Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex no.80 Xalapa ago. 2000
Article
Sex-steroids associated with the reproductive cycle in male and female bolson tortoise, Gopherus flavomarginatus
Rolando González Trápaga1, Gustavo Aguirre 2 and Gary A. Adest3
1 Instituto de Ecología, Centro Regional Durango, Apdo.Postal 632, Durango CP 34000, Durango, México.
2 Instituto de Ecología, Apdo. Postal 63, Xalapa CP 91000, Veracruz, México.
3 P.O. Box 879, Springville, CA 93265 USA.
Recibido: 18 de noviembre 1997
Aceptado: 13 de octubre 1999
Resumen
Estudiamos los ciclos de esteroides sexuales de machos y hembras en estado silvestre de la tortuga del Bolsón en la Reserva de la Biósfera de Mapimí (Durango). Tomamos muestras de sangre por venipunción de la yugular a intervalos bisemanales durante un período de 14 meses, determinándose los niveles circulantes de esteroides por medio de radioinmunoensayos. Los niveles hormonales mostraron tendencias estacionales. En las hembras, la testosterona tuvo un valor máximo al salir de la brumación, en asociación con el incremento en la receptividad sexual, y el nivel de estradiol aumentó después de la emergencia asociado con la maduración folicular. El nivel de estos dos esteroides se incrementó con las lluvias estacionales, cuando ocurrieron el crecimiento folicular y la vitelogénesis. La progesterona mostró niveles bajos a la emergencia, los cuales aumentaron con el incremento de la duración del dia y la temperatura, llegando al máximo antes del pico de la oviposición. La caída en los niveles de las tres hormonas estuvo asociada con la oviposición. Las hembras probablemente almacenan esperma durante parte del otoño y el invierno para usarlo en la siguiente estación reproductiva. Los machos salieron de la hibernación con bajos niveles de testosterona, pero exhibieron conductas reproductivas poco después. Junto con un incremento de la testosterona, el cortejo y la frecuencia de apareamientos se incrementaron con estímulos ambientales, prolongándose hasta principios del otoño. Los niveles de testosterona y la actividad reproductiva disminuyeron conforme se aproximaba el inicio de la hibernación. Los niveles máximos de testosterona en machos de esta especie son mayores que los conocidos para cualquier otro vertebrado.
Palabras clave: Gopherus flavomarginatus,Reproducción, Ciclos estacionales, Hormonas.
Abstract
This study describes sex-steroid cycles of free-living male and female Bolson tortoises at the Mapimi Biosphere Reserve in northeastern Durango, Mexico. Blood samples were taken at biweekly intervals during a 14 month period by jugular venipuncture, and plasma levels of steroids were determined using radioimmunoassays. Hormone levels showed seasonal trends. In females testosterone peaked following emergence, associated with increased sexual receptivity, and estradiol increased after emergence in association with follicular maturation. These two steroids increased with seasonal rains, when follicular enlargement and vitellogenesis occurred. Females emerged from brumation with low levels of progesterone, which increased with daylength and temperature and reached a maximum before the peak of egg-laying. Declines in all three hormones were associated with oviposition. Females probably store sperm during brumation for use during the following season. Males emerged after brumation with low testosterone levels and soon exhibited reproductive behavior. Along with a testosterone rise, mating and courtship increased with environmental cues and extended until early fall. Testosterone levels and reproductive activity decreased as brumation approached. Male peak testosterone levels are higher than those reported for any vertebrate.
Key words: Gopherus flavomarginatus,Reproduction, Seasonal cycles, Hormones.
Introduction
Within chelonians, there have been few organisms studied with sufficient depth to give us a general idea of the endocrinology of reproduction. One of the most studied turtle species has been Chrysemys picta (Callard et al. 1976a, b, 1978; Klicka and Mahmoud, 1977; Ganzhorn and Licht, 1983; Licht et al. 1985; Gist et al. 1990). Other species of chelonians in which the endocrinology of reproduction has been characterized are Chelydra serpentina (Klicka and Mahmoud, 1972; Lewis et al. 1979; Mahmoud et al. 1987, 1989), Sternotherus odoratus (McPherson et al. 1982; Mendonça, 1987a, b), Chelonia mydas (Licht et al., 1979, 1980; Owens and Morris, 1985), Caretta caretta (Owens and Morris, 1985; Wibbels et al. 1987a, b, 1990), Testudo hermanni (Kuchling et al. 1981), Geochelone nigra (Schramm et al. 1999) Gopherus polyphemus (Taylor, 1982) and G. agassizii (Rostal et al. 1994; Lance et al. 1995).
In this study, we documented sexual steroids concentrations in a sample of free-living males and females of the Bolson tortoise, G. flavomarginatus, at the Mapimi Biosphere Reserve in the state of Durango, in the central Chihuahuan Desert. Populations of G. flavomarginatus have declined and its range has contracted as a result of multiple factors, including climatic change and increasing anthropogenic impacts (Morafka, 1977, 1988; Aguirre et al. 1997). It is the largest terrestrial reptile in North America (Legler and Webb, 1961), and was recognized as a distinct species by Legler (1959). It has been listed as endangered by both the Mexican wildlife law and the U.S Endangered Species Act (Aguirre et al. 1997).
