the FST.
-
Álvarez-Silva & Fernández-Guasti, 2019The combination of mirtazapine plus venlafaxine reduces immobility in the forced swim test and does not inhibit female sexual behaviorPharmacology, Biochemistry and Behavior, 2019
Procedures
Mirtazapine (MTZ) was dissolved in physiological saline with .5% acetic acid. Venlafaxine (VLF) hydrochloride was dissolved in physiological saline. Both drugs were administered intraperitoneally using a subchronic schedule (23, 5, and 1 hour before the evaluation) in a volume of 4 ml/kg. The subchronic scheme was chosen based on previous results (Detke et al., 1995; Hernández & Fernández-Guasti, 2018; Rénéric, Bouvard, & Stinus, 2002; Álvarez-Silva & Fernández-Guasti, 2019). All the experiments were performed during the first four hours of the awake phase of the animals.
-
Detke et al., 1995Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressantsPsychopharmacology, 1995
-
Hernández & Fernández-Guasti, 2018Male rats with same-sex preference show higher immobility in the forced swim test, but similar effects of fluoxetine and desipramine than males that prefer femalesPharmacology, Biochemistry and Behavior, 2018
-
Rénéric, Bouvard, & Stinus, 2002In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressedEuropean Neuropsychopharmacology, 2002
-
Álvarez-Silva & Fernández-Guasti, 2019The combination of mirtazapine plus venlafaxine reduces immobility in the forced swim test and does not inhibit female sexual behaviorPharmacology, Biochemistry and Behavior, 2019
Forced swim test
Forced swimming is a widely used test for the evaluation of antidepressants. This test is based on the observation that rats, when placed in a cylinder with water and forced to swim, eventually make only the minimal movements necessary to keep their heads above the water level, a behavior known as immobility. The use of the FST as a model of depression has been controverted by the suggestion that it might reflect learning rather than despair (Molendijk & de Kloet, 2015). However, it is still considered an appropriate test to assess the potential antidepressant-like effect of drugs (Slattery & Cryan, 2012).
-
Molendijk & de Kloet, 2015Immobility in the forced swim test is adaptative and does not reflect depressionPsychoneuroendocrinology, 2015
-
Slattery & Cryan, 2012Using the rat forced swim test to assess antidepressant-like activity in rodentsNature Protocols, 2012
The procedure consisted in placing the rats in individual glass cylinders (45 cm tall × 20 cm diameter) that had been filled with water 30 cm deep (Detke et al., 1995). Two swimming sessions were conducted: a 15-minute pretest, followed 24 hours later by a 5-minute test. Antidepressant treatments were administered between these sessions. After each session, rats were removed from the cylinders, dried with towels, placed in warm cages, and later returned to their home cages. Test sessions were videotaped for later scoring of three different behaviors: immobility (passive behavior), swimming, and climbing (active behaviors). The actions were scored after a 5-seconds period, so 60 counts were obtained over the 5-minute test sessions (Estrada-Camarena, Rivera, Berlanga, & Fernández-Guasti, 2008; Hernández & Fernández-Guasti, 2018; Álvarez-Silva & Fernández-Guasti, 2019).
-
Detke et al., 1995Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressantsPsychopharmacology, 1995
-
Estrada-Camarena, Rivera, Berlanga, & Fernández-Guasti, 2008Reduction in the latency of action of antidepressants by 17-β estradiol in the forced swimming testPsychopharmacology, 2008
-
Hernández & Fernández-Guasti, 2018Male rats with same-sex preference show higher immobility in the forced swim test, but similar effects of fluoxetine and desipramine than males that prefer femalesPharmacology, Biochemistry and Behavior, 2018
-
Álvarez-Silva & Fernández-Guasti, 2019The combination of mirtazapine plus venlafaxine reduces immobility in the forced swim test and does not inhibit female sexual behaviorPharmacology, Biochemistry and Behavior, 2019
Rota Rod test
To assess possible motor coordination effects of drug treatments, all groups evaluated in the FST were tested in the Rota Rod. This test consisted in placing the rats on a rotating cylinder at a constant speed of 11 rotations/minute. The animals were subjected to two training sessions in consecutive days before the test. The final evaluation was made right before the FST and the total of drop-offs in a period of five minutes were counted for each rat.
