Introduction
Maize leaf blight, caused by Exserohilum turcicum, is one of the most important leaf diseases in the world. The development of the disease is favored by high relative humidity (> 90%) and a mean daily temperature of 18-26 °C. Typical symptoms are long lesions (2.5 and 15 cm in length), elliptical, and grayish green in color. As the disease spreads, lesions become tan with dark areas and coalesce to form the blight. The reduction of the photosynthetic area may reach up to 65%, leading to losses between 15 and 75% in yield (Félix-Gastélum et al., 2018).
The current management of maize blight implies the use of synthetic fungicides of the dithiocarbamate, nitrile, triazole and strobilurin chemical groups (De Rossi et al., 2020). However, the ordinary use of synthetic fungicides increases the selection pressure on the population of the pathogen, leading to the development of resistance to fungicides and inconsistencies in chemical control (Wise and Mueller, 2011).
In recent years, bioprotection has arisen as an alternative to the chemical control of multiple plant diseases. Some rhizobacteria have a great potential for the suppression of phytopathogens, due to their capacity to produce a wide range of lytic enzymes and diffusible and volatile antimicrobial compounds, that limit the growth and infection of phytopathogens, as well as stimulating the growth of plants with the production and modulation of phytohormones (Sartori et al., 2015, 2017; Sehrawat et al., 2022).
Volatile organic compounds (VOC) are secondary metabolites of low molecular weights and low polarity, making them easily diffusible in the soil and the atmo- sphere (Zhao et al., 2023). Several VOCs emitted by rhizobacteria are able to inhib- it the growth of phytopathogenic fungi, hence their potential to be used to control diseases in planta and post-harvest (Poulaki and Tjamos, 2023; Zhao et al., 2023). This antifungal effect is attributed to the alteration of the cell wall integrity, the cell membrane fluidity, the disruption of the redox balance, and even to the alteration of the transcriptome of the pathogen, reducing the expression of the virulence and energetic metabolism genes.
Some of the main VOCs-producing rhizobacteria genera with antifungal ac- tivity include Bacillus, Pseudomonas, Paenibacillus, Brevibacillus and Ralstonia (Zhao et al., 2023). Alongside its antifungal activity, some bacterial VOCs promote growth and induce systemic resistance in plants, which allows them to defend fast- er and more effectively against pathogens (Poulaki and Tjamos, 2023).
Hydrocyanic acid, or hydrogen cyanide (HCN), is a VOC with antimicrobial activity that joins cytochrome c oxidase and interferes with cell respiration (Anand et al., 2023). Some rhizobacteria such as Pseudomonas use this molecule as one of its main mechanisms to fight pathogens in plants (Anand et al., 2020).
Due to this, the aims of this study were to: 1) obtain and characterize maize Exserohilum turcicum isolates; 2) evaluate the effect of the VOCs produced by rhizobacteria on mycelial growth and the in vitro infection of E. turcicum; and 3) determine the production of hydrocyanic acid or hydrogen cyanide (HCN), an im- portant VOC with antifungal effect, produced by rhizobacteria.
Materials and Methods
Obtaining and purifying fungal isolates. In February and March, 2020, maize leaves with typical symptoms of leaf blight were gathered from the municipal areas of Ahome, El Fuerte and Guasave, in Sinaloa, Mexico. The leaves were cut into small pieces (25 mm2) from the edge of the lesion, disinfested for 3 min in a 0.5% sodium hypochlorite solution and washed three times with sterile distilled water. The pieces of disinfested tissue were placed in Petri dishes with Potato Dextrose Agar (PDA; MCD LAB, Tlalnepantla, State of Mexico), supplemented with neomycin (0.1125 mg/mL) and streptomycin sulphate (0.5 mg/mL), and incubated at 25 °C for 3 days. The fungal cultures were transferred to dishes with PDA and incubated at 25 °C for 14 days. Monosporic cultures were obtained and stored in filter paper at 4 °C (Hiruma and Saijo, 2016).