G. flavomarginatus spends the vast majority of its life in extensive burrows. The seasonal cycle of this species is typified by a minor activity peak prior to seasonal rains, a major peak following them and restricted activity in fall and winter (Morafka et al. 1981; Adest et al. 1989a). Information regarding the Bolson tortoise's reproductive biology has been compiled (Morafka et al. 1981; Morafka, 1982; Adest and Aguirre, 1995). Preliminary hormonal data on the reproductive cycle of G. flavomarginatus were reported by González (1995).
This paper concentrates on the reproductive endocrinology of the Bolson tortoise. The objectives of this study were to describe the annual reproductive cycle based on variation of sexual steroids, and to relate the hormonal cycle to reproductive behavior observed in the field.
Materials and methods
All field work was conducted at the Laboratorio del Desierto, located within the Mapimi Biosphere Reserve in the state of Durango, Mexico (260º 20'-260º 52' N/103º 58'-103º 32' W). Barbault and Halffter (1981) present a description of climate, vegetation and community structure of the reserve.
We captured tortoises in their burrows or while active in the surface. Field techniques for tortoise capture, transport, blood sampling, chemical immobilization, cloacal palpation, inguinal palpation of gravid females to detect eggs, and behavioral field observations are described in Aguirre et al. (1984), Adest et al. (1988, 1989b) and González (1994). Following Adest et al. (1989a) we considered animals with straight line carapace length >250 mm as adults. Tortoises were sexed on the basis of shell secondary sexual characteristic and cloacal palpation. In addition, inguinal palpation to detect the ocurrence of shelled an unshelled eggs was practiced in all females.
Heparinized samples of 3 ml of blood were withdrawn from tortoises by jugular venipuncture, maintained in ice, and centrifuged for five minutes in a tabletop clinical centrifuge. Plasma was removed and frozen in liquid nitrogen and shipped to the Laboratory of Reptilian Endocrinology at Universtiy of California, Berkeley for sex steroid radioimmunoassay as detailed in Licht et al. (1979). Female testosterone, estradiol and progesterone levels and male testosterone levels were measured. Blood samples from field-caught subjects were withdrawn approximately every two weeks between April 1985 and May 1986. Sampling was not feasible during January 1986 when tortoises were dormant in their burrows. A total of 148 adult females and 59 adult males were sampled in the course of this study. Most individuals were sampled within 24 hours of capture (100 females, 38 males). However, blood sampling was not completed within this time frame in some tortoises and re-sampling was done later. These individuals were housed in an outdoor enclosure with access to underground shelter and natural forage. Forty-eight females and 21 males were sampled this way. Females were kept up to 43 days (46 individuals one to 10 days, and two individuals 11 to 43 days) and males were kept up to 25 days (20 individuals one to 7 days and one up to 25 days) under these conditions. All animals were released in the burrow or location of capture.
We conducted statistical analyses using the computer program Data Desk4® (Velleman, 1992). We log10-transformed all steroid data to approach normality. We used one-way ANOVA to examine steroid levels of tortoises sampled within 24 hours and more than 24 hours. We examined variation in steroid levels among the 13 biweekly sampling periods with one way-ANOVA, followed by pairwise comparisons of sampling periods using Fishers's least significant difference (LSD). Means are reported ± 1 SE.
Results
No significant differences existed between steroid levels of organisms according to time elapsed to get blood, and all samples of each steroid were pooled into a single group for each sex (female testosterone: F(1,145)=3.18, P=0.076; female estradiol: F(1,146)=0.29, P=0.59; female progesterone: F(1,145)=1.45, P=0.23; male testosterone: F(1,57)=0.013, P=0.91). We describe the annual cycle separately for each steroid and sex.
Female Annual Cycle
Testosterone: A mean average value of 23.76 ± 11.89 ng/ml was recorded at the beginning of April 1985, followed by a gradual and significant decrease to the minimum mean of 2.54 ± 1.05 ng/ml observed in early July, coincident with the end of the oviposition period (early April vs. early July: P=0.006, Fisher's LSD). Following this, testosterone levels rose almost constantly (Fig. 1) displaying a significant increase in October to 28.90 ± 8.39 ng/ml (late August/early September vs. October: P=0.026, Fisher's LSD). At the beginning of brumation levels fell slightly, and then gradually increased during the remaining inactive period reaching a maximum mean during the transition between March and April 1986 (48.95 ± 12.57 ng/ml). At the end of April and beginning of May there was a decrease in circulating levels to values comparable to those observed in the early April/late May sample of the previous year.
