Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.42 no.2 Texcoco may. 2024 Epub 24-Feb-2025
https://doi.org/10.18781/r.mex.fit.2401-2
Phytopathological Note
Fungal causal agents of the Black Spot of the cactus (Opuntia ficus-indica) in Colima, Mexico
1 Facultad de Ciencias Biológicas y Agropecuarias, Universidad de Colima, Autopista Colima-Manzanillo km 40, C.P. 28930, Tecomán, México;
2 Centro Nacional de Referencia Fitosanitaria, Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA), km 37.5 Carretera Federal México-Pachuca, Av. Centenario de la Educación, Col. Santa Ana, C.P. 55740, Tecámac, Estado de México, México;
33 Facultad de Ciencias Biológicas y Agropecuarias, Universidad de Colima, Autopista Colima-Manzanillo km 40, C.P. 28930, Tecomán, México.
Objective/Background:
The prickly pear cactus (Opuntia ficus-indica) holds significant economic, social, and cultural importance in Mexico. However, it is recurrently affected by Black Spot disease (BS), caused by various phytopathogenic fungi. Identifying the causal agents of BS in commercial prickly pear crops is crucial for efficient agronomic management of the disease. The objective of this study was to identify the phytopathogenic fungi responsible for BS in prickly pear plantations in the Colima state, Mexico.
Materials and Methods:
Fifty cladodes from 50 plants exhibiting BS symptoms were collected from commercial plantations in Colima. The pathogenicity of the isolated fungi was verified using Koch’s postulates, and those causing the most severe BS symptoms were molecularly identified.
Results:
Thirty-five fungi were isolated from plants with BS symptoms, of which 20 exhibited distinct mycelial growth. Only six fungi induced BS symptoms; three of them were responsible for severe symptoms in cladodes: Alternaria alternata, Corynespora cassiicola, and Neoscytalidium dimidiatum.
Conclusion:
BS is caused by various phytopathogenic fungi, but this is the first report of C. cassiicola and N. dimidiatum as causal agents of BS in prickly pear cactus.
Key words: Corynespora; Neoscytalidium; Alternaria; pathogenicity; ITS.
Objetivo/Antecedentes:
El nopal verdura (Opuntia ficus-indica) es una planta de importancia económica, social y cultural en México, sin embargo, una de las enfermedades recurrentes a esta especie vegetal es la Mancha Negra (MN), la cual se genera por el ataque de diferentes hongos fitopatógenos, de ahí que la identificación de los agentes causales de la MN en los cultivos comerciales de nopal sea una etapa relevante para el manejo agronómico eficiente de la MN. El objetivo de este estudio fue identificar los hongos fitopatógenos causales de la MN en plantaciones de nopal verdura en el estado de Colima, México.
Materiales y métodos:
Se recolectaron 50 cladodios de 50 plantas con síntomas de MN en plantaciones comerciales de Colima, de los hongos aislados se comprobó la patogenicidad de ellos mediante los postulados de Koch, así mismo se identificaron molecularmente aquellos hongos que generaron los síntomas más severos de MN.
Resultados:
Se aislaron 35 hongos de plantas sintomáticas de MN, de los cuales 20 presentaron un crecimiento micelial distinto. Solo seis hongos generaron síntomas de MN; siendo tres de ellos los responsables de generar síntomas severos en cladodios: Alternaria alternata, Corynespora cassiicola, and Neoscytalidium dimidiatum.
Conclusión:
La MN es generada por diferentes hongos fitopatógenos, pero este es el primer reporte de C. cassiicola y N. dimidiatum como agentes causales de la MN en nopal verdura.
