Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.42 no.2 Texcoco may. 2024 Epub 24-Feb-2025
https://doi.org/10.18781/r.mex.fit.2310-2
Phytopathological Note
Detection and molecular characterization of a 16SrII group phytoplasma associated with ‘witches broom’ disease in cactus (Opuntia sp.)
1 Postgrado en FitosanidadFitopatología, Colegio de Postgraduados Campus Montecillo. Km 36.5 Carretera México-Texcoco. Montecillo, Texcoco, Estado de México, México. C. P. 56264.
Objective/Background
Phytoplasmas are obligate plant pathogens that exhibit strong specificity with their hosts. Typical symptoms induced by these pathogens include stunted growth and general decline, among others, and they rarely lead to plant death. The aim of this research was to determine the phytoplasma associated with the ‘witch’s broom’ symptom in an ornamental cactus (Opuntia sp.).
Materials and Methods
Four samples of ornamental cacti exhibiting ‘witch’s broom’ symptoms were collected from four commercial nurseries in Texcoco, State of Mexico. DNA extraction was performed on the samples, followed by PCR using specific primers for phytoplasmas (P1/P7 and R16F2n/R16R2). Phytoplasma determination was carried out through PCR, in vitro RFLP, sequencing, and phylogenetic analysis.
Results
According to the various analyses conducted, it was determined that the phytoplasma associated with the ornamental cactus belongs to the subgroup 16SrII-C.
Conclusion
Based on the obtained results, it is established that a phytoplasma from the 16SrII-C subgroup is associated with the ‘witch’s broom’ symptom in the ornamental cactus (Opuntia sp.).
Keywords: deformation; uncultivable pathogens; in vitro RFLP
Objetivo/Antecedentes.
Los fitoplasmas son patógenos obligados de plantas, tienen una fuerte especificidad con sus hospedantes, los síntomas típicos inducidos por estos patógenos incluyen reducción de crecimiento y declinamiento generalizado, entre otros, y rara vez ocasionan muerte de la planta. El objetivo de esta investigación fue determinar el fitoplasma asociado al síntoma de ‘escoba de bruja’ en un cactus ornamental (Opuntia sp.).
Materiales y Métodos.
En cuatro viveros comerciales en Texcoco, Estado de México, se tomaron cuatro muestras de cactus ornamental con síntomas de ‘escoba de bruja’. Se realizó extracción de ADN de las muestras y se sometieron a PCR con iniciadores específicos para fitoplasmas (P1/P7 y R16F2n/R16R2). La determinación del fitoplasma en estudio se realizó por PCR, RFLP in vitro, secuenciación y análisis filogenético.
Resultados.
De acuerdo con los diferentes análisis que se realizaron, se determinó que el fitoplasma asociado al nopal ornamental pertenece al subgrupo 16SrII-C.
Conclusión.
Con base en los resultados obtenidos, se establece que un fitoplasma del subgrupo16SrII-C está asociado con el síntoma de ‘escoba de bruja’ del cactus ornamental (Opuntia sp.).
Palabras clave deformación; patógenos no cultivables; RFLP in vitro
Introduction
Different cacti have been planted and introduced in some parts of the world as ornamental and edible plants. Cacti are susceptible to infections by phytoplasmas and they develop diseases with symptoms characterized by growth in the shape of a witch’s broom and a green mosaic pattern on the epidermis (Cai et al., 2008). The global movement of bulbs, trimmings and seeds of ornamental and for human consumption has enabled the introduction of new pathogens to diverse ecological niches (Miedaner and Garbelotto, 2024). When the native flora is susceptible to an introduced pathogen, this can give rise to a rapid decline, which may lead to its extinction (Miedaner and Garbelotto, 2024). In recent years, increasing amounts of ornamental plants have been found to have symptoms of phytoplasmas, pathogens with a broad genetic plasticity, which helps them inhabit and infect any type of plant, an example of which is ‘Candidatus Phytoplasma asteris’, which has been found in over 80 monocotyledonous and dicotyledonous species in different parts of the world (Lee et al., 2009). Phytoplasmas are obligate plant and insect parasites, and in most cases, they need both hosts to disperse in nature (Whitcomb and Tully, 1989). These bacteria are characterized by having a small genome, and as a consequence, they have a limited metabolic capacity, thus they necessarily require an insect vector and a host plant to reproduce. Phytoplasmas are pathogens that have colonized both the animal and plant kingdoms, and the symptoms they induce interfere with plant development, since they cause proliferation, virescence and phyllody primarily (Bertaccini, 2015).