Estradiol: Figure 2 describes a slight non-significant increase in this hormone at the end of April and beginning of May 1985, followed by a constant significant decrease to the cycle minimum of 2.5 ± 0.52 ng/ml in June (late April/early May vs. late June: P=0.04, Fisher's LSD). In July the levels increased significantly from 3.03 ± 0.42 ng/ml to 6.46 ±0.91 ng/ml (early July vs. late July: P=0.03, Fisher's LSD) and to 9.74 ± 0.91 ng/ml during late August-early September (early July vs. late August/early September: P#0.00001, Fisher's LSD). Increasing values continued until November with a mean maximum of 13.13 ± 2.65 ng/ml, after which there was a significant decrease in February 1986 to 6.81 ± 2.22 ng/ml (November vs. February: P=0.03, Fisher's LSD). No significant changes occurred for the rest of the cycle, a slight increase in levels ocurring during late March-early April 1986 followed by a drop to levels comparable to those measured in the late April/early May sample of the previous year.
Progesterone: Hormone levels doubled between the beginning of April 1985 (1.36 ± 0.60 ng/ml) and late April-early May (3.04 ± 0.71 ng/ml), the difference being very nearly significant (P=0.052, Fisher's LSD) (Fig. 3). Values continued to decline, reaching a mean low of 1.33 ± 0.23 ng/ml at the beginning of July, which was significantly different from the previous month (late June vs. early July: P=0.03, Fisher's LSD). For the rest of the year, minor, non-significant fluctuations in progesterone occurred, reaching a minimum mean value of 1.5 ± 0.34 ng/ml in February 1986. Thereafter, a significant change occurred at the end of March and beginning of April when values increased to a maximum mean of 3.53 ± 0.55 ng/ml (February vs. late March/early April: P=0.03, Fisher's LSD). This maximum was similar to that measured in the late April/early May sample of the previous year.
Male Annual Cycle
Testosterone: During early April 1985 there was a reduction in hormone levels from 327 ng/ml to the lowest level detected in late April/early May, 133.15 ng/ml (Fig. 4). Beginning in late May, concentrations increased constantly during the subsequent months, reaching the mean maximum of 1028 ± 108.66 ng/ml in late July. The differences between the two levels in early and late July were not statistically significant (P=0.10, Fisher's LSD) in spite of the fact that the first value was almost the half of the second (597.34 ± 140.20 ng/ml), probably due to small sample size. After the maximum, hormone levels dropped constantly reaching a mean low of 310.31 ± 124.41 ng/ml in November. The difference between October and November was significant (P=0.002, Fisher's LSD). During the rest of brumation testosterone values did not change significantly. Small sample sizes prohibited analysis of the brumation-activity transition although there was an increasing trend in the mean testosterone level in the late March/early April and late April/early May 1986 samples.
Discussion
Circulating sexual steroids levels in blood from free-living tortoises obtained within 24 hours of capture were not different from levels in samples obtained over different periods of time after initial capture. These results differ from those reported on the effects of confinement on physiological processes in reptiles (Lance, 1984, 1990; Licht et al. 1985; Lance and Elsey, 1986; Whittier et al. 1987; Wibbels et al. 1987a, b; Elsey et al. 1990, 1991; Cree et al. 1991). Bolson tortoises do not exhibit a marked corticosterone-mediated stress response, and variations in steroid levels observed in both sexes mainly reflect the energetic demands imposed by environmental conditions (González, 1995). We supposed that our protocol did not induce any distinct stress response to capture and/or temporary confinement, or if present, we failed to detect such a response with the sampling periodicity used.
Histological observations on the gonadal condition related to endocrine profiles have been made for G. polyphemus (Taylor, 1982) and G. agassizii (Rostal et al. 1994; Lance et al. 1995), but not for G. flavomarginatus. We used the histological information on those species to infer gonadal activity in association with steroid levels in G. flavomarginatus, coupled with information recorded by inguinal palpation on the ocurrence of eggs, and reproductive behavior observed in the field.
Female Annual Cycle
Testosterone: In female Bolson tortoises, the cycle of testosterone is similar to that reported in other female chelonians. Two maxima occur, one at the beginning of the annual activity cycle which can be related to ovarian growth before oviposition, and the second, of lesser intensity, prior to brumation and related to vitellogenesis of the following year's clutch. Basal levels occur toward the end of oviposition (July) and correlate well with data from oxytocin-induced clutches on the timing of clutch deposition (Adest et al. 1989b). The majority of wild Bolson tortoise females do not lay eggs in any given year and the average number of annual clutches is 1.4 (Adest et al. 1989a) in contrast to an average of 2.0 in captive females (Janulaw, 1978). Female captive Bolson tortoise in the Mapimi Biosphere Reserve occasionally lay up to three clutches per year (pers. obs.)