Key words: Corynespora; Neoscytalidium; Alternaria; patogenicidad; ITS
Introduction
Mexico is the leading global producer of vegetable prickly pear cactus (Opuntia spp.) with 13 thousand hectares of cultivated land, yielding a production of 869,956 t and generating profits of 2,962 million pesos in 2021. The states of Morelos, Mexico City, and Mexico State contribute around 75.6% to the national production (SIAP, 2022). This vegetable boasts an annual per capita consumption of 6.3 kg and serves as a rich source of carbohydrates (3-7%), fibre (1-2%), lipids (0.2%), vitamin C (10-15 mg/100 g), proteins (0.5-1%), and minerals (1-2-%) (Martins et al., 2023). Prickly pear cactus plantations face challenges posed by diseases caused by various fungi, with Black Spot (BS) being one of the most common (Hernández-Sánchez et al., 2014). BS leads to total crop loss and is characterized by symptoms manifesting in two ways: circular spots and map-like patterns. The former begins with the appearance of small green spots, which, after a week, turn green reddish to dark brown and increase in size, forming circular spots of 3 to 4 cm in diameter. Subsequently, these circular spots turn black with yellow margins, traversing the prickly pear cactus cladode and causing desiccation in the affected areas (Quezada-Salinas et al., 2006). On the other hand, the expression of symptoms in map form is characterized by partial or total invasion of the cladode with irregularly shaped spots, exhibiting symptoms like those of the circular form (Quezada-Salinas et al., 2013). Various fungi have been reported as causal agents of BS in different species of the Cactaceae family. For instance, Colletotrichum gloeosporioides and Fusarium lunatum, Alternaria alternata and Curvularia lunata in Opuntia plantations in Tlalnepantla, Morelos, Mexico (Flores-Flores et al., 2013); Pseudocercospora opuntiae in plantations in the state of Jalisco, Mexico (Ochoa et al., 2015); Alternaria longipes, Colletrotrichum fructicola, Lasiodiplodia euphorbicola, L. iraniensis, L. jatrophicola, Neofusicoccum batangarum, N. hyalinum, N. batangarum, Neopestalotiopsis sp., and Nigrospora in Nopalea cochenillifera in Brazil (Conforto et al., 2019; Feijo et al., 2019). In this context, the identification of fungi causing diseases in economically significant crops, including Opuntia plantations, is crucial for developing effective management and control strategies, thus enhancing crop productivity and sustainability. In Colima, Mexico, BS remains a significant challenge for vegetable prickly pear cactus cultivation, yet causal fungal agents remain unidentified. This gap in specific knowledge hinders yield optimization and the creation of effective pathogen management strategies. Consequently, this study aims to isolate, assess, and identify the fungal species responsible for BS in prickly pear cactus plantations.
In April and May 2021, the collection of prickly pear cactus cladodes was conducted in commercial vegetable prickly pear cactus (O. ficus-indica) plantations located in five communities in the state of Colima: Agua Dulce (19°17’85.2’’N, 103°52’10.7’’W), Juluapan (19°18’41.2’’N, 103°49’94.2’’W), La Limonera (19°18’50.0’’N, 103°49’17.2’’W), El Espinal (10°16’17.9’’N, 103°47’51.1’’W), and Las Guásimas (19°07’83.8’’N, 103°43’27.4’’W). Disease incidence was visually determined in each plantation by calculating the percentage of symptomatic plants from the total analysed. For this evaluation, 10 plants showing symptoms of BS were selected, with one symptomatic cladode collected each for analysis. These cladodes were individually placed in plastic bags and transported to the laboratory in a cooler within the first 24 h after collection. The cladodes were washed with tap water and dried with paper towels. Subsequently, five to six fragments (0.5 cm2) of symptomatic tissue were cut, disinfested with 1.5% sodium hypochlorite (v/v) for 1.5 min, rinsed three times for one minute each with sterile distilled water, and dried with sterile paper towels in a laminar flow hood (Labconco Inc., USA). To assess the efficacy of disinfection, 100 µL of the last rinse water was taken, plated on potato dextrose agar (PDA, MCD Lab, Mexico), and incubated for seven days. From the disinfested fragments, another six fragments were obtained and distributed in a Petri dish with PDA supplemented with chloramphenicol (500 mg L-1) (Quezada- Salinas et al., 2006; Alonso-Díaz et al., 2007); the latter were incubated at 25 °C in the dark for five to seven days and observed at 24-hour intervals. From each emerging fungal colony, a sample was taken, placed in the centre of a Petri dish with PDA, and incubated under the same conditions. A pathogenicity test was performed with each isolated fungal strain to confirm if they were causal agents of prickly pear cactus BS. For this, cladodes without disease symptoms were collected from commercial plantations and disinfested following the procedure reported by Quezada-Salinas et al. (2006). Incisions (five per cladode) were made on the cladode surfaces with a sterile scalpel, and 5 mm mycelium disks from each fungal strain with seven days of growth were inoculated onto these wounds. As a control treatment, the mycelium disk was replaced with a PDA disk. Each disk was covered with cotton moistened with sterile water for 24 h, the cladodes were placed inside a plastic bag to retain moisture, and they were kept at 25 °C with observations made at 24-hour intervals. From cladodes showing BS symptoms, the re-isolation of the fungal strain was performed to verify Koch’s postulates (Volcy, 2008; Flores-Flores et al., 2013). The pathogenicity tests were conducted in triplicate. The molecular identification of fungi causing the most severe disease symptoms was carried out using the primers ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5-’TCCTCCGCTTATTGATATGC-3’) to amplify a fragment of 500 bp from the ITS region of rDNA. The PCR conditions were as follows: an initial denaturation at 95 °C for 3 min, followed by 25 cycles at 58 °C for 30 sec (annealing), 72 °C for 2 min (extension), 95 °C for 30 sec (denaturation), and a final extension at 72 °C for 10 min (White et al., 1990). The amplified PCR products were sequenced using the Sanger sequencing method on the 3130 Genetic Analyzer (Applied Biosystems) at the Integral Plant Health Diagnosis Laboratory (LADIFIT) (https:// www.colpos.mx/posgrado/fitosanidad/ladifit.php) in the State of Mexico, Mexico. For identification, the fungal sequences were compared with the database deposited in GenBank NCBI (National Center for Biotechnology Information).