Open-field test
Locomotor activity was measured individually using an actimeter (Panlab LE 8825), that consisted in a polypropylene box (45 × 45 × 20 cm) with two infrared frames. The rat was placed in the center of the box, left for five minutes, and the counts of general activity, stereotyped movements and rearings were obtained. At the end of each individual test, the box was cleaned with 70% alcohol.
Statistical analysis
Data are presented as means ± standard errors. Differences were considered statistically significant when the p value was ≤ .05. The comparisons between control and treated animals were made using the Mann-Whitney U test.
Ethical considerations
All experimental procedures were performed in accordance with the Mexican Official Norm for animal care and handling (NOM-062-ZOO-1999) and approved by the Institutional Ethics Committee of the CINVESTAV-IPN.
Results
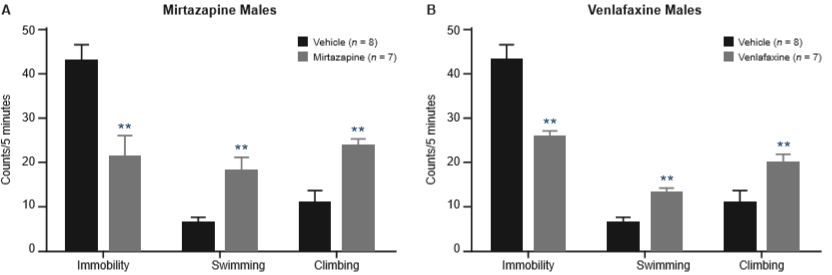
The effect of mirtazapine on immobility, swimming, and climbing on male rats is shown in Figure 1A. Mirtazapine produced a significant decrease in immobility (U = 4.5, p = .004), with an increase of both swimming (U = 3.5, p = .002), and climbing (U = 5.5, p = .006). Figure 1B shows that venlafaxine administration to males also reduced immobility (U = 3.5, p = .002) and significantly increased both active behaviors (swimming: U = 6, p = .008; climbing: U = 5.5, p = .006).
Thumbnail
Figure 1
Effect of mirtazapine 40 mg/kg (A) and venlafaxine 60 mg/kg (B) in the FST on male rats. Values are presented as mean (± SEM) counts of immobility, swimming, and climbing behaviors assessed every 5s during the 5-minute test. Data were analyzed using the Mann-Whitney U test. ** p < .01 vs. control group.
Effect of mirtazapine 40 mg/kg (A) and venlafaxine 60 mg/kg (B) in the FST on male rats. Values are presented as mean (± SEM) counts of immobility, swimming, and climbing behaviors assessed every 5s during the 5-minute test. Data were analyzed using the Mann-Whitney U test. ** p < .01 vs. control group.
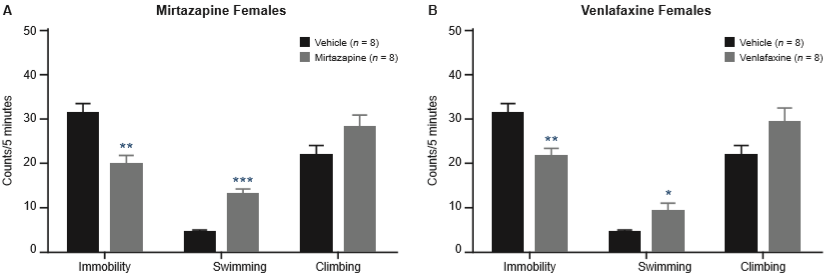
In female rats, mirtazapine also reduced immobility (U = 6, p = .004) and increased swimming (U = 0, p = .0002), but not climbing behavior (U = 17, ns) (Figure 2A). Venlafaxine showed a similar profile, reducing immobility (U = 7.5, p = .004) with an increased swimming (U = 9.5, p = .017), but with no significant increases in climbing (U = 14, ns) (Figure 2B).