Testosterone appears to play a role as precursor in the biosynthesis of estradiol in female reptiles (Chieffi and Pierantoni, 1987). In Chrysemys picta there are two testosterone maxima coincident with estrogen levels, in the spring and fall, associated with ovarian growth and ovarian recrudescence, and supporting a precursor model (Callard et al. 1978). In Sternotherus odoratus testosterone is maximal before ovulation of the first clutch and remains high during successive clutches. Following oviposition testosterone levels decrease until a subsequent rise coincident with vitellogenesis. Low levels of testosterone suggest that in reptiles with multiple clutches there is a rapid aromatization of androgen so as to counteract its possible antivitellogenic effect (McPherson et al. 1982). In female Chelonia mydas there is an increase in circulating testosterone during the mating season, one month before oviposition, suggesting its role in stimulating sexual receptivity, followed by a decrease during the course of the breeding season (Licht et al. 1979). In comparison, the role of testosterone fluctuation seems more complex in G. flavomarginatus. Increases in testosterone observed at the end of brumation and the beginning of the active season may function as estradiol precursors for the oviposition period which follows, however the role of testosterone as stimulator of sexual receptivity does not correspond to behavior observed in the field. While courting and mating have been observed as early as March 20, the peak of mating behavior occurs after the onset of the summer rains during July and August and some mating has been documented as late as October. Thus, in Bolson tortoises, testosterone may play a role as estrogen precursor prior to oviposition early in the year and a subsequent role to increase sexual receptivity and stimulate vitellogenesis may occur later in the season.
Estradiol: Annual estrogen cycles with peaks pre- and post-brumation in female Bolson tortoises correspond to known estradiol functions in chelonians: stimulation of vitellogenesis, final maturation of ovarian follicles prior to their release, and recrudescence of the ovary after courtship and mating (Callard et al. 1978; Iverson, 1980; Taylor, 1982; Palmer and Guillette, 1990; Rostal et al. 1994; Lance et al. 1995). In G. agassizii vitellogenesis and ovarian maturation are coincident with peak air temperatures (Rostal et al. 1994). In G. flavomarginatus a similar relationship was observed where increased estradiol levels coincide with peak air temperatures and precipitation (Fig. 5).
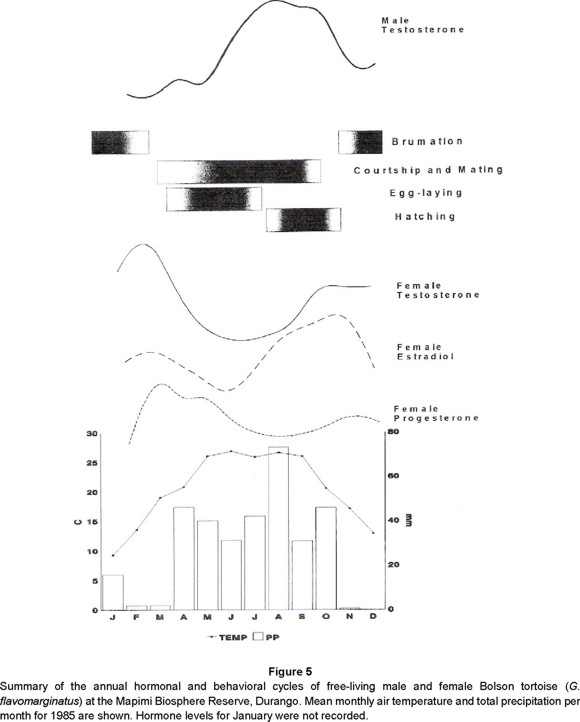
Progesterone: The pattern we observed in G. flavomarginatus females fitted those reported for congeners and correlated well with our field data. Maximum progesterone levels occured in the transition between the end of brumation and the beginning of activity, presumably associated with maturation of follicles. We were able to palp soft, oviductal eggs as early as February 24 and the first hard-shelled eggs were detected March 13 while progesterone levels peaked during March and April. Progesterone levels were lowest in early July and we found maximum abundance of palpable eggs during May and declines in palpable eggs during June and July. The latest detected clutch was July 22. Taylor (1982) reported a progesterone maximum in April in G. polyphemus, when follicles are ready for ovulation and a minimum in September following a regular and constant decline. After October, the decline was more pronounced until November and then levels remained almost constant until February. Taylor suggested that increasing progesterone levels prevented ovulation and ended follicular growth and that oviposition occured only when progesterone levels were low. Lance et al. (1995) also reported low levels of progesterone in female G. agassizii during the year and a decided peak during April around the time of ovulation.
Progesterone is also synergistic with ovarian estrogen in influencing female sexual receptivity through the development of visual and olfactory cues to which males respond (Moore, 1987). We observed a slight increase in female progesterone during late July and a peak in copulations during July and August. This is also the time of maximal rainfall and elevated ambient temperatures (Fig. 5).
We interpret female hormone levels and behavior as producing the following annual reproductive cycle in Bolson tortoises: Female tortoises emerge from brumation with low levels of progesterone but enlarged ovarian follicles. This permits the development of ovarian follicles. Progesterone levels increase with increasing daylength and temperature and reach a maximum before the peak of the oviposition period. Testosterone peaks following emergence, associated with estradiol synthesis and increased sexual receptivity. Estradiol increases after emergence and produces follicular maturation. Some courtship occurs at this time as does the detection of shelled eggs. Declines in all three hormones are associated with oviposition. Following the onset of seasonal rains, testosterone and estradiol increase and are associated with female receptivity during the peak season of mating, follicular enlargement, and vitellogenesis of the next year's eggs. Clutches laid during the previous months hatch following the onset of seasonal rains; the mean incubation time of hatching is around four months, peaking in September (Adest et al. 1989b; Adest and Aguirre, 1995). We hypothetize that female Bolson tortoises store sperm during brumation for use during the following season. This explains the presence of palpable soft and hard-shelled oviductal eggs shortly after emergence and prior to the onset of significant epigean or reproductive activity in this species. Anatomical and experimental evidence of sperm retention in female Gopherus has been reported. Palmer and Guillette (1988, 1990) observed sperm storage tubules associated with the submucosal uterine glands of G. polyphemus. Palmer et al. (1998) used allozyme data to infer paternity and identify cases where stored sperm was used to fertilize eggs in G. agassizii.