Among the analysed plantations, El Espinal exhibited the highest incidence of BS with 60% of plants affected, followed by Agua Dulce (40%), La Limonera (35%), Las Guásimas (20%), and Juluapan (10%) (Figure 1). From the collected cladodes, 35 fungi were isolated, 20 of which exhibited distinct morphological characteristics in terms of colour, texture, and the presence of rings. The majority of the isolates displayed circular growth, complete margins, cottony texture, and flat elevation (Figure 2). There was also variation in the colony’s growth time; 14 fungi covered the Petri dish surface within the first five days, while the remaining ones did so after 10 days. In the pathogenicity tests, only six out of the 20 isolates induced symptoms associated with BS, and three of these caused severe disease symptoms on prickly pear cactus cladodes. This included softening of the cladodes and the development of circular and irregular black spots throughout the plant tissue. Overall, the cladodes exhibited lesions of up to 5 cm in length 10 days after the fungal infection. The fungi that induced more severe symptoms of BS
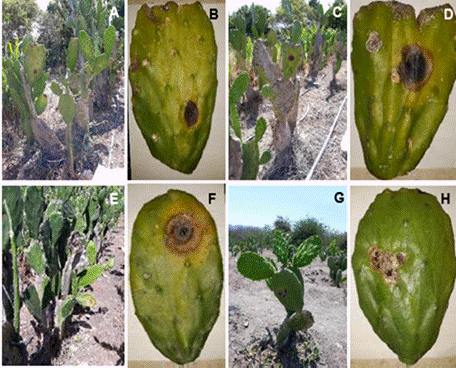
Figure 1 Plants and cladodes with symptoms of Black Spot in vegetable prickly pear cactus (O. ficus-indica) crops in various plantations in Colima state, Mexico. A) El Espinal, B) Agua Dulce, C) Las Guásimas, D) Juluapan.
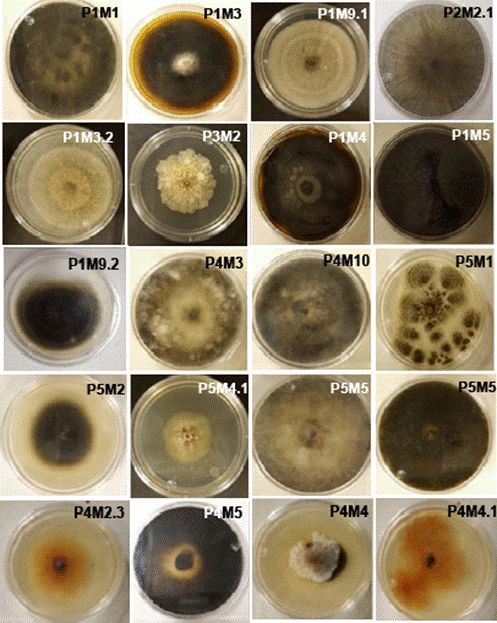
Figure 2 Fungi isolated from prickly pear cactus cladodes showing symptoms of Black Spot. The isolates are deposited in the mycological collection of the Faculty of Biological and Agricultural Sciences at the University of Colima for subsequent morphological and molecular characterization.
were identified as Alternaria alternata (GenBank ID: OP038904), Corynespora cassiicola (OP038906), and Neoscytalidium dimidiatum (OP038907) (Figure 3).