Considering the importance of generating scientific knowledge of the emerging pathogens with the potential of spreading easily and efficiently by insect vectors onto crops of economic interest, it is necessary to know the different groups of phytoplasmas that can be found in ornamental cacti that are widely sold in Mexico due to their phenotypic characteristics, making them a source of inoculum. Therefore, the aim of this investigation was to detect and identify the phytoplasma related to the thickening and proliferation cladodes in an ornamental cactus.
Detection of phytoplasmas. Four cactus samples with “witches’ broom” symptom were obtained from four nurseries in Texcoco, State of Mexico (Figure 1B-C). DNA extraction was carried out using the CTAB 2% method, and rDNA amplification (100 ng µL-1) was carried out using universal primers for phytoplasmas, P1 (5´-AAGAGTTTGATCCTGGCTCAGGATT-3´) and P7 (5´-CGTCCTTCATCGGCTCTT-3´) (Deng y Hiruki, 1991; Schneider et al., 1995) which amplify 1.8 kb, followed by a second PCR (nested reaction) with the pair R16F2n (5´-GAAACGACTGCTAAGACTGG-3´) and R16R2(5´TGACGGGCGGTGTGTACAAACCCCG-3´) (Gundersen and Lee, 1996) which amplify 1.2 kb. The PCR reactions were carried out in a Techne® TC-300
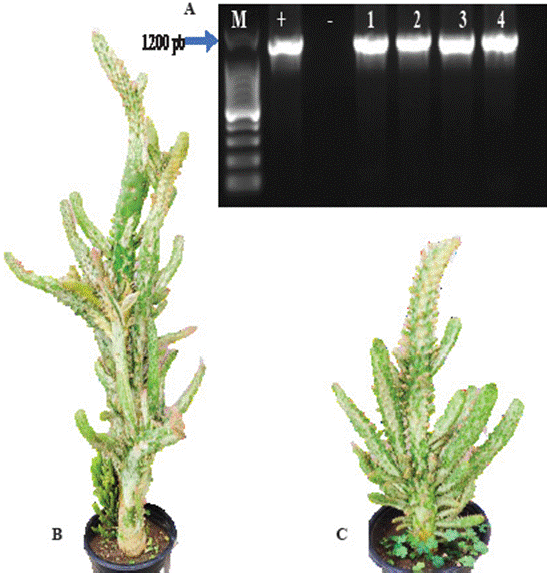
Figure 1 A) Amplifications of 16S rDNA of phytoplasmas obtained using primers R16F2n/R16R2. Lane M; Molecular marker 100 pb, lane +; DNA from Dimorphotheca sinuata infected with “Candidatus Phytoplasma asteris” (16SrI-B), lane -; Negative control, PCR without a template, lane 1-4; samples of cactus (Opuntia sp.) with “witches’ broom” syndrome, from nurseries located in Texcoco, State of Mexico; B-C) “Witches’ broom” symptoms in an ornamental cactus.
thermocycler, with an amplification protocol described earlier by Ortega-Acosta et al. (2019). As a positive control, DNA from Dimorphotheca sinuata infected with ‘Candidatus Phytoplasma asteris’ (16SrI-B) was used. From the nested PCR, 5 µL of amplified product were loaded and observed on 1% agarose gel stained with ethidium bromide and viewed on a UV transilluminator.