Male Annual Cycle
Testosterone: Reproductive activity of male Bolson tortoises lasts up to eight months (Fig. 5). Male tortoises court and copulate females following emergence from brumation in late March. This behavior peaks from July to September, and ends during October. The testosterone cycle is seasonal, reaching its highest average in July, just before the bout of maximum male reproductive activity, but five months after the onset of courtship and mating. In Bolson tortoises, annual testosterone cycles correspond to those reported in other species of Gopherus. In G. agassizii there are two maxima, one in April and the other in August. These correspond to times of mating activity as well as seasonal variation in chin glands (Alberts et al. 1994). Mating in Desert tortoises is reported to occur immediately after spring emergence (Rostal et al. 1994) and also in late summer (Alberts et al. 1994). Likewise, testosterone maximum coincident with emergence and prior to mating, and a second peak in fall corresponding to spermatogenesis occur in G. polyphemus (Taylor, 1982). We measured plasma testosterone levels, in replicated samples from male Bolson tortoises, as high as 1,329 ng/ml. This level occurred on July 8, and is more than two times greater than that reported for any other vertebrate. Within the genus Gopherus, Lance et al. (1995) found levels as high as 550 ng/ml in captive G. agassizii from Nevada, and Taylor (1982) measured 89.9 ng/ml in G. polyphemus. High circulating testosterone levels in reptiles have been attributed to 1) modification of the permeability in the testicular blood vessels, producing a greater discharge of testosterone into the circulation 2) the presence of a transport protein with high affinity for testosterone which decreases the metabolism of this steroid (Xavier, 1982), and 3) intratubular synthesis of androgen by Sertoli cells (Courty and Dufaure, 1979, 1980).
In several vertebrates, including temperate reptiles, gamete production and sexual behavior is expressed in two different patterns (Volsoe, 1944; Lofts, 1977, 1987; Crews, 1984; Licht, 1984). The first pattern, dissociated reproductive tactic or post-nuptial gametogenesis, involves storage of germ cells in the reproductive tract of one or both sexes during brumation to be used in the following cycle. The second pattern, associated reproductive tactic or pre-nuptial gametogenesis, does not imply such a temporal separation and the germ cells are produced and utilized within the same reproductive cycle.
The dissociated tactic has been documented in G. polyphemus (Taylor, 1982) and G. agassizii (Rostal et al. 1994; Lance et al. 1995). Reproductive tactic in male Bolson tortoises can be interpreted as associated if the observed temporal coincidence of hormonal and behavioral maxima during summer are stressed. However, considering the extent of courtship and mating, a dissociated tactic would better describe reproductive pattern in G. flavomarginatus. Although one long period of mating activity is apparent, it has not been demonstrated if insemination occurs equally throughout all the period. We documented the presence of oviductal shelled eggs during early March following emergence, suggesting summer and fall mating and sperm storage play an important function in Bolson tortoise reproduction. No histological documentation of spermatogenesis is available for this species, and it is required for a better understanding of the gametogenic function in male Bolson tortoises.
We interpret the reproductive cycle of the male Bolson tortoise as follows: Males emerge after brumation with low testosterone levels, regressed testes, and mature spermatozoa. Testosterone increases along with daylength and ambient temperature and courtship occurs with limited frequency. Testosterone increases steadily toward July as the testes recrudesce although surface activity is limited. The onset of the rainy season significantly increases epigean activity, including mating, and spermatogenesis peaks. Testosterone levels decrease as brumation approaches and the testes regress.
Acknowledgments
This project was supported by grants from the World Wildlife Fund (3109), Consejo Nacional de Ciencia y Tecnología (PCECBEU-023346), and National Science Foundation Latin American International Program (NSF INT-8504154.) We especially thank Paul Licht and the personnel of the Laboratory of Reptilian Endocrinology at UC Berkeley for steroid radioimmunoassays. Helpful comments from V. A. Lance and two anonymous reviewers improved the paper. Invaluable help was provided by D.J. Morafka, J.V. Jarchow and J. Flanagan throughout the study. We also thank all those who assisted in the collection of specimens, especially Adalberto Herrera and Juan Francisco Herrera. Permits for blood collecting and export were issued by the Secretaria de Desarrollo Urbano y Ecologia (412.2120-1845 and 413.7- 1239) and the U.S. Fish and Wildlife Service. This research is a Mexican contribution to Man and Biosphere-UNESCO Biosphere Reserve Program.