The BS in prickly pear cactus is a disease caused by various fungi, making the identification of the causal agent a crucial step for implementing appropriate control strategies. This is the first report on fungi causing BS in commercial prickly pear cactus plantations in Colima. The fungal species A. alternata, C. cassiicola, and N. dimidiatum were identified as the ones causing the most severe damage to prickly pear cactus cladodes. In this study, 50 cladodes with apparent BS symptoms were collected, and 20 fungi were isolated, exhibiting differences in colour, texture, and the presence of rings. This number is lower than that reported by Conforto
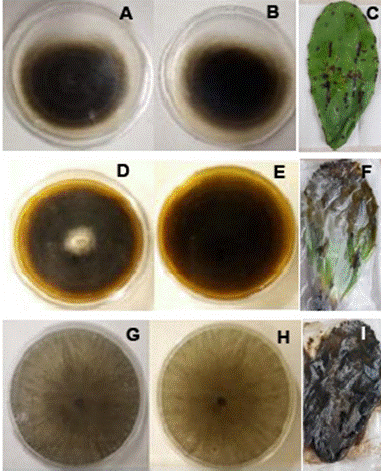
Figure 3 Fungi isolated from cladodes with Black Spot symptoms that induced severe lesions in the pathogenicity test. Growth on PDA of Alternaria alternata (obverse, A; reverse B) and symptoms induced on the cladode (E), Corynespora cassiicola (D, E, F), and Neoscytalidium dimidiatum (G, H, I).
et al. (2019), who obtained 50 isolates from 180 cladodes. The discrepancy in the number of analysed cladodes may explain the difference in the number of isolated fungi in both studies. Regarding the identified fungi in cladodes with BS, Oliveira et al. (2018) reported various Colletotrichum species (C. siamense, C. fructicola, and C. karstii) in N. cochenillifera in Brazil; however, these authors did not evaluate the pathogenicity of the fungi. Quezada-Salinas et al. (2006) isolated and identified C. gloeosporioides in O. ficus-indica cladodes with BS symptoms in Mexico; nevertheless, this fungus did not induce disease in healthy prickly pear cactus plants. On the other hand, the fungi C. gloeosporioides, A. alternata, F. lunatum, C. lunata (Flores-Flores et al., 2013), and Pseudocercospora sp. isolated from symptomatic cladodes did induce the disease in healthy plants (Quezada- Salinas et al., 2006; Ochoa et al., 2015), meaning they are causal agents of BS. Of these species, only A. alternata aligns with one of the isolated fungi in Colima plantations, while C. cassiicola and N. dimidiatum have not been reported as causal agents of BS in prickly pear cactus plantations. Souza et al. (2010) analysed the diversity of fungi causing diseases in forage palm (O. ficus-indica) cultivated in dry regions of northeastern Brazil. Based on morphological characterization, these authors found that Scytalidium lignicola, Alternaria tenuis, Macrophomina phaseolina, Cladosporium cladosporioides, Lasiodiplodia theobromae, Fusarium oxysporum f. sp. opuntiarum, C. lunata, Aspergillus niger, Nigrospora sphaerica, C. gloeosporioides, Exserohilum turcicum, Pestalotia pitospora, Rhizopus stolonifer, Rhizoctonia solani, and Sphaceloma protearum are the most common fungi in plants with disease symptoms. Recently, Conforto et al. (2019) identified Alternaria tenuissima, A. longipes, C. gloeosporioides, C. fructicola, C. siamense, F. lunatum, F. verticillioides, F. incarnatum, F. iraniensis, L. euphorbicola, L. pseutheobromae, L. theobromae, N. batangarum, Neopestalotiopsis australis, N. protearum, N. sphaerica, and N. hainanensis as causal agents of BS in N. cochenillifera. Among these fungi, those of the Alternaria genus were the most common in cladodes with disease symptoms, while L. iraniensis and F. lunatum caused the most severe damage. In contrast to previous findings, the current research identifies A. alternata, C. cassiicola, and N. dimidiatum as the fungi responsible for the most severe symptoms in prickly pear cactus cladodes. These differences may be due to the studied plant species. Additionally, the various fungal species identified as causal agents of BS may be attributed to different factors such as (i) environmental