Morphological characterization of fungal isolates. In order to evaluate the morphological characteristics of the isolates, they were cultivated on PDA and V8- agar and incubated at 25 °C for 10 and 15 días, respectively. The mycelial growth rate and the macroscopic characteristics of the cultures (texture, type of edge and pigmentation of front and back) were evaluated on PDA. The shape, number of septa, length and width of 30 conidia of each isolate, cultivated on V8, was evaluated under an Axio Imager M2 microscope (Carl Zeiss).
Molecular identification of fungal isolates
DNA extraction, PCR and sequencing. The genomic DNA was extracted from 50 mg of fresh mycelia, of PDA cultures grown for 10 days, using the YeaStarTM Genomic DNA kit (N. cat D2002, Zymo Research, Irvine, CA, USA), following the manufacturer’s protocol. The amplification of the ITS region, the actin (act) and the second largest subunit of the polymerase RNA II (rpb2) genes, was carried out using the primers ITS1/ ITS4 (White et al., 1990), Ex-actF/Ex-actR (5’ - CCCCGAGCAGTCTTCCGTA - 3’/5’ - GTACGTCCAGAGGCGTACAG - 3’; 480 pb) y Ex-rpb2F/Ex-rpb2R (5’ - CTTCGTCGAACAAAYACWCCTG - 3’/5’ - CRCAGTGRGTRTAGGCATGG - 3’; 760 pb), respectively. The primers for act and rpb2 were designed for this study, using the program Primer3 (Untergasser et al., 2012). The PCR was carried out in an Apollo ATC-201 thermocycler (Nyx Technik, San Diego, CA, USA) with 1 ng of DNA, 1.5 mM of MgCl2, 0.2 mM of each dNTP, 0.5 mM of each primer and 1 U of polymerase DNA (Invitrogen, Brazil, Cat. Nº 11615-050) in a final volume of 25 mL. The PCR program consisted of 5 min of initial denaturation at 94 °C, followed by 35 denaturation cycles at 95°C for 40 s, annealing at 55 °C for ITS and 60 °C for act and rpb2 for 40 s, a 1 min extension at 72 °C and a final extension at 72 °C for 5 min. The PCR products were sequenced at Macrogen Inc. (Seoul, South Korea).
Multigene phylogenetic analysis. The sequences were edited in BioEdit v 7.0.5.3 (Hall, 1999) and compared in the NCBI data base using the BLASTn algorithm. A concatenated alignment act+ITS+rpb2 was generated with MUSCLE (Edgar, 2004), implemented in MEGA X (Kumar et al., 2018). The partition scheme was evaluated in PartitionFinder v 1.1.1 (Lanfear et al., 2012), based on Akaike’s Information Criterion (AIC). Phylogenetic reconstruction was performed with the Maximum Likelihood (ML) method in RAXML v 7.2.8 (Stamatakis, 2006), using the model GTRGAMMAI and 1000 bootstrap replicates. The phylogram was edited using iTOL (Letunic and Bork, 2021; https://itol.embl.de/).
Pathogenicity test
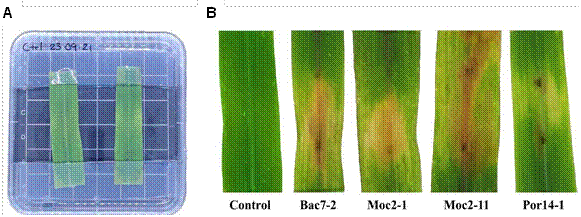
Detached leaf assay. The pathogenicity of four E. turcicum isolates was evaluated following the methodology by Perochon and Doohan (2016), with modifications. Leaf fragments of 6 cm in length, were taken from the second true leaf of 20- day old maize plants, grown under controlled conditions in the laboratory. The leaf fragments were disinfested superficially with a 0.75% sodium hypochlorite solution for 1 min, followed by four washes with sterile distilled water. After being disinfected, they were placed in squared Petri dishes (100 mm × 100 mm), adaxial side up. The tips of the leaves were placed between two layers of water agar supplemented with 50 μg/mL of 6-benzylaminopurine (BAP; Sigma-Aldrich, Steinheim, Germany), as in a “sandwich” (Aregbesola et al., 2020).