Literature cited
Adest, G.A. & G. Aguirre. 1995. Natural and life history of the Bolson tortoise, Gopherus flavomarginatus. Publ. Soc. Herpetol. Mex. 2:1-5. [ Links ]
Adest, G.A., G. Aguirre L., D.J. Morafka & J.V. Jarchow. 1989a. Bolson tortoise (Gopherus flavomarginatus) conservation: I. Life history. Vida Silv. Neotrop. 2:7-13. [ Links ]
––––––––––. 1989b. Bolson tortoise (Gopherus flavomarginatus) conservation: II. Husbandry and reintroduction. Vida Silv. Neotrop. 2:14-20. [ Links ]
Adest, G.A., J. Jarchow & B. Brydolf. 1988. A method for manual ventilation of tranquilized tortoises. Herp. Review, 19:80. [ Links ]
Aguirre, G. G. A. Adest & D.J. Morafka. 1984. Home range and movement patterns of the Bolson Tortoise, Gopherus flavomarginatus. Acta Zool. Mex., (n.s.) 1: 1-28. [ Links ]
Aguirre, G., D.J. Morafka & G.A. Adest. 1997. Conservation strategies for the Bolson tortoise, Gopherus flavomarginatus, in the Chihuahuan Desert. Pp. 333-338 In : Van Abbema J. (ed.). Proceedings: Conservation, Restoration, and Management of Tortoises and Turtles- An International Conference. State University of New York . Purchase, New York, USA. [ Links ]
Alberts, A.C., D.C. Rostal & V. Lance. 1994. Studies on the chemistry and social significance of chin gland secretions in the desert tortoise, Gopherus agassizii. Herp. Monogr. 8:116-124. [ Links ]
Barbault, R. & G. Halffter (Eds.). 1981. Ecology of the Chihuahuan Desert. Publ. Inst. Ecol. No. 8, México. [ Links ]
Callard, I.P., G.V. Callard, V. Lance & S. Eccles. 1976a. Seasonal changes in testicular structure and function and the effects of gonadotropins in the freshwater turtle, Chrysemys picta. Gen. Comp. Endocrinol. 30:347-356. [ Links ]
Callard, I.P., V. Lance, A.R. Salhanick & D. Barad. 1978. The annual ovarian cycle of Chrysemys picta: Correlated changes in plasma steroids and parameters of vitellogenesis. Gen. Comp. Endocrinol. 35:245-257. [ Links ]
Callard, I.P., I. McChesney, C. Scanes & G.V. Callard. 1976b. The influence of mammalian and avian gonadotropins on in vitro ovarian steroid synthesis in the turtle (Chrysemys picta). Gen. Comp. Endocrinol. 28:2-9. [ Links ]
Chieffi, G. & R. Pierantoni. 1987. Regulation of ovarian steroidogenesis. In: Norris, D.O. and R.E. Jones (Eds.), Hormones and reproduction in fishes, amphibians, and reptiles. Plenum Press, New York:117-144. [ Links ]
Courty, Y. & J.P. Dufaure. 1979. Levels of testosterone in the plasma and testis of the viviparous lizard (Lacerta vivipara Jacquin) during the annual cycle. Gen. Comp. Endocrinol. 39:336-342. [ Links ]
––––––––––. 1980. Levels of testosterone, dihydrotestosterone and androstenedione in the plasma and testis of a lizard (Lacerta vivipara Jacquin) during the annual cycle. Gen. Comp. Endocrinol. 42:325-333. [ Links ]
Cree, A., L.J. Guillette, Jr. & J.F. Cockrem. 1991. Identification of female Tuatara in ovulatory condition using plasma sex steroid concentrations. New Zealand J. Zool. 18:421-426. [ Links ]
Crews, D. 1984. Gamete production, sex hormone secretion, and mating behavior uncoupled. Horm. Behav. 18:22-28. [ Links ]
Elsey, R.M., T. Joanen, L. McNease & V. Lance. 1990. Stress and plasma corticosterone levels in the American Alligator: Relationships with stocking density and nesting success. Comp. Biochem. Physiol. 95A:55-63. [ Links ]
Elsey, R.M., V. Lance, T. Joanen & L. McNease. 1991. Acute stress suppresses plasma estradiol levels in female Alligators (Alligator mississippiensis). Comp. Biochem. Physiol. 100A:649-651. [ Links ]
Ganzhorn, D. & P. Licht. 1983. Regulation of seasonal gonadal cycles by temperature in the Painted turtle, Chrysemys picta. Copeia, 1983:347-358. [ Links ]
Gist, D.H., J.A. Michaleson & J.M. Jones. 1990. Autumn mating in the Painted turtle, Chrysemys picta. Herpetologica, 46:331-336. [ Links ]
González T., R.G. 1994. Reproducción de la tortuga de Mapimí, Gopherus flavomarginatus (Legler 1959). Tesis de licenciatura. Facultad de Ciencias, U.N.A.M., México. [ Links ]