RFLP analyses. Approximately 500 ng of each one of the products obtained from the second PCR with the primers R16F2n/R16R2 underwent a Restriction fragment length polymorphism (RFLP) using the restriction enzymes RsaI, MseI (Tru 91), KpnI, EcoRI (Promega, USA) and HaeIII (Sigma-Aldrich, USA) at 37 °C for 4 h. For this analysis, only the key enzymes used to identify groups of phytoplasmas were considered. The number and size of the resulting fragments were analyzed by electrophoresis in 3% agarose gel. The restriction patterns obtained were compared with those published earlier (Lee et al., 1998) and with those obtained with the tool iPhyClassifier from a reference strain from subgroup 16SrII-C (Zhao et al., 2009).
Sequencing and phylogenetic analysis. The products obtained from the nested PCR were purified and sequenced in both directions (Macrogen Inc. Korea) and afterwards, a phylogenetic tree was generated using the neighbor-joining method in MEGA X (Kumar et al., 2018) using sequences from different groups and subgroups of phytoplasmas. In this case, Acheloplasma laidlawii was used as an external group.
The results indicated the presence of phytoplasmas in the four samples of ornamental cacti with symptoms of “witches’ broom” (Figure 1A). The consensual sequence obtained in this study was deposited in the in NCBI (National Center for Biotechnology Information) GeneBank with accession number 0N413680.1. The BLAST analysis of the sequence indicated a 100% similarity with cactus witches’ broom phytoplasma (group 16SrII, subgroup C) (accession numbers MH644006MH644007). The analysis of electrophoretic patterns with restriction enzymes confirmed that the phytoplasma under study is a member of subgroup 16SrII-C (Figure 2B). On the other hand, the in silico electrophoretic profile of reference strain AJ293216.2 of subgroup 16SrII-C (Figure 2C), which considers 17 restriction
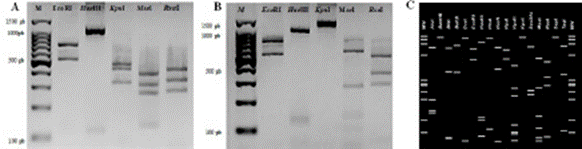
Figure 2 RFLP analysis of the 16S rDNA of phytoplasmas amplified using primers R16F2n/R16R2 and digested with five restriction enzymes: EcoRI, HaeIII, KpnI, MseI and RsaI M: molecular marker 100 pb (Promega, USA); A) Positive control ‘Candidatus Phytoplasma asteris’ (I-B); B) Symptomatic sample of cactus from this study (Accession number: 0N413680); C) Restriction patterns in silico, generated from the sequences of gene 16S rDNA of the Cactus witches’-broom phytoplasma 16SrII-C (Accession number: AJ293216.2) of the reconnaissance sites for 17 restriction enzymes.
enzymes, coincided with the result of the RFLP obtained with the digestion carried out in the laboratory with the five key enzymes that provide certainty of the group to which a certain phytoplasma belongs. The phylogenetic analysis placed the phytoplasma under study (0N413680) in subgroup 16Sr II-C (Figure 3).