The leaves were inoculated with three 5-mL drops (~ 120 conidia) of a conidial suspension at 2.4 × 104 conidia/mL, obtained from a 15-day old culture on V8 agar. Leaves inoculated with a sterile 1% Tween 80 solution were used as a control. The plates were incubated at 25 °C, with a 12 h light/12 h dark photoperiod for 6 days in a completely randomized design, with three repetitions per treatment. The assay was carried out twice.
Antifungal effect of the bacterial VOCs on E. turcicum
Bacterial strains. The antagonistic effect of six rhizobacteria, previously isolated and characterized by Morales-Ruíz (2022), was evaluated. Strains B2 (Staphylococcus warneri), B3 (Bacillus aryabhattai), B9 (Staphylococcus saccharolyticus), B11 (Acinetobacter radioresistens) and B15 (Bacillus velezensis) were isolated from the rhizosphere of Arundo donax (carrizo), and strain B95 (Pseudomonas aeruginosa) was taken from the rhizosphere of maize.
Divided Petri dish assay. The assay of antagonism by VOC was carried out in Petri dishes with two divisions (90 mm in diameter), containing Nutrient Agar (NA) and PDA, respectively. The bacterial strains were streak-inoculated on the NA side of the dish and incubated at 25 °C for 12 h. A PDA disk with mycelial growth of the fungal isolate (5 mm in diameter) was then inoculated in the side of the Petri dish containing PDA. Immediately afterwards, the dishes were sealed and incubated at 25 °C for 10 days, in a completely randomized arrangement, with 5 repetitions for each bacteria/fungus combination. Petri dishes with PDA inoculated with the fungal isolate in the absence of bacteria were used as controls. The diameter of the fungal cultures was measured and the percentage of inhibition was calculated using the formula proposed by Vincent (1947). The assay was carried out twice, independently, in different times.
Detached leaf assay. The bacteria that displayed inhibition percentages ≥40%, with at least two E. turcicum isolates in the divided dish assay, were selected to evaluate their effect in the reduction of maize leaves infection by four E. turcicum isolates. The assay was performed in divided Petri dishes (100 mm × 15 mm); on one side, the maize leaf was placed and inoculated with a suspension of fungal conidia, following the methodology previously described for the pathogenicity test. A 35 mm diameter Petri dish, containing AN medium with the bacterial strain streak-inoculated, was placed on the other side of the plate. Pathogenicity controls with no bacteria inoculated on the 35 mm Petri dish containing sterile AN medium, were used for each fungal isolate. Additionally, non-inoculated leaves, exposed to bacterial VOCs were used as bacterial controls to rule out a possible phytotoxic effect of the bacterial VOCs on the maize leaf, which could interfere with the estimation of the damage caused by the fungus. The plates were tightly sealed to prevent VOC leakage. Four replicates were performed per treatment. The plates were distributed in a completely randomized design and incubated at 25 ºC, with a photoperiod of 16 h light/ 8 h darkness, for six days. The percentage of leaf area affected (%LAA) was estimated using the Image J software, version 1.8.0 (Schindelin et al., 2012). The assay was carried out twice, independently and at different times.