Thumbnail
Figure 2
Effect of mirtazapine 40 mg/kg (A) and venlafaxine 60 mg/kg (B) in the FST on female rats. Values are presented as mean (± SEM) counts of immobility, swimming, and climbing behaviors assessed every 5s during the 5-minute test. Data were analyzed using the Mann-Whitney U test. *p < .05; **p < .01; ***p < .001 vs. control group.
Effect of mirtazapine 40 mg/kg (A) and venlafaxine 60 mg/kg (B) in the FST on female rats. Values are presented as mean (± SEM) counts of immobility, swimming, and climbing behaviors assessed every 5s during the 5-minute test. Data were analyzed using the Mann-Whitney U test. *p < .05; **p < .01; ***p < .001 vs. control group.
Regarding locomotor activity, mirtazapine reduced spontaneous activity in both male and female rats. On the other hand, venlafaxine did not affect locomotion (Table 1). Neither mirtazapine nor venlafaxine modified the number of drops from the Rota Rod (data not shown).
Table 1
Locomotor activity after the administration of mirtazapine or venlafaxine in male and female rats
Locomotor activity after the administration of mirtazapine or venlafaxine in male and female rats
| Treatment | Males | Females |
|---|---|---|
| Vehicle | 1714 ± 325 | 1818 ± 440 |
| Mirtazapine 40 mg/kg | 401 ± 98*** | 327.5 ± 159*** |
| Venlafaxine 60 mg/kg | 1462 ± 287 | 1192 ± 413 |
Discussion and conclusion
In this study, we analyzed the antidepressant-like effect of mirtazapine and venlafaxine on male and female rats using the FST. The main findings were:
-
Both antidepressants similarly reduced immobility in male and female rats.
-
In males, mirtazapine and venlafaxine increased both active behaviors, whereas in females these antidepressants enhanced only swimming.
-
Mirtazapine reduced locomotor activity in both sexes, while venlafaxine had no effect.
The antidepressant-like effect of mirtazapine in male rats was reported in previous studies (Rénéric et al., 2002; Rogóz, Kabziἠski, Sadaj, Rachwalska, & Gadek-Michalska, 2012). Our results agree with those reported by Rénéric et al. (2002) showing that subchronic mirtazapine reduced immobility at an expense of increasing both swimming and climbing. It is known that mirtazapine, through the α2-adrenergic receptor blockade (de Boer, Ruigt, & Berendsen, 1995; de Montigny, Haddjeri, Mongeau, & Blier, 1995), increases the serotonergic and noradrenergic neurotransmission. The enhancement of both neurotransmission systems may produce greater antidepressant-like effects than those produced by selective antidepressants (Rénéric et al., 2002). The negative effect of mirtazapine on locomotor activity may be produced by its high affinity for histaminergic H1 receptors, which activation induces sedation (Davis & Wilde, 1996). However, this effect did not interfere with the increase in active behaviors seen in the FST nor with the motor coordination tested in the Rota Rod.