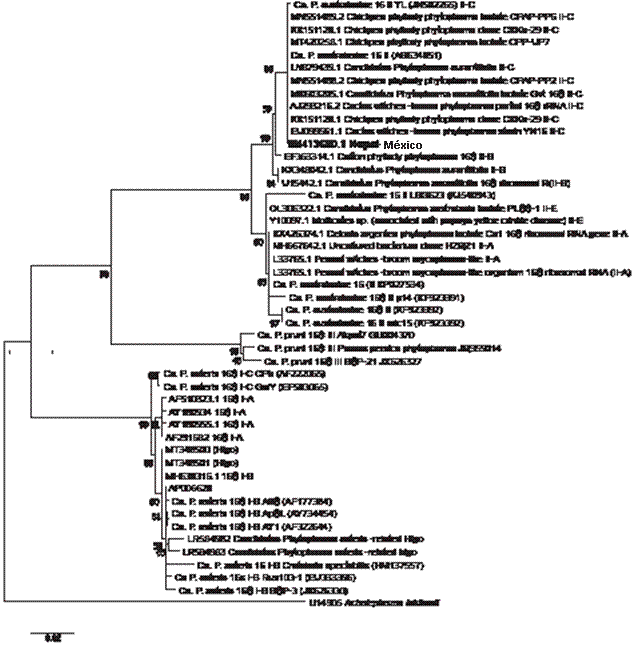
Figure 3 Phylogenetic tree created using the neighbor-joining method, with sequences of the 16S rDNA deposited in the GeneBank, showing the relationship between the phytoplasmas for groups 16SrI and 16SrII with the phytoplasma that induced “witches’ broom” in cactus (Opuntia sp.) (Accession number: 0N413680.1). The bar indicates the number of substitutions per nucleotides.
For their reproduction, phytoplasmas require diverse hosts, whether plants or insects. Some insects reported as phytoplasma vectors are Macrosteles quadrilineatus, Haplaxius crudus, Dictyophara europaea, Euscelidius variegatus and Hyalesthes obsoletus, among other (Alma et al., 2019). In plants, they are found in the phloem, including immature cells of this tissue that still keep their nuclei, whereas in vector insects, they are found in diverse tissues, intra or extracellularly (Bertaccini et al., 2014). In ornamental plants, 14 groups of phytoplasmas have been found, out of the 16 existing ones, as well as 30 subgroups, all of which induce different symptoms (Bellardi et al, 2018). ‘Candidatus Phytoplasma asteris’ is the main group that infects ornamental plants worldwide. To date, 60 ornamental plant species have been reported to be infected with phytoplasmas globally (Madhupriya, 2016).
In Turkey, ‘Candidatus Phytoplasma aurantifolia’-related strain (16SrII-B group) was recently reported to have been found in an ornamental cactus that displayed symptoms similar to those observed in plants in this study (Ayvaci et al., 2021). In Mexico, several groups of phytoplasmas have been reported as related to the prickly pear cactus with “thickening of the cladode” or “male plant,” the most important plant in this crop, since it significantly reduces the production in all cactus-producing areas of the country (Hernández-Pérez et al., 2009; Suaste et al., 2012; Aguilar, 2019). The symptoms of this disease consist of a partial yellowing of the plant, a gradual reduction in the size of the leaf and fruit, thickening and cordiform development of the cladode, as well as the inhibition of the floral and vegetative sprouting, and in the final stages, plant production stops without it dying. In these symptoms, different groups of phytoplasmas have been found to be related, including 16SrII (Hernández-Pérez et al., 2009), 16SrI (‘Candidatus Phytoplasma asteris’) (Zak et al., 2011), 16SrXIII (Suaste et al., 2012) and 16SrVI (‘Candidatus Phytoplasma trifolii’) (Aguilar, 2019). Despite the wide geographic distribution of phytoplasmas, its range of hosts and importance as phytopathogenic microorganisms, there is little understanding of the defense routes the plant has to avoid its establishment, which can give rise to alternative strategies for its management, thus reducing its impact on agriculture. On the other hand, there are norms that regulate the establishment and mobility of this type of ornamental plants, such as the General Wildlife Law (2000) and the Official Mexican Norm for the Protection of Wild Native Mexican Flora and Fauna Species (2002). Likewise, the international trade of cacti is regulated by the Convention on International Trade in Endangered Species of Wild Flora and Fauna (2008). Regarding phytotechnical resources for agriculture and food, we have the Federal Seed Production, Certification and Trade Law (2007). In trade, the use of distinctive signs is regulated internationally by the Agreement on Trade-Related Aspects of Intellectual Property Rights in the World Trade Organization and in Mexico, unlike other countries, these pathogens have been allowed to establish in different ornamental cacti species, which can become an important source of inoculum for other crops. Alongside this, which vector insects may be involved in its transmission to other agriculturally important crops is unknown.