Hydrocyanic acid (HCN) production test. The production of HCN in four bacterial strains was evaluated following the methodology reported by Anand et al. (2020). Filter paper squares (1 cm2) were dipped in the Feigl and Anger reagent, which consists of 10 mL of chloroform (CTR Scientific, Mexico City, Mexico), 50 mg of copper ethyl acetoacetate (II) (Thermo scientific, Waltham, Massachusetts, USA) and 50 mg of 4,4-methylene bis (N, N-dimethylaniline) (Sigma Aldrich, San Luis, Missouri, USA), until saturation. The filter paper was removed from the reagent and left to dry all night at room temperature (25 °C). The bacteria were streaked on AN medium in Petri dishes (60 mm in diameter), the filter paper was placed on the inner side of the Petri dish lid. Three replicates were performed per treatment. The plates were sealed and incubated at 25 °C for 48 h. Strains were considered positive for HCN production if the filter paper turned blue.
Statistical analysis. The statistical analyses were carried out in R Studio version 1.4.1106 (RStudio Team 2020), using the “agricolae” package (de Mendiburu, 2020). The diameter of the fungal culture and the %LAA were analyzed with an analysis of variance (ANOVA) and Tukey’s post hoc means separation test (a = 0.05). The %LAA was transformed with the arcsine formula before the statistical analysis. The normality of the residuals was corroborated using the Shapiro Wilks test and with a Normal QQ plot.
Results
Morphological characterization of fungal isolates. Four monosporic isolates were obtained, with morphological characteristics that varied slightly (Table S1, S2). Growth rates fluctuated between 7.56 and 19.89 mm/day. The cultures were cottony, olive-green color in the front, and fimbriate margin (Figure 1A). On the opposite side, they were olivaceous black in color, with white- or cream-colored edges (Figure 1B). The conidia of the four isolates were tapered, slightly curved to straight, elongated, olive colored, with a prominent hilum (Figure 1C), a length of 54.49 to 124.34 µm (91.77 µm ± 16.75), a width of 11.81 to 29.86 µm (21.01 µm ± 3.24), and an average of six septa (Table S2).
Molecular identification of fungal isolates. The sequences comparison of the act and rpb2 genes, as well as the ITS region, displayed percentages identity of 96.63
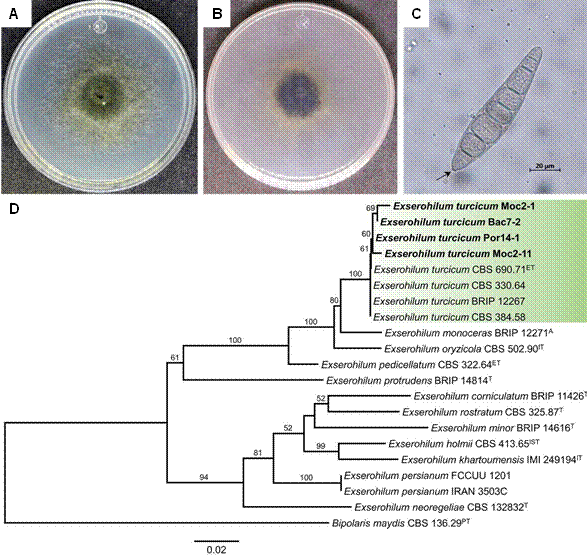
Figure 1 Characterization of Exserohilum turcicum isolates. A-C) Morphological characteristics of representative isolate Bac7-2. A) Frontal view of the culture in PDA; B) Reverse view of the culture in PDA; C) Conidium; D) Maximum Likelihood phylogram inferred from the combined matrix of the markers act+ITS+rpb2 from Exserohilum.
to 100% with the species Exserohilum turcicum. The sequences of the isolates were deposited in the GenBank data base and the accession numbers are shown in Table S1.
Figure 1D shows the phylogram inferred from the concatenated alignment act+ITS+rpb2, where isolates Bac7-2, Por14-1, Moc2-1 and Moc2-11 are shown to belong to Exserohilum turcicum, since they group into the same clade as the E. turcicum CBS 330.64 type isolate (bootstrap of 100%).