-
Rénéric et al., 2002In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressedEuropean Neuropsychopharmacology, 2002
-
Rogóz, Kabziἠski, Sadaj, Rachwalska, & Gadek-Michalska, 2012Effect of co-treatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim testPharmacological Reports, 2012
-
Rénéric et al. (2002)In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressedEuropean Neuropsychopharmacology, 2002
-
de Boer, Ruigt, & Berendsen, 1995The α2-selective adrenoceptor antagonist org 3770 - mirtazapine, Remeron® enhances noradrenergic and serotonergic transmissionHuman Psychopharmacology: Clinical and Experimental, 1995
-
de Montigny, Haddjeri, Mongeau, & Blier, 1995The effects of mirtazapine on the interactions between central noradrenergic and serotonergic systemsCNS Drugs, 1995
-
Rénéric et al., 2002In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressedEuropean Neuropsychopharmacology, 2002
-
Davis & Wilde, 1996Mirtazapine: A review of its pharmacology and therapeutic potential in the management of major depressionCNS Drugs, 1996
Venlafaxine is a dual reuptake inhibitor, five times more potent to inhibit serotonin reuptake than noradrenaline reuptake (Holliday & Benfield, 1995). However, at high doses, venlafaxine may also inhibit dopamine reuptake (Rénéric & Lucki, 1998). Accordingly, Rénéric & Lucki (1998) using the rat FST, found that low doses of venlafaxine (10, 20 and 40 mg/kg) enhanced swimming, while higher doses (80 mg/kg) also increased climbing. Present results showing that 60 mg/kg of venlafaxine in males reduced immobility at an expense of increasing swimming and climbing agree with these data. Furthermore, Millan et al. (2001) showed that venlafaxine at doses higher than 40 mg/kg increased the concentrations of serotonin and noradrenaline in the brain frontal cortex up to four to six times compared with lower doses. As shown, the venlafaxine dose here used (60 mg/kg) reduced immobility without altering locomotion, suggesting that its effect may be considered as selective antidepressant-like.
-
Holliday & Benfield, 1995Venlafaxine: A review of its pharmacology and therapeutic potential in depressionDrugs, 1995
-
Rénéric & Lucki, 1998Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming testPsychopharmacology, 1998
-
Rénéric & Lucki (1998)Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming testPsychopharmacology, 1998
-
Millan et al. (2001)S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramineJournal of Pharmacology and Experimental Therapeutics, 2001
The present result shows that mirtazapine in female rats reduced immobility and increased swimming agrees with the report of Melo et al. (2012), who showed that this antidepressant did not increase climbing in this sex. However, these findings contrast with the report of Rénéric & Lucki (1998) using the same dose in males, stressing the importance of sex and possibly of gonadal hormones in the neurochemical effects of mirtazapine and other antidepressants (Estrada-Camarena, Fernández-Guasti, & López-Rubalcava, 2003; Vega-Rivera, López-Rubalcava, & Estrada-Camarena, 2013b).
-
Melo et al. (2012)Antidepressants differentially modify the extinction of an aversive memory task in female ratsProgress in Neuro-psychopharmacology and Biological Psychiatry, 2012
-
Rénéric & Lucki (1998)Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming testPsychopharmacology, 1998
-
Estrada-Camarena, Fernández-Guasti, & López-Rubalcava, 2003Antidepressant-like effect of different estrogenic compounds in the forced swimming testNeuropsychopharmacology, 2003
-
Vega-Rivera, López-Rubalcava, & Estrada-Camarena, 2013bThe antidepressant-like effect of ethynyl estradiol is mediated by both serotonergic and noradrenergic systems in the forced swimming testNeuroscience, 2013
The antidepressant-like effect of venlafaxine in the FST in female rats agrees with the report of Estrada-Camarena et al. (2008). In that and in the present study, the co-administration of venlafaxine and estradiol to OVX rats reduced immobility and increased swimming, without modifying climbing. These results suggest that the presence of estradiol may increase the sensitivity to the serotonergic actions of venlafaxine in female rats.
-
Estrada-Camarena et al. (2008)Reduction in the latency of action of antidepressants by 17-β estradiol in the forced swimming testPsychopharmacology, 2008
The results of this study suggest that estrogens favor in females the serotonergic actions of both mirtazapine and venlafaxine. There are several mechanisms by which estradiol regulates the serotonergic system. For example, estradiol inhibits SERT (Bertrand et al., 2005; Koldzic-Zivanovic, Seitz, Watson, Cunningham, & Thomas, 2004), increases the serotonin synthesis, and promotes the desensitization of 5-HT1A receptors (Vega-Rivera et al., 2013b), and induces the uncoupling of the 5-HT1A receptor from its G protein (Mize, Young, & Alper, 2003). These actions enhance the serotonergic transmission that might underlie the effects of mirtazapine and venlafaxine on swimming.