––––––––––. 1995 Reproduction of the Bolson Tortoise, Gopherus flavomarginatus, Legler 1959. Publ. Soc. Herpetol. Mex. 2: 32-36. [ Links ]
Iverson, J.B. 1980. The reproductive biology of Gopherus polyphemus (Chelonia: Testudinidae). Amer. Midl. Nat. 103:353-359. [ Links ]
Janulaw, J. 1978. Captive maintenence and breeding of the Bolson tortoise. Pp. 157-163. In: Trotter, M. y C.G. Jackson Jr. (Eds.). Desert Tortoise Council Proceedings of 1978 Symposium. [ Links ]
Klicka, J. &I.Y. Mahmoud. 1972. Conversion of pregnenolone-414C to progesterone-414C by turtle corpus luteum. Gen. Comp. Endocrinol. 19:367-369. [ Links ]
––––––––––. 1977. The effects of hormones on reproductive physiology of the Painted turtle, Chrysemys picta. Gen. Comp. Endocrinol. 31:407-413. [ Links ]
Kuchling, G., R. Skolek-Winnisck & E. Bamberg. 1981. Histochemical and biochemical investigation on the annual cycle of testis, epididymis, and plasma testosterone of the tortoise, Testudo hermanni Gmelin. Gen. Comp. Endocrinol. 44:194-201. [ Links ]
Lance, V.A. 1984. Endocrinology of reproduction in male reptiles. Symp. Zool. Soc. Lond. 52:357-383. [ Links ]
––––––––––. 1990. Stress in reptiles. In: Epple, A., C.G. Scanes and M.H. Stetson (Eds.), Progress in comparative endocrinology. Wiley-Liss, Inc., New York:461-466. [ Links ]
Lance, V.A. & R.M. Elsey. 1986. Stress-induced suppression of testosterone secretion in male Alligators. J. Exp. Zool. 239:241-246. [ Links ]
Lance, V.A., D.C. Rostal, J.S. Grumbles & L. Morici. 1995. Endocrine profiles of the reproductive cycle of male and female Desert tortoises. Publ. Soc Herpetol. Mex. 2:45-49. [ Links ]
Legler, J.M. 1959. A new tortoise, genus Gopherus, from north-central Mexico. Univ. Kansas Publ. Mus. Nat. Hist. 11:335-343. [ Links ]
Legler, J.M. & R.G. Webb. 1961. Remarks on a collection of Bolson tortoises, Gopherus flavomarginatus. Herpetologica, 17:26-37. [ Links ]
Lewis, J., I.Y. Mahmoud & J. Klicka. 1979. Seasonal fluctuations in the plasma concentrations of progesterone and oestradiol-17â in the female Snapping turtle (Chelydra serpentina). J. Endocr. 80:127-131. [ Links ]
Licht, P. 1984. Reptiles. In: Lamming, G.E. (Ed.), Marshall's Physiology of Reproduction. Churchill Livingstone, Edinburgh:206-282. [ Links ]
Licht, P., G.L. Breitenbach & J.D. Congdon. 1985. Seasonal cycles in testicular activity, gonadotropin, and thyroxine in the Painted turtle, Chrysemys picta, under natural conditions. Gen. Comp. Endocrinol. 59:130-139. [ Links ]
Licht, P., W. Rainey & K. Cliffton. 1980. Serum gonadotropin and steroids associated with breeding activities in the Green sea turtle, Chelonia mydas. II. Mating and nesting in natural populations. Gen. Comp. Endocrinol. 40:116-122. [ Links ]
Licht, P., J. Wood, D.W. Owens & F. Wood. 1979. Serum gonadotropins and steroids associated with breeding activities in the Green sea turtle Chelonia mydas. I. Captive animals. Gen. Comp. Endocrinol. 39:274-289. [ Links ]
Lofts, B. 1977. Patterns of steroidogenesis and spermatogenesis in male reptiles. In: Calaby, J.H. and C.H. Tyndale-Biscoe (Eds.), Reproduction and Evolution. Australian Acad. Sci., Canberra:127-136. [ Links ]
––––––––––. 1987. Testicular function. In: Norris, D.O. and R.E. Jones (Eds.), Hormones and reproduction in fishes, amphibians, and reptiles. Plenum Press, New York:283-325. [ Links ]
Mahmoud, I.Y., R.V. Cyrus & D.L. Wright. 1987. The effect of arginine vasotocin and ovarian steroids on uterine contractility in the Snapping turtle, Chelydra serpentina. Comp. Biochem. Physiol. 86A:559-564. [ Links ]
Mahmoud, I.Y., L.J. Guillette, Jr., M.E. McAsey & C. Cady. 1989. Stress-induced changes in serum testosterone, estradiol-17â and progesterone in the turtle, Chelydra serpentina. Comp. Biochem. Physiol. 93A:423-427. [ Links ]
McPherson, R.J., L.R. Boots, R. MacGregor III & K.R. Marion. 1982. Plasma steroids associated with seasonal reproductive changes in a multiclutched freshwater turtle, Sternotherus odoratus. Gen. Comp. Endocrinol. 48:440-451. [ Links ]