Pathogenicty tests. The four E. turcicum isolates produced typical lesions on the leaves, in the detached leaf assay (Figure 2). The first lesions, such as chlorotic spots in the inoculated area, were observed four days after the inoculation and progressed to necrotic lesions (Figure 2). The leaves of the control treatment remained free of lesions during the assay (Figure 2A).
Antifungal effect of the bacterial VOCs on E. turcicum
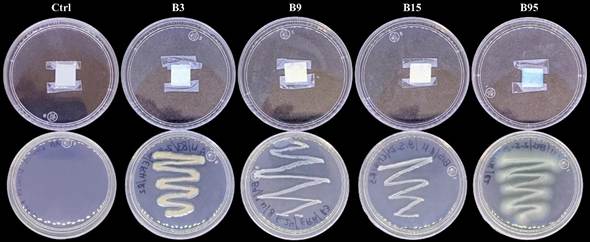
Divided Petri dish assay. The mycelial growth of the E. turcicum isolates, except for isolate Moc2-11, was reduced by the VOCs of at least one rhizobacteria (Figure 3; Table 1). The VOCs of strains B3 and B95 inhibited fungal isolates in a greater proportion. Strains B9 and B15 only inhibited the growth of isolates Bac7-2 and Moc2-1, and favored the growth of isolate Por14-1. Strain B15 showed greater inhibition of the isolate Bac7-2 (63.29%). Strains B2 and B11 only reduced the growth of isolate Bac7-2 and favored the growth of isolate Por14-1 (Table 1).
Detached leaf assay. Based on the results of the divided plate assay, strains B3, B9, B15 and B95 were chosen to evaluate the protective effect of their VOCs against infections caused by the E. turcicum isolates on detached maize leaves. The %LAA exposed to the bacterial VOCs was statistically lower (p < 0.05) to the one recorded
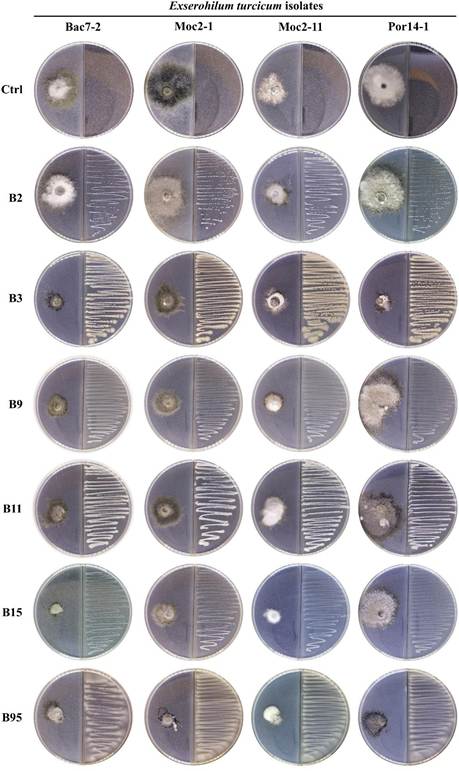
Figure 3 Effect of the bacterial volatile organic compounds on the mycelial growth of Exserohilum turcicum in divided Petri dishes.
Table 1 Effect of the bacterial volatile organic compounds (VOCs) on the mycelial growth of Exserohilum turcicum in divided plates.