-
Bertrand et al., 2005The effect of low estrogen state on serotonin transporter function in mouse hippocampus: a behavioral and electrochemical studyBrain Research, 2005
-
Koldzic-Zivanovic, Seitz, Watson, Cunningham, & Thomas, 2004Intracellular signaling involved in estrogen regulation of serotonin reuptakeMolecular and Cellular Endocrinology, 2004
-
Vega-Rivera et al., 2013bThe antidepressant-like effect of ethynyl estradiol is mediated by both serotonergic and noradrenergic systems in the forced swimming testNeuroscience, 2013
-
Mize, Young, & Alper, 2003Uncoupling of 5-HT1A receptors in the brain by estrogens: regional variations in antagonism by ICI 182,780Neuropharmacology, 2003
In addition to the prevalent action of the serotonergic transmission in females, it must be considered that males have a higher catecholamine response after the administration of idazoxan (an α2-adrenergic blocker) in comparison to females (Schmidt et al., 1997). Such sex difference may explain why mirtazapine and venlafaxine increased climbing in males.
-
Schmidt et al., 1997Gender differences in brain metabolic and plasma catecholamine responses to alpha2-adrenoceptor blockade.Neuropsychopharmacology, 1997
It is important to highlight that, without any antidepressant treatment, male rats showed a higher number of immobility counts than females, and this difference was statistically significant (U = 11, p = .02). This was one of the reasons why a 2-way ANOVA was not performed (the other reason was that not all data followed a normal distribution). This sex difference in immobility has been previously reported (Fernández-Guasti, Olivares-Nazario, Reyes, & Martínez-Mota, 2017; Gómez, Martínez-Mota, Estrada-Camarena, & Fernández-Guasti, 2014; Kokras et al., 2009) and may respond to sex variations in dopamine (Dalla et al., 2008), serotonin (Dalla, Pitychoutis, Kokras, & Papadopoulou-Daifoti, 2010), and 5-HT1A receptors (Drossopoulou et al., 2004) in brain areas that modulate stress-related behaviors. These differences may also explain the higher sensitive serotonergic system (primarily to external factors) in women as compared to men (Khan, Brodhead, Schwartz, Kolts, & Brown, 2005).
-
Fernández-Guasti, Olivares-Nazario, Reyes, & Martínez-Mota, 2017Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim testPharmacology, Biochemistry and Behavior, 2017
-
Gómez, Martínez-Mota, Estrada-Camarena, & Fernández-Guasti, 2014Influence of the brain sexual differentiation process on despair and antidepressant-like effect of fluoxetine in the rat forced swim testNeuroscience, 2014
-
Kokras et al., 2009Sex-related differential response to clomipramine treatment in a rat model of depressionJournal of Psychopharmacology, 2009
-
Dalla et al., 2008Sex differences in the effects of two stress paradigms on dopaminergic neurotransmissionPhysiology & Behavior, 2008
-
Dalla, Pitychoutis, Kokras, & Papadopoulou-Daifoti, 2010Sex differences in animal models of depression and antidepressant responseBasic & Clinical Pharmacology & Toxicology, 2010
-
Drossopoulou et al., 2004Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in ratsNeuroscience, 2004
-
Khan, Brodhead, Schwartz, Kolts, & Brown, 2005Sex differences in antidepressant response in recent antidepressant clinical trialsJournal of Clinical Psychopharmacology, 2005
In conclusion, mirtazapine and venlafaxine showed a similar antidepressant-like effect in both male and female rats. However, both antidepressants increased swimming and climbing behaviors in males, while in female rats, only swimming was enhanced, suggesting the sex differential participation of noradrenaline and serotonin.
Acknowledgments
We thank Dr. Jorge Ocampo from “Laboratorios Bioquimed, México” for mirtazapine and venlafaxine donation.