On the other hand, the advantage in the identification with phylogenetic and molecular criteria helps provide more accurate knowledge on the association of more than one phytoplasma in hosts with the same or different symptoms or the symptoms caused by one phytoplasma group, such as 16SrI. This has allowed the concept of disease in the case of phytoplasmas to change; in many cases, it is pointed out that the characteristic biological properties of a particular phytoplasma are linked to an established group or subgroup with phylogenetic criteria, so these biological properties can only be used as secondary criteria to consider a phytoplasma as the causal agent of a disease.
Based on our results, a phytoplasma of the subgroup 16SrII-C is established to be related with the “witches’ broom” symptom in an ornamental cactus (Opuntia sp.).
Literatura Citada
Aguilar, P.N.Y. (2019). Manejo integrado del engrosamiento del cladodio en tres cultivares de nopal tunero en Teotihuacán, Estado de México. [Tesis de Doctorado en Ciencias, Colegio de Postgraduados], México. [ Links ]
Alma, A., Lessio, F., y Nickel, H. (2019). Insects as Phytoplasma Vectors: Ecological and Epidemiological Aspects. En Bertaccini, A., Weintraub, P., Rao, G., y Mori, N. (Eds.), Phytoplasmas: Plant Pathogenic Bacteria II. Springer, Singapore. 10.1007/978-981-13-2832-9_1 [ Links ]
Ayvaci, H., Simsek, E., Akkurak, H., Dikilitas, M., y Guldur, M.E. (2021). First report of a ‘Candidatus Phytoplasma aurantifolia’-related strain associated with Cactus witches’ broom disease in Opuntia sp. in Turkey. New Disease Report, 44(1). 10.1002/ndr2.12031 [ Links ]
Bellardi, M.G., Bertaccini, A., Rao, G.P., y Madhupriya (2018). Phytoplasma diseases in ornamental crops. En Rao, G.P., Bertaccini, A., Fiore, N., y Liefting, L.W. (Eds.), Phytoplasmas: Plant Pathogenic Bacteria-I. Characterization and Epidemiology of Phytoplasma Associated Diseases. Springer, Singapore, pp. 191-233. [ Links ]
Bertaccini, A. (2015). Phytoplasma research between past and future: what directions?. Phytopathogenic Mollicutes, 5, S1-S4. [ Links ]
Bertaccini, A., Duduk, B., Paltrinieri, S., y Contaldo, N. (2014). Phytoplasmas and phytoplasma diseases: severe threat to agriculture. American Journal of Plant Sciences, 5, 1763-1788. 10.4236/ajps.2014.512191 [ Links ]
Cai, H., Wei, W., Davis, R.E., Chen, H., y Zhao, Y. (2008). Genetic diversity among phytoplasmas infecting Opuntia species: virtual RFLP analysis identifies new subgroups in the peanut witches’-broom phytoplasma group. International Journal of Systematic and Evolutionary Microbiology, 58, 1448-1457. [ Links ]
Convención sobre el Comercio Internacional de Especies Amenazadas de Fauna y Flora Silvestres (2008). https://cites.org/esp/disc/text.php (consulta, agosto 2023). [ Links ]
Deng, S. y Huriki (1991). Amplification of 16Sr RNA genes from culturable and nonculturable mollicutes. Journal of Microbiological Methods, 14, 53-61. [ Links ]
Gundersen, D.E. y Lee, I.-M. (1996). Ultrasensitive detection of phytoplasmas by nested PCR assays using two universal primer pairs. Phytopathology Mediterranea, 35, 114-51. https://www.jstor.org/stable/42685262 [ Links ]