| Strain | Bac7-2 | Moc2-1 | Moc2-11 | Por14-1 | ||||
|---|---|---|---|---|---|---|---|---|
| Colony diameter | %MGI | Colony diameter | %MGI | Colony diameter | %MGI | Colony diameter | %MGI | |
| B2 | 35.21*,b | 26.13 ± 9.41 | 52.95a | - | 32.38a | - | 49.48a | - |
| B3 | 20.86cd | 56.22 ± 3.08 | 31.35bc | 37.52 ± 8.38 | 15.08b | 39.48 ± 13.81 | 19.56c | 33.83 ± 5.40 |
| B9 | 19.91cd | 58.22 ± 2.19 | 25.10c | 49.99 ± 2.08 | 18.02ab | 27.68 ± 7.70 | 48.75a | - |
| B11 | 37.14b | 22.09 ± 3.33 | 42.40ab | 15.52 ± 10.47 | 25.42ab | - | 47.39a | - |
| B15 | 17.50d | 63.29 ± 1.46 | 23.96c | 52.26 ± 2.19 | 21.66ab | 13.10 ± 4.84 | 52.04a | - |
| B95 | 28.14bc | 40.96 ± 5.70 | 23.25c | 53.67 ± 2.34 | 15.87b | 36.33 ± 3.63 | 19.12c | 35.32 ± 8.74 |
| CTRL | 47.67a | - | 50.19a | - | 24.92ab | - | 29.57b | - |
* Means are shown.
%MGI Percentage of mycelial growth inhibition.
- Indicates no inhibition.
Data followed by different letters in the same column are statistically different, according to Tukey’s test (p < 0.05).
in the leaves of the control group (Figure 4, Table 2). The lowest %LAA values were recorded in the treatments with the B95 strain, with reductions of the infection that ranged between 95.58 and 98.63% in the leaves infected with isolates Por14-1 and Moc2-1, respectively. The VOCs of strain B15 reduced the infection (>80%) in the maize leaves after being inoculated with the fungal isolates. No phytotoxic effects by the bacterial VOCs were observed in the maize leaves (Figure 4).
Production of hydrocyanic acid (HCN). HCN production was found in strains B3 and B95. After 24 h of incubation of the rhizobacteria, a color change was observed (from white to blue) in the filter paper on the culture of the B95 strain, which intensified after 48 h of incubation. In strain B3, a slight blue color was observed in the filter paper after 48 h. The intensity of the blue color in strain B95 suggests that it produces a higher concentration of this VOC than strain B3 (Figure 5).
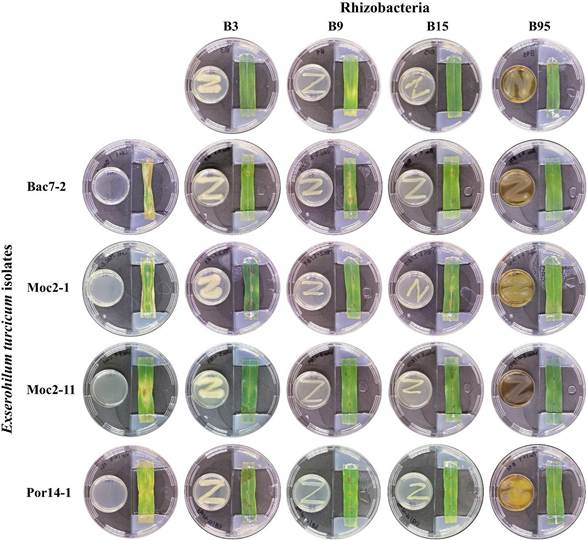
Figure 4 Effect of the bacterial volatile organic compounds on the infective capacity of Exserohilum turcicum on detached maize leaves.
Table 2 Effect of the bacterial volatile organic compounds (VOCs), in the infective capacity of Exserohilum turcicum in detached maize leaves.
| Strain | Bac7-2 | Moc2-1 | Moc2-11 | Por14-1 | ||||
|---|---|---|---|---|---|---|---|---|
| %LAA | %RI | %LAA | %RI | %LAA | %RI | %LAA | %RI | |
| B3 | 4.23*,c | 92.82 ± 1.92 | 8.93b | 84.17 ± 4.79 | 4.47b | 89.98 ± 4.74 | 16.63b | 63.74 ± 12.08 |
| B9 | 18.41b | 68.82 ± 7.24 | 4.44bc | 92.12 ± 2.80 | 5.48b | 87.71 ± 3.98 | 11.47b | 74.97 ± 7.59 |
| B15 | 7.12bc | 87.92 ± 4.22 | 7.59b | 86.55 ± 4.17 | 8.48b | 80.98 ± 4.88 | 4.85b | 89.40 ± 5.26 |
| B95 | 1.54c | 97.37 ± 1.32 | 0.76c | 98.63 ± 0.28 | 1.23b | 97.24 ± 1.04 | 2.02b | 95.58 ± 1.85 |
| CTRL | 59.06a | - | 56.48a | - | 44.65a | - | 45.87a | - |
%LAA Percentage of leaf area affected.