References
- Addis, M. E. (2008). Gender and depression in men. Clinical Psychology: Science and Practice, 15(3), 153-168. doi: 10.1111/j.1468-2850.2008.00125.x Links
- Álvarez-Silva, A. A., & Fernández-Guasti, A. (2019). The combination of mirtazapine plus venlafaxine reduces immobility in the forced swim test and does not inhibit female sexual behavior. Pharmacology, Biochemistry and Behavior, 187, 172817 doi: 10.1016/j.pbb.2019.172817 Links
- Anttila, S. A., & Leinonen, E. V. (2001). A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Reviews, 7(3), 249-264. doi: 10.1111/j.1527-3458.2001.tb00198.x Links
- Barros, H. M., & Ferigolo, M. (1998). Ethopharmacology of imipramine in the forced swimming test: gender differences. Neuroscience & Biobehavioral Reviews, 23(2), 279-286. doi: 10.1016/S0149-7634(98)00029-3 Links
- Berlanga, C., & Flores-Ramos, M. (2006). Different gender responses to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. Journal of Affective Disorders, 95(1-3), 119-123. doi: 10.1016/j.jad.2006.04.029 Links
- Bertrand, P. P., Paranavitane, U. T., Chavez, C., Gogos, A., Jones, M., & van den Buuse, M. (2005). The effect of low estrogen state on serotonin transporter function in mouse hippocampus: a behavioral and electrochemical study. Brain Research, 1064(1-2), 10-20. doi: 10.1016/j.brainres.2005.10.018 Links
- Bogdanova, O. V., Kanekar, S., D’Ancy, K. E., & Renshaw, P. F. (2013). Factors influencing behavior in the forced swim test. Physiology and Behavior, 118, 227-239. doi: 10.1016/j.physbeh.2013.05.012 Links
- Cochran, S. V., & Rabinowitz, F. E. (2000). Men and depression: Clinical and empirical perspectives. San Diego, California: Academic Press. ISBN 0-12-177540-2 Links
- Dalla, C., Antoniou, K., Kokras, N., Drossopoulou, G., Papathanasiou, G., Bekris, S., ... Papadopoulou-Daifoti, Z. (2008). Sex differences in the effects of two stress paradigms on dopaminergic neurotransmission. Physiology & Behavior, 93(3), 595-605. doi: 10.1016/j.physbeh.2007.10.020 Links
- Dalla, C., Pitychoutis, P. M., Kokras, N., & Papadopoulou-Daifoti, Z. (2010). Sex differences in animal models of depression and antidepressant response. Basic & Clinical Pharmacology & Toxicology, 106(3), 226-233. doi: 10.1111/j.1742-7843.2009.00516.x Links
- Davis, R., & Wilde, M. I. (1996). Mirtazapine: A review of its pharmacology and therapeutic potential in the management of major depression. CNS Drugs, 5(5), 389-402. doi: 10.2165/00023210-199605050-00007 Links
- de Boer, T., Ruigt, G. S. F., & Berendsen, H. H. G. (1995). The α2-selective adrenoceptor antagonist org 3770 (mirtazapine, Remeron®) enhances noradrenergic and serotonergic transmission. Human Psychopharmacology: Clinical and Experimental, 10(S2), S107-S118. doi: 10.1002/hup.470100805 Links
- de Montigny, C., Haddjeri, N., Mongeau, R., & Blier, P. (1995). The effects of mirtazapine on the interactions between central noradrenergic and serotonergic systems. CNS Drugs, 4(1), 13-17. doi: 10.2165/00023210-199500041-00004 Links
- Detke, M. J., Rickels, M., & Lucki, I. (1995). Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology, 121(1), 66-72. doi: 10.1007/BF02245592 Links
- Drossopoulou, G., Antoniou, K., Kitraki, E., Papathanasiou, G., Papalexi, E., Dalla, C., & Papadopoulou-Daifoti, Z. (2004). Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience, 126(4), 849-857. doi: 10.1016/j.neuroscience.2004.04.044 Links
- Estrada-Camarena, E., Fernández-Guasti, A., & López-Rubalcava, C. (2003). Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology, 28(5), 830-838. doi: 10.1038/sj.npp.1300097 Links