Mendonça, M.T. 1987a. Photothermal effects on the ovarian cycle of the Musk turtle, Sternotherus odoratus. Herpetologica, 43:82-90. [ Links ]
––––––––––. 1987b. Timing of reproductive behavior in male Musk turtles, Sternotherus odoratus: Effects of photoperiod, temperature and testosterone. Anim. Behav. 35:1002-1014. [ Links ]
Moore, F.L. 1987. Regulation of reproductive behaviors. In: Norris, D.O. and R.E. Jones (Eds.), Hormones and reproduction in fishes, amphibians, and reptiles. Plenum Press, New York:505-522. [ Links ]
Morafka, D.J. 1977. A biogeographical analysis of the Chihuahuan Desert through its herpetofauna. Dr. W. Junk B.V. Publisher. The Hague. 313 pp. [ Links ]
––––––––––. 1982. The status and distribution of the Bolson tortoise (Gopherus flavomarginatus). Pp. 71-94 In : Bury, B.E (Ed.) North American Tortoises :Conservation and Ecology. Wildlife research report 12, U.S. Dpt. of the Interior, Washington, D.C., USA. [ Links ]
––––––––––. 1988. Historical biogeography ot the Bolson Tortoise. Annals Carnegie Museum 57: 47-72. [ Links ]
Morafka, D.J., G.A. Adest, G. Aguirre & M. Recht. 1981. The ecology of the Bolson tortoise, Gopherus flavomarginatus. In: Barbault, R. and G. Halffter (Eds.), Ecology of the Chihuahuan Desert. Publ. Inst. Ecol. No. 8:35-78. [ Links ]
Owens, D.Wm. & Y.A. Morris. 1985. The comparative endocrinology of sea turtles. Copeia, 1985:723-735. [ Links ]
Palmer, B.D. & L.J. Guillette Jr. 1988. Histology and functional morphology of the female reproductive tract of the tortoise Gopherus polyphemus. Am. J. Anat., 183:200-211. [ Links ]
––––––––––. 1990. Morphological changes in the oviductal endometrium during the reproductive cycle of the tortoise, Gopherus polyphemus. J. Morphol. 204:323-333. [ Links ]
Palmer, K.S., D.C Rostal, J.S. Grumbles & M. Mulvey. 1998. Long-term sperm storage in the desert tortoise (Gopherus agassizzi). Copeia 1998(3):702-705. [ Links ]
Rostal, D.C., V.A. Lance, J.S. Grumbles & A.C. Alberts. 1994. Seasonal reproductive cycle of the Desert tortoise (Gopherus agassizii) in the eastern Mojave desert. Herp. Monogr. 8:72-82. [ Links ]
Schramm, B.G., M. Casares & V. Lance. 1999. Steroid levels and reproductive cycle of the Galapagos tortoise, Geochelone nigra, living under seminatural conditions on Santa Cruz Island (Galapagos). Gen. Comp. Endocrinol. 114: 108-120. [ Links ]
Taylor, R.W., Jr. 1982. Seasonal aspects of the reproductive biology of the Gopher tortoise, Gopherus polyphemus. Ph.D. Dissertation. The University of Florida. [ Links ]
Velleman, P. 1992. Data Desk 4 Handbook. Data Description Inc. Ithaca, New York. [ Links ]
Volsoe, H. 1944. Structure and seasonal variation of the male reproductive organs of Vipera berus (L.). Spolia Zool. Mus. Hauniensis, 5:1-172. [ Links ]
Whittier, J.M., R.T. Mason & D. Crews. 1987. Plasma steroid hormone levels of female Red-sided garter snakes, Thamnophis sirtalis parietalis: Relationship to mating and gestation. Gen. Comp. Endocrinol. 67:33-43. [ Links ]
Wibbels, T., D.W. Owens & M.S. Amoss. 1987a. Seasonal changes in the serum testosterone titers of Loggerhead sea turtles captured along the Atlantic coast of the United States. In: Witzell, W.N. (Ed.), Ecology of east Florida sea turtles. U.S. Dep. Commer., N.O.A.A. Tech. Rep. NMFS53:59-64. [ Links ]
Wibbels, T., D.W. Owens, C.J. Limpus, P.C. Reed & M.S. Amoss, Jr. 1990. Seasonal changes in serum gonadal steroids associated with migration, mating, and nesting in the Loggerhead sea turtle (Caretta caretta). Gen. Comp. Endocrinol. 79:154-164. [ Links ]
Wibbels, T., D.W. Owens, Y.A. Morris & M.S. Amoss. 1987b. Sexing techniques and sex ratios for immature Loggerhead sea turtles captured along the Atlantic coast of the United States. In: Witzell, W.N. (Ed.), Ecology of east Florida sea turtles. U.S. Dep. Commer., N.O.A.A. Tech. Rep. NMFS53:65-74. [ Links ]
Xavier, F. 1982. Progesterone in the viviparous lizard Lacerta vivipara: Ovarian biosynthesis, plasma levels, and binding to transcortin-type protein during the sexual cycle. Herpetologica, 38:62-70. [ Links ]