%RI Percentage reduction of the infection.
* Means are shown.
Data followed by different letters in the same column are statistically different, according to Tukey’s test (p < 0.05).
Discussion
The morphometric and microscopic characteristics of fungal isolates were similar to those reported for other E. turcicum isolates in the region (Félix-Gastélum et al., 2018) and worldwide (Hernández-Rastrepo et al., 2018). The phylogenetic multigene analysis verified the identity of the four pathogenic fungal isolates.
The observed variation of the diseased severity induced by the isolates, has been reported in other studies and it has been attributed to the high genetic diversity in the populations of this fungal species, along with the susceptibility of the maize varieties and/or the combination of both (Ahangar et al., 2016; Nieuwoudt et al., 2018; Cui et al., 2022; Bankole et al., 2023).
Strains of bacterial genera such as Pseudomonas, Bacillus, Pantoea, Paenibacillus and Sinomonas have been proven to inhibit the mycelial growth of E. turcicum by producing diffusible compounds and hydrolytic enzymes (Sartori et al., 2020; Chen et al., 2022). However, the effect of the bacterial VOCs on this phytopathogen has been scarcely explored.
This study shows the capacity of VOCs of bacterial strains (B3, B9, B15 and B95), to inhibit the mycelial growth of E. turcicum and protect maize leaves against the attack of this fungus by reducing the leaf area affected. The antifungal activity of these bacterial strains was previously evaluated against Rhizoctonia zeae and recorded inhibitions of the mycelial growth between 40% and 50% via diffusible compounds (Morales-Ruiz et al., 2022). The same authors reported that strain B95 was inefficient in the inhibition of R. zeae, which contrasts with the results of this study, since this strain displayed high inhibition values by VOCs and diffusible compounds (Data not shown), against all E. turcicum isolates. In addition, the VOCs of strain B95 reduced the %LAA in maize leaves inoculated with the fungal isolates by more than 90%.
Sartori et al. (2020) reported mycelial growth inhibition of E. turcicum by VOCs emitted by Curtobacterium, Pantoea and Bacillus strains, the latter genus displaying the greatest fungal inhibition. Among the VOCs produced by these bacteria we can mention 2-Nonadecanone, indole, D-limonene, 2-Tridecanone and dimethyl trisulfide, whose fungal antifungal activity has already been shown (Wu et al., 2019). Gao et al. (2017) reported inhibitions between 83 and 91% of Alternaria alternata and Botrytis cinerea, respectively, using VOCs from B. velezensis, ZSY-1 strain. This antifungal effect was attributed to the VOCs pyrazine, benzothiazole, phenol and 1,1-dimethylethyl. The mycelial growth inhibition of the E. turcicum isolates by B. velezensis strain B15 VOCs, is similar to the values reported by Gao et al. (2017).
Pseudomonas species such as P. aureaginosa and P. fluorescens produce VOCs such as 2,5-Dimethyl-3(2H)-furanone, silanediol, 2,4,4-Trimethyl-1-pentene, HCN, dimethyl disulfide (DMDS), dimethyl trisulfide (DMTS), with proven anti- fungal activity against Botryosphaeria rhodina, R. solani, Pythium aphaniderma- tum, Penicillium italicum, and others (Michelsen and Stougaard, 2012; Wang et al., 2020: Morales et al., 2023). The variations regarding the percentages of inhibition (4 and 80%) reported by these authors for P. aureaginosa and P. fluorescens were attributed to the bacterial (antagonist) and fungal strains (pathogen). The inhibition values of E. turcicum isolates by P. aureaginosa B95 were high, especially against isolate Moc2-1, which was inhibited by 98%. This value is greater to those docu- mented by the authors mentioned above.