differences in the study area, (ii) activities implemented in each zone for plantation yield, and (iii) the application of fertilizers and agrochemicals (Rodríguez, 2001; Pacasa-Quisbert et al., 2017). Furthermore, the degree of pathogenicity of the causal agents may be attributed to the production of a variety of proteins, organic acids, and secondary metabolites with cytotoxic and phytotoxic activity. For example, Alternaria species produce host-specific toxins (i.e., AK, AAL, and AF), which determine the pathogenicity of these fungi (Castaldi et al., 2023). Specifically, AK-toxins and AF-toxins targets the plasma membrane, leading to electrolyte loss and membrane damage in susceptible plants (Nakashima et al., 1985; Otani et al., 1995). It has also been reported that C. cassiicola produces phytotoxins known as cassiicolins, terpenoids, polyketides, and nitrogenous metabolites that are relevant to the pathogenesis of the fungus (Yang et al., 2022). In conclusion, this study identifies A. alternata, C. cassiicola, and N. dimidiatum as the primary fungi responsible for BS diseases in commercial prickly pear cactus plantations in Colima, making the first report of C. cassiicola and N. dimidiatum in this context. Despite previous findings of various fungi associated with BS in different regions and plant species, these three fungi were found to cause the most severe symptoms in the studied plantations. This discrepancy highlights the impact of various factors on disease prevalence and severity. The identification of these causal agents is crucial for developing targeted management strategies to mitigate BS in prickly pear cacti.
Literatura Citada
Alonso-Díaz, M. A., García, L., Galindo-Velasco, E., Lezama-Gutiérrez, R., Ángel-Sahagún, C. A., Rodríguez-Vivas, R. I. y Fragoso-Sánchez, H. (2007). Evaluation of Metarhizium anisopliae (Hyphomycetes) for the control of Boophilus microplus (Acari: Ixodidae) on naturally infested cattle in the Mexican tropics. Veterinary Parasitology, 147(3-4), 336-340. https://doi.org/10.1016/j.vetpar.2007.03.030 [ Links ]
Castaldi, S., Zorrilla, J. G., Petrillo, C., Russo, M. T., Ambrosino, P., Masi, M., Cimmino, A. y Isticato, R. (2023). Alternaria alternata isolated from infected pears (Pyrus communis) in Italy produces non-host toxins and hydrolytic enzymes as infection mechanisms and exhibits competitive exclusion against Botrytis cinerea in co-infected host Fruits. Journal of Fungi, 9, 326. https://doi.org/10.3390/jof9030326 [ Links ]
Colegio de Postgraduados (2022). Laboratorios de diagnóstico integral fitosanitario (LADIFIT). https://www.colpos.mx/posgrado/fitosanidad/ladifit.php [ Links ]
Conforto, C., Lima, N. B., Araújo, F. J., Saraiva, M. P., Maharachchikumbura, S. y Michereff, S. J. (2019). Characterization of fungal species associated with cladode brown spot on Nopalea cochenillifera in Brazil. European Journal of Plant Pathology, 155(4), 1179-1194. https://doi.org/10.1007/s10658019-01847-3 [ Links ]
Feijo, F., Silva, M., Nascimento, A., Infante, N., Ramos, R., Assunção, I. y Lima, G. S. (2019). Botryosphaeriaceae species associated with the pickly pear cactus Nopalea cochenillifera. Tropical Plant Pathology, 44(5), 452-459. https://doi.org/10.1007/s40858-019-00299-8 [ Links ]
Flores-Flores, R., Velázquez-del Valle, M. G., León-Rodríguez, R., Flores-Moctezuma, H. E. y Hernández-Lauzardo, A. N. (2013). Identification of fungal species associated with cladode spot of prickly pear and their sensitivity to chitosan. Journal of Phytopathology, 161(7-8), 544-552. https://doi.org/10.1111/jph.12104 [ Links ]