- Estrada-Camarena, E., López-Rubalcava, C., Hernández-Aragón, A., Mejía-Mauries, S., & Picazo, O. (2011). Long-term ovariectomy modulates the antidepressant-like action of estrogens, but not of antidepressants. Journal of Psychopharmacology, 25(10), 1365-1377. doi: 10.1177/0269881111408456 Links
- Estrada-Camarena, E., Rivera, N. V., Berlanga, C., & Fernández-Guasti, A. (2008). Reduction in the latency of action of antidepressants by 17-β estradiol in the forced swimming test. Psychopharmacology, 201(3), 351-360. doi: 10.1007/s00213-008-1291-8 Links
- Fernández-Guasti, A., Olivares-Nazario, M., Reyes, R., & Martínez-Mota, L. (2017). Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim test. Pharmacology, Biochemistry and Behavior, 152, 81-89. doi: 10.1016/j.pbb.2016.01.011 Links
- Gómez, M. L., Martinez-Mota, L., Estrada-Camarena, E., & Fernandez-Guasti, A. (2014). Influence of the brain sexual differentiation process on despair and antidepressant-like effect of fluoxetine in the rat forced swim test. Neuroscience, 261, 11-22. doi: 10.1016/j.neuroscience.2013.12.035 Links
- Gray, V. C., & Hughes, R. N. (2015). Drug-, dose- and sex-dependent effects of chronic fluoxetine, reboxetine and venlafaxine on open-field behavior and spatial memory in rats. Behavioural Brain Research, 281, 43-54. doi: 10.1016/j.bbr.2014.12.023 Links
- Hernández, A., & Fernández-Guasti, A. (2018). Male rats with same-sex preference show higher immobility in the forced swim test, but similar effects of fluoxetine and desipramine than males that prefer females. Pharmacology, Biochemistry and Behavior, 171, 39-45. doi: 10.1016/j.pbb.2018.05.017 Links
- Hernández-Munive, A. K., Rebolledo-Solleiro, D., Ventura-Aquino, E., & Fernández-Guasti, A. (2018). Reduced lordosis and enhanced aggression in paced and non-paced mating in diabetic female rats. The Journal of Sexual Medicine, 15(2), 124-135. doi: 10.1016/j.jsxm.2017.11.018 Links
- Holliday, S. M., & Benfield, P. (1995). Venlafaxine: A review of its pharmacology and therapeutic potential in depression. Drugs, 49(2), 280-294. doi: 10.2165/00003495-199549020-00010 Links
- Khan, A., Brodhead, A. E., Schwartz, K. A., Kolts, R. L., & Brown, W. A. (2005). Sex differences in antidepressant response in recent antidepressant clinical trials. Journal of Clinical Psychopharmacology, 25(4), 318-324. doi: 10.1097/01.jcp.0000168879.03169.ce Links
- Kokras, N., Antoniou, K., Dalla, C., Bekris, S., Xagoraris, M., Ovestreet, D. H., & Papadopoulou-Daifoti, Z. (2009). Sex-related differential response to clomipramine treatment in a rat model of depression. Journal of Psychopharmacology, 23(8), 945-956. doi: 10.1177/0269881108095914 Links
- Koldzic-Zivanovic, N., Seitz, P. K., Watson, C. S., Cunningham, K. A., & Thomas, M. L. (2004). Intracellular signaling involved in estrogen regulation of serotonin reuptake. Molecular and Cellular Endocrinology, 226(1-2), 33-42. doi: 10.1016/j.mce.2004.07.017 Links
- Kornstein, S. G., Schatzberg, A. F., Thase, M. E., Yonkers, K. A., McCullough, J. P., Keitner, G. I., ... Keller, M. B. (2000). Gender differences in treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry, 157(9), 1445-1452. doi: 10.1176/appi.ajp.157.9.1445 Links
- Martenyi, F., Dossenbach, M., Mraz, K., & Metcalfe, S. (2001). Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: a double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. European Neuropsychopharmacology, 11(3), 227-232. doi: 10.1016/S0924-977X(01)00089-X Links