The use of members of the Staphyloccocus genus as a biological control agent is not frequent. However, some species that produce VOCs with antifungal ac- tivity have been identified. Alijani et al. (2019) documented a 34.52% mycelial growth inhibition and 82.81% inhibition in the conidia germination of Colletotri- chum nymphaeae, due to the effect of the VOCs emitted by Staphylococcus sciuri. Mesityl oxide, acetic acid, toluene, o-xylene, 4-methtldecane and 2-methylpropyl ester were the most commonly produced VOCs by S. sciuri, all with verified anti- microbial activity (Nakkeeran et al. 2020).
The inhibition values of the B2 (S. warneri) and B9 (S. saccharolyticus) strains in this study are different to those reported by Alijani et al. (2019). Strain B9 in- hibited isolates Bac7-2 and Moc2-1 to greater extents, whereas strain B2 only in- hibited isolate Bac7-2 by 26.13%. The reduced %LAA observed in maize leaves inoculated with the E. turcicum isolates and then exposed to the VOCs of strain B9, may be due to an inhibition of the conidial germination of the E. turcicum isolates, since these were used as inoculants to infect the maize leaves.
HCN is a VOC with an antifungal effect, which interferes with cell respiration by inhibiting the cytochrome c oxidase. It is produces by rhizobacteria, especially by Pseudomonas, although cyanogenic strains of Aeromonas, Chromobacterium, Burkholderia and Bacillus have also been reported (Sehrawat et al., 2022). Antifungal species P. aeruginosa, P. fluorescens, P. chlororaphis, P. putida, B. cereus, B. subtilis, B. paramycoides and B. aryabhattai have been reported as HCN producers (Anand et al., 2020; Shastri et al., 2020; Sehrawat et al., 2022; Riera et al., 2023). Based on the ability of strains B95 and B3 to produce HCN, it could be suggested that their antagonistic effect can be attributed to this VOC, especially in strain B95. However, it is possible that other VOCs are involved. On the other hand, the antifungal effect of strains B9 and B15 is caused by VOCs other than HCN, such as those reported by Alijani et al. (2019) for Staphyloccocus (mesityl oxide, acetic acid, toluene, o-xylene, 4-methyldecane, 2-methylpropyl ester), or those reported by Sartori et al. (2020) for Bacillus (2-Nonadecanone, indole, D-limonene, 2-Tridecanone and dimethyl trisulfide). However, the involvement of non-previously reported VOCs cannot be ruled out.
Conclusions
The fungal isolates recovered from maize leaves with leaf blight symptoms were identified as E. turcicum, and their pathogenicity was verified. The mycelial growth and infective ability of E. turcicum were affected by the VOCs emitted by the evaluated rhizobacteria. Strains B3 (Bacillus aryabhattai), B15 (Bacillus velezensis) and B95 (Pseudomonas aeruginosa) were the most efficient in the mycelial growth inhibition of the fungal isolates and in the reduction of the %LAA. Strain B95 has a higher potential as a biocontrol agent against E. turcicum, and its further study is needed to verify its effectiveness in planta. The HCN produced by strains B3 and B95 may be involved in the antifungal effect on E. turcicum. Strains B9 and B15 produced no HCN, suggesting that the antagonism against E. turcicum may be due to other antifungal VOCs. It is necessary to study the volatile profile of the four bacterial strains and their individual and collective (mixed) effects on E. turcicum, in order to explore a possible synergic effect between VOCs and between bacterial strains.











 texto en
texto en