Hernández-Pérez, R., Noa-Carrazana, J.C., Gaspar, R., Mata, P., y Flores-Estévez, N. (2009). Detection of Phytoplasma on Indian Fig (Opuntia ficus-indica Mill) in Mexico Central Region. OnLine Journal of Biological Sciences, 9(3), 62-66. 10.3844/ojbsci.2009.62.66 [ Links ]
Kumar, S., Stecher, G., Li, M., Knyaz, C., y Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular Biology and Evolution, 35(6), 1547-1549. 10.1093/molbev/msy096 [ Links ]
Lee, I.-M., Gundersen-Rindal, D.E., Davis, R.E., y Bartoszyk, M.I. (1998). Revised classification scheme of phytoplasmas based on RFLP analyses of 16S rRNA and ribosomal protein gene sequences. International Journal of Systematic Bacteriology, 48, 1153-1169. https://pubag.nal.usda.gov/download/26444/PDF [ Links ]
Lee, S., Han, S., y Cha, B. (2009). Mixed infection of 16S rDNA I and V groups of phytoplasma in a single jujube tree. The Plant Pathology Journal, 25, 21-25. 10.5423/PPJ.2009.25.1.021 [ Links ]
Ley Federal de Producción, Certificación y Comercio de Semillas (2007). https://www.diputados.gob.mx/LeyesBiblio/pdf/LFPCCS_110518.pdf (consulta, agosto 2023). [ Links ]
Ley general de vida silvestre (2000). https://www.diputados.gob.mx/LeyesBiblio/pdf/146_200521.pdf (consulta, agosto 2023). [ Links ]
Madhupriya (2016). Molecular characterization of phytoplasmas associated with important ornamental plant species in Northern India. [Thesis submitted at, Amity University, Haryana]. [ Links ]
Miedaner, T. y Garbelotto, M. (2024). Human-mediated migration of plants, their pathogens and parasites. Journal of Plant Pathology. 10.1007/s42161-024-01589-0 [ Links ]
Norma Oficial Mexicana de Protección a Especies Nativas de México de Flora y Fauna Silvestre (2002). https://dof.gob.mx/nota_detalle.php?codigo=735036&fecha=06/03/2002#gsc.tab=0 (consulta, agosto 2023). [ Links ]
Ortega-Acosta, C., Ochoa-Martínez, D.L., Rojas-Martínez, R.I., y Gutiérrez-Gallegos, J.A. (2019). Phyllody of daisy (Dimorphotheca sinuata) associated to ‘Candidatus phytoplasma asteris’. Revista Mexicana de Fitopatología, 37(3), 444-453. 10.18781/R.MEX.FIT.1905-3 [ Links ]
Schneider, B., Seemüller, E., Smart, C.D., y Kirkpatrick, B.C. (1995). Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. En Razin, S. y Tully, J.G. (Eds.), Molecular and diagnostic procedures in mycoplasmology. Academic Press, San Diego. [ Links ]
Suaste-Dzul, A., Rojas-Martínez, R.I., Zavaleta-Mejía, E., y Pérez-Brito, D. (2012). Detección molecular de fitoplasmas en nopal tunero (Opuntia ficus-indica) con síntomas de engrosamiento del cladodio. Revista Mexicana de Fitopatología, 30, 1-9. https://www.redalyc.org/articulo.oa?id=61225129007 [ Links ]
Zak, L.F., Yáñez-Morales, M.J., Alanis-Martínez, I., y González-Pérez, E. (2011). New hosts of 16SrI phytoplasma group associated with edible Opuntia ficus-indica crop and its pests in Mexico. African Journal of Microbiology Research, 5, 910-918. [ Links ]
Zhao, Y., Wei, W., Lee, I.-M., Shao, J., Suo, X., y Davis, R.E. (2009). Construction of an interactive online phytoplasma classification tool, iPhyclassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). International Journal of Systematic and Evolutionary Microbiology, 59, 2582-2593. 10.1099/ijs.0.010249-0 [ Links ]
Received: October 02, 2023; Accepted: February 19, 2024











 texto en
texto en 