- Melo, T. G., Izídio, G. S., Ferreira, L. S., Sousa, D. S., Macedo, P. T., Cabral, A., ... Silva, R. H. (2012). Antidepressants differentially modify the extinction of an aversive memory task in female rats. Progress in Neuro-psychopharmacology and Biological Psychiatry, 37(1), 33-40. doi: 10.1016/j.pnpbp.2012.01.012 Links
- Millan, M. J., Gobert, A., Lejeune, F., Newman-Tancredi, A., Rivet, J. M., Auclair, A., & Peglion, J. L. (2001). S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. Journal of Pharmacology and Experimental Therapeutics, 298(2), 565-580. Links
- Mize, A. L., Young, L. J., & Alper, R. H. (2003). Uncoupling of 5-HT1A receptors in the brain by estrogens: regional variations in antagonism by ICI 182,780. Neuropharmacology, 44(5), 584-591. doi: 10.1016/S0028-3908(03)00044-3 Links
- Molendijk, M. L., & de Kloet E. R. (2015). Immobility in the forced swim test is adaptative and does not reflect depression. Psychoneuroendocrinology, 62, 389-391. doi: 10.1016/j.psyneuen.2015.08.028 Links
- Rénéric, J. P., & Lucki, I. (1998). Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology, 136(2), 190-197. doi: 10.1007/s002130050555 Links
- Rénéric, J. P., Bouvard, M., & Stinus, L. (2002). In the rat forced swimming test, NA-system mediated interactions may prevent the 5-HT properties of some subacute antidepressant treatments being expressed. European Neuropsychopharmacology, 12(2), 159-171. doi: 10.1016/S0924-977X(02)00007-X Links
- Rogóz, Z., Kabziἠzki, M., Sadaj, W., Rachwalska, P., & Gᶏdek-Michalska, A. (2012). Effect of co-treatment with fluoxetine or mirtazapine and risperidone on the active behaviors and plasma corticosterone concentration in rats subjected to the forced swim test. Pharmacological Reports, 64(6), 1391-1399. doi: 10.1016/S1734-1140(12)70936-2 Links
- Šagud, M., Hotujac, L. J., Mihaljević-Pelleš, A., & Jakovljević, M. (2002). Gender differences in depression. Collegium Antropologicum, 26(1), 149-157. Links
- Schmidt, M. E., Matochik, J. A., Goldstein, D. S., Schauten, J. L., Zametkin, A. J., & Potter, W. Z. (1997). Gender differences in brain metabolic and plasma catecholamine responses to alpha2-adrenoceptor blockade. Neuropsychopharmacology, 16(4), 298-310. doi: 10.1016/S0893-133X(96)00264-3 Links
- Slattery, D. A., & Cryan, J. F. (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nature Protocols, 7(6), 1009-1014. doi: 10.1038/nprot.2012.044 Links
- Sramek, J. J., Murphy, M. F., & Cutler, N. R. (2016). Sex differences in the psychopharmacological treatment of depression. Dialogues in Clinical Neuroscience, 18(4), 447-457. Links
- Vega-Rivera, N. M., Fernández-Guasti, A., Ramírez-Rodríguez, G., & Estrada-Camarena, E. (2013a). Acute stress further decreases the effect of ovariectomy on immobility behavior and hippocampal cell survival in rats. Psychoneuroendocrinology, 38(8), 1407-1417. doi: 10.1016/j.psyneuen.2012.12.008 Links
- Vega-Rivera, N. M., López-Rubalcava, C., & Estrada-Camarena, E. (2013b). The antidepressant-like effect of ethynyl estradiol is mediated by both serotonergic and noradrenergic systems in the forced swimming test. Neuroscience, 250, 102-111. doi: 10.1016/j.neuroscience.2013.06.058 Links
- World Health Organization. (2018). Depresión. Retrieved from: https://www.who.int/es/news-room/fact-sheets/detail/depression Links
- Xing, Y., He, J., Hou, J., Lin, F., Tian, J., & Kurihara, H. (2013). Gender differences in CMS and the effect of antidepressant venlafaxine in rats. Neurochemistry International, 63(6), 570-575. doi: 10.1016/j.neuint.2013.09.019 Links