Hernández-Sánchez, E., Mora-Aguilera, G., Tlapal, B., Rodríguez-Leyva, E. y Alvarado, D. (2014). Effect of initial disease intensity of cactus black spot (Opuntia ficus-indica) in the temporal and spatial characterization. Revista Mexicana de Fitopatología, 32(2), 132-146. https://www.smf.org.mx/rmf/Vol3222014/AR/32-2_05.pdf [ Links ]
Martins, M., Ribeiro, M. H. y Almeida, C. M. M. (2023). Physicochemical, nutritional, and medicinal properties of Opuntia ficus-indica (L.) Mill. and its main agro-industrial use: A review. Plants (Basel), 3012(7), 1512. https://doi.org/10.3390/plants12071512 [ Links ]
Nakashima, T., Ueno, T., Fukami, H., Taga, T., Masuda, H., Osaki, K., Otani, H., Kohmoto, K. y Nishimura, S. (1985). Isolation and Structures of AK-Toxin I and II, Host-specific phytotoxic metabolites produced by Alternaria alternata japanese pear pathotype. Agricultural and Biological Chemistry, 49, 807-815. https://doi.org/10.1271/BBB1961.49.807 [ Links ]
Ochoa, M. J., Rivera, L. A., Arteaga-Garibay, R. I., Martínez-Peña, D., Ireta, J. y Portillo, L. (2015). Black spot caused by Pseudocercospora opuntiae in cactus pear productive systems of Jalisco, Mexico. Journal of the Professional Association for Cactus Development, 17, 1-12. https://doi.org/10.56890/jpacd.v17i.58 [ Links ]
Oliveira, L. F., Feijó, F. M., Mendes, A. L., Neto, J. D., Netto, M. S., Assunção, I. P. y Lima, G. S. (2018). Identification of Colletotrichum species associated with brown spot of cactus prickly pear in Brazil. Tropical Plant Pathology, 43(3), 247-253. https://doi.org/10.1007/s40858-018-0215-3 [ Links ]
Otani, H., Kohmoto, K. y Kodama, M. (1995). Alternaria toxins and their effects on host plants. Botany, 73, 453-458. https://doi.org/10.1139/B95-282 [ Links ]
Pacasa-Quisbert, F., Loza-Murguia, M. G., Bonifacio-Flores, A., Vino-Nina, L. y Serrano-Canaviri, T. (2017). Comunidad de hongos filamentosos en suelos del Agroecosistema de K’iphak’iphani, Comunidad Choquenaira-Viacha. Journal of the Selva Andina Research Society, 8(1), 2-25. http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S2072-92942017000100002 [ Links ]
Quezada-Salinas, A., Sandoval-Islas, J. S., Alvarado-Rosales, D. y Cárdenas-Soriano, E. (2006). Etiología de la mancha negra del nopal (Opuntia ficus-indica Mill) en Tlalnepantla, Morelos, México. Agrociencia, 40(5), 641-653. https://www.redalyc.org/pdf/302/30240509.pdf [ Links ]
Rodríguez, M. D. P. (2001). Biodiversidad de los hongos fitopatógenos del suelo de México. Acta Zoológica Mexicana, 1, 53-78. https://doi.org/10.21829/azm.2001.8401846 [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera) (2022). Panorama Agroalimentario. https://www.gob.mx/siap/ [ Links ]
Souza, A. E. F., Nascimento, L. C., Araújo, E., Lopes, E. B. y Souto, F. M. (2010). Ocorrência e identificação dos agentes etiológicos de doenças em palma forrageira (Opuntia ficus-indica Mill.) no semiárido Paraibano. Biotemas, 23(3), 11-20. https://doi.org/10.5007/21757925.2010v23n3p11 [ Links ]
Volcy, C. (2008). Génesis y evolución de los postulados de Koch y su relación con la fitopatología. Una revisión. Agronomía Colombiana, 26(1), 107-115. http://www.scielo.org.co/pdf/agc/v26n1/v26n1a13.pdf [ Links ]
White, T. J., Bruns, T., Lee, S. y Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. En M. A. Innis, D. H. Gelfand, J. J. Sninsky y T. J. White (Eds.), Protocols: a guide to methods and applications (pp. 315-322). Academic Press, New York. https://doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Yang, X., Guo, Z., Yang, Y., Abulaizi, A., Xiong, Z., Zhang, S., Li, B. y Huang, G. (2022). Isolation and identification of secondary metabolites produced by phytopathogenic fungus Corynespora cassiicola from Hevea brasiliensis. Molecules, 27, 7360. https://doi.org/10.3390/molecules27217360 [ Links ]
Received: January 18, 2024; Accepted: March 06, 2024











 texto en
texto